Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zili Zhai | -- | 1451 | 2023-03-07 21:36:52 | | | |
| 2 | Conner Chen | Meta information modification | 1451 | 2023-03-09 04:15:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhai, Z.; Yamauchi, T.; Shangraw, S.; Hou, V.; Matsumoto, A.; Fujita, M. Exposure to Ethanol and Acetaldehyde Cause Skin Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/41945 (accessed on 07 February 2026).
Zhai Z, Yamauchi T, Shangraw S, Hou V, Matsumoto A, Fujita M. Exposure to Ethanol and Acetaldehyde Cause Skin Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/41945. Accessed February 07, 2026.
Zhai, Zili, Takeshi Yamauchi, Sarah Shangraw, Vincent Hou, Akiko Matsumoto, Mayumi Fujita. "Exposure to Ethanol and Acetaldehyde Cause Skin Cancer" Encyclopedia, https://encyclopedia.pub/entry/41945 (accessed February 07, 2026).
Zhai, Z., Yamauchi, T., Shangraw, S., Hou, V., Matsumoto, A., & Fujita, M. (2023, March 07). Exposure to Ethanol and Acetaldehyde Cause Skin Cancer. In Encyclopedia. https://encyclopedia.pub/entry/41945
Zhai, Zili, et al. "Exposure to Ethanol and Acetaldehyde Cause Skin Cancer." Encyclopedia. Web. 07 March, 2023.
Copy Citation
Acetaldehyde (AcAH) is a carcinogenic byproduct of ethanol metabolism. Ethanol-associated malignancies commonly occur in the upper gastrointestinal tract exposed to AcAH after ethanol ingestion. Unexpectedly but true, emerging epidemiological evidence supports a link between alcohol consumption and cutaneous melanoma, suggesting skin exposure to ethanol and AcAH as potential causes of skin cancer.
melanoma
ethanol
acetaldehyde
1. Introduction
Melanoma is the most serious type of skin cancer, with an increasing incidence worldwide [1][2]. Cumulative evidence indicates that the risk of melanoma correlates with genetic factors [3][4], personal lifestyles [5][6], and phenotypic risk factors that reflect gene/personal lifestyle interactions [7][8]. Individual lifestyle factors associated with melanoma risk include UV exposure, cigarette smoking, alcohol use, overweight and obesity, poor diet, environmental pollution, and stress [5][6]. Nearly half (14 out of 29) of the studies on the relationship between alcohol consumption and melanoma, including 10 cohort and 19 case-control studies, have shown a positive correlation, while only 2 showed a negative correlation. Further, of 20 studies assessing alcohol dose effects, 13 (65%) demonstrated an association between alcohol dose and melanoma risk [9]. These associations became stronger in multiple meta-analyses with larger sample sizes [10][11][12][13]. A systematic meta-analysis by Gandini et al. found that individuals in the highest category of recent alcohol intake had a 30% increased risk of melanoma compared to those in the lowest category, and a nearly two-fold increased risk of melanoma was found with cumulative alcohol consumption [14].
Approximately 4% of cancer cases worldwide are caused by alcohol consumption [15]. The main culprit for this is acetaldehyde (AcAH), an immediate metabolite of ethanol. AcAH is a mutagen and carcinogen implicated in a wide range of cancers by forming adducts with proteins and DNA and disrupting cellular functions [16][17]. However, ethanol and AcAh are derived not just from alcohol drinking but from various sources, some of which exist naturally [18][19]. In fact, our skin is exposed to ethanol and AcAH every day, regularly at extremely low and safe levels [18][19][20]. While our skin, like the liver, is equipped with ethanol metabolism mechanisms to reduce the concentration of ethanol and AcAH [18][20], dysfunction of these ethanol and AcAH metabolizing enzymes in the skin may greatly influence the skin biology and increase the risk of ethanol/AcAH-associated skin diseases.
2. Exposure to Ethanol and AcAH
Humans are in a chemical and toxicological environment [21] and are exposed to ethanol and AcAH in many ways. Once in the bloodstream, ethanol and AcAH can reach many organs and tissues, including the skin [22]. The skin is also directly exposed to alcohols and aldehydes from natural chemicals or industrialized products [19][20].
2.1. Sources of Ethanol
Ethanol is not only the active ingredient of alcoholic beverages (beers, wines, and spirits) but also is a ubiquitous substance from various sources (Figure 1). It is one of the main indoor and outdoor pollutants [19]. In addition to alcoholic beverages and air pollutants, non-alcoholic beverages on the market can contain as much as 0.5% ethanol [23]. Certain herbal medicines, including those used to treat coughs, colds, and gastrointestinal (GI) diseases, are also sources of ethanol [24]. Furthermore, many foods contain ethanol, which is produced from sugar through fermentation. Examples include fermented foods (i.e., bread, yogurt, vinegar, and kimchi), preservatives, bakery products, fruit, and fruit juices [25]. Brewers and bakers use yeast to make a variety of alcoholic beverages and expand the dough.
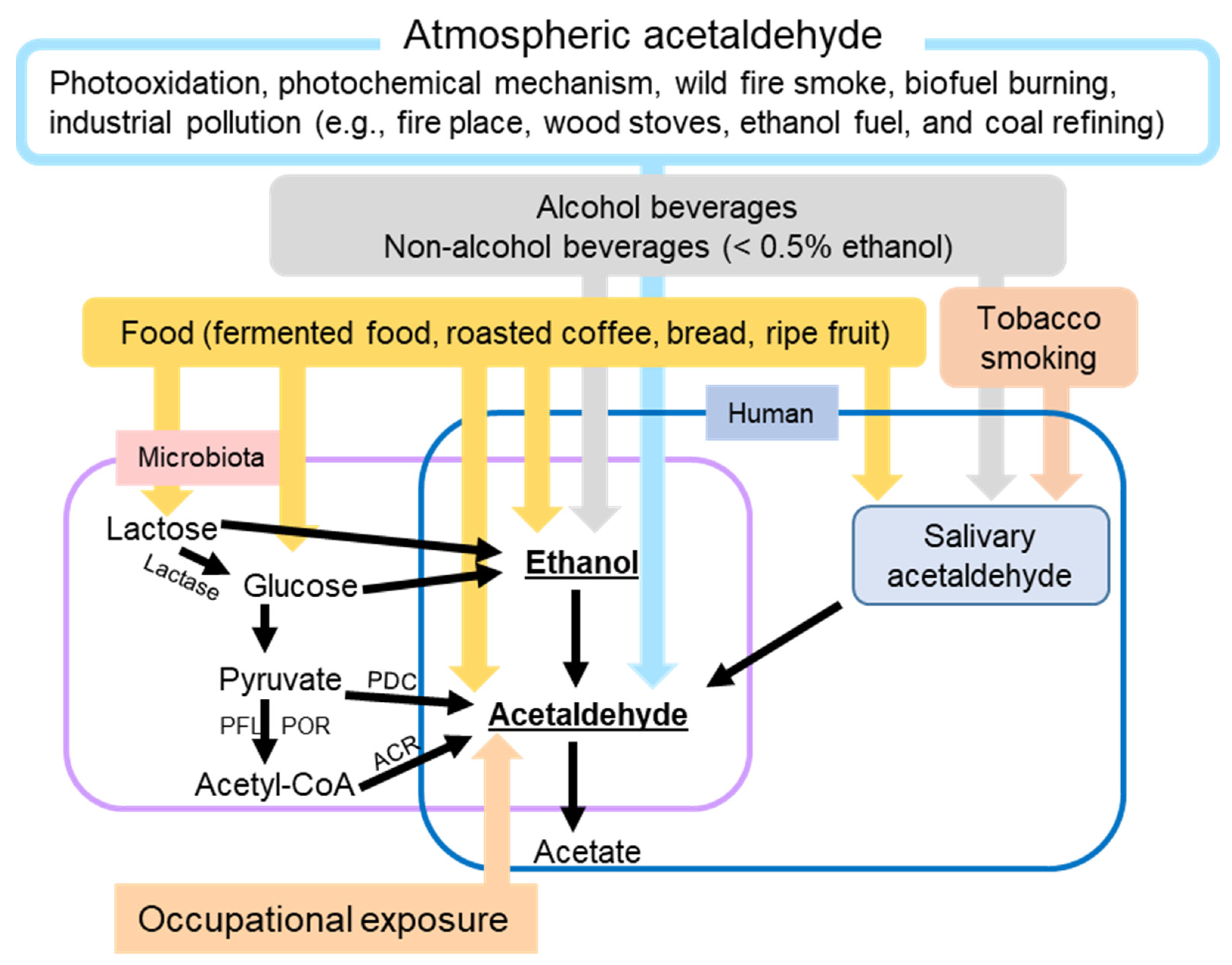
Figure 1. Schematic overview of the major factors leading to human skin exposure to ethanol and/or acetaldehyde. ACR, acetyl-CoA reductase; PDC, pyruvate decarboxylase; PFL, pyruvate formate-lyase; POR, pyruvate ferredoxin oxidoreductase.
Even without the exogenous ethanol intake mentioned above, our bodies contain low levels of ethanol. Baseline ethanol levels in the blood and breath can reach 0.02–0.15 mg/dL and 0.07–0.39 mg/L, respectively, without consuming alcohol [26]. These low levels of ethanol are generated by microbial fermentation. Fermenting yeasts such as Saccharomyces and Candida (C.) and fermenting bacteria such as Zymomonas mobilis and Sarcina ventriculi are present in our oral cavity and digestive tract. These microbiotas use anaerobic respiration to convert non-ethanol, carbohydrate-rich foods such as glucose and lactose to ethanol by fermentation in the oral cavity, GI system, or urinary system [27]. This microbial ethanol production is particularly interesting in certain medical conditions.
In auto-brewery syndrome (ABS), also known as gut fermentation syndrome, microbiota-derived ethanol concentrations in the body are comparable to those produced by directly consuming alcoholic beverages [28][29]. In a study conducted by Malik et al., the blood alcohol concentration (BAC) in an ABS patient reached 400 mg/dL [30], which is comparable to a BAC that can cause respiratory depression, coma, and death [31]. The species causing the ABS include Klebsiella pneumoniae, C. albicans, C. glabrata, Saccharomyces cerevisiae, C. intermedia, C. parapsilosis, and C. kefyr [32]. ABS is a rare condition. This syndrome could be underdiagnosed, as the symptoms may be mood changes, delirium, and brain fog or mimic a food allergy [30][32][33]. Triggers of this ABS include meals high in carbohydrates, psychological stress, and reduced dietary intake. ABS may also be related to pre-existing conditions, including a history of antibiotic use and comorbidities, such as type 2 diabetes, obesity, liver cirrhosis, and Crohn’s disease [27].
Ethanol can also be produced from AcAH by microbial reduction [34].
2.2. Sources of AcAH
Similar to ethanol exposure, human exposure to AcAH is not only from alcohol consumption but also from other diverse sources (Figure 1). AcAH can be produced endogenously in any tissue with high ethanol metabolizing enzyme activity [35]. Bodily AcAH may come from oral and gut microbes that metabolize ethanol to AcAH. Salivary AcAH levels reached up to 140 µM after ingesting a moderate amount of ethanol (0.5 g/kg body weight) [35]. Long-term exposure to locally produced AcAH in saliva may explain the higher risk of upper GI cancers in heavy drinkers [35].
The atmospheric AcAH are from photochemical production, net ocean emissions, biogenic emissions, biomass burning emissions, and anthropogenic emissions, accounting for 60%, 27%, 11%, 1.6%, and < 1% of global AcAH production, respectively [36]. The largest source is the photo-oxidation of volatile organic compounds such as alkanes and alkenes [36]. The second-largest source is water bodies that degrade dissolved organic compounds through the photochemical mechanism and emit AcAH into the atmosphere [37]. Most plant cells and some microorganisms use anaerobic respiration to break down glucose to AcAH and release carbon dioxide (biogenic emissions). Biomass burning emissions are from wildfire smoke and biofuel burning. Another source of AcAH includes urban and industrial pollution, such as residential fireplaces, woodstoves, ethanol fuel, vehicle exhaust fumes, coal refining, and waste processing. Therefore, like ethanol, AcAH is a common noxious environmental pollutant [19]. Nevertheless, sinks of atmospheric AcAH include reaction with hydroxyl radical, photolysis, and wet and dry deposition, leading to an overall atmospheric lifetime for AcAH of approximately 20 h [36][38].
AcAH sources also include occupational exposure. Individuals may be at risk of higher AcAH exposure when working in gas stations [39], transportation vehicles [40], waterpipe café [41], bakeries [42], and beauty salons [43], as well as in industries using AcAH as a solvent for perfumes, polyester resins, acetic acid, mirror silvering, tanning leather, denaturing alcohol, fuel compositions, gelatin fiber hardeners, glue and casein products, paper, cosmetics, aniline dyes, plastics, and synthetic rubber [44].
AcAH is also contained in various foods (e.g., fermented food, roasted coffee, bread, and ripe fruit) and beverages and is used as a flavoring agent and a preservative for fruits and fish [45][46].
Furthermore, AcAH is a byproduct of tobacco smoking [47]. When coupled with nicotine, AcAH has been shown to increase the addictive potential of smoking [48][49]. Nieminen et al. reported that the concentration of AcAH in saliva remains as high as 261 μM with one cigarette, which is higher than the AcAH concentration after drinking high-concentration (40%) ethanol for a short period [47]. Smoking can increase AcAH production from ethanol in saliva by 60–75%; for heavy drinking, the increase in AcAH is up to 100% [47].
Another source of AcAH is pyruvate, an important energy source produced during glycolysis. In anaerobic conditions, yeast use pyruvate decarboxylase (PDC) to convert pyruvate to AcAH, and C. albicans, an opportunistic pathogenic yeast, has been shown to contribute to oral squamous cell carcinoma progression by producing high levels of AcAH from glucose under low oxygen conditions [50][51]. Some bacteria also use PDC to convert pyruvate to AcAH. However, as a common pathway in bacteria, pyruvate is first decarboxylated to acetyl-CoA by pyruvate ferredoxin oxidoreductase and/or pyruvate formate-lyase. Acetyl-CoA is then converted into AcAH by acetylating acetyl-CoA reductase in bacteria [52]. Since the enzymes to produce AcAH from pyruvate have not been reported in humans, pyruvate-derived AcAH is likely produced from bodily microbiota rather than human cells.
Collectively, ethanol and AcAH are part of our life. The skin is exposed to these toxic metabolites through the air, water, land, smoke, food, and bodily microbiota, among other means. Since ethanol and AcAH are readily degraded in the environment or metabolized in the body, the anticipated skin exposure levels are very low or safe. However, when the body’s metabolic process becomes dysfunctional, high levels of ethanol and AcAH can cause health consequences, including skin diseases.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The growing burden of invasive melanoma: Projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171.
- Read, J.; Wadt, K.A.; Hayward, N.K. Melanoma genetics. J. Med. Genet. 2016, 53, 1–14.
- Hawkes, J.E.; Truong, A.; Meyer, L.J. Genetic predisposition to melanoma. Semin. Oncol. 2016, 43, 591–597.
- Sawada, Y.; Nakamura, M. Daily lifestyle and cutaneous malignancies. Int. J. Mol. Sci. 2021, 22, 5527.
- Batta, N.; Shangraw, S.; Nicklawsky, A.; Yamauchi, T.; Zhai, Z.; Menon, D.R.; Gao, D.; Dellavalle, R.P.; Fujita, M. Global melanoma correlations with obesity, smoking, and alcohol consumption. JMIR Dermatol. 2021, 4, e31275.
- Ribero, S.; Glass, D.; Bataille, V. Genetic epidemiology of melanoma. Eur. J. Dermatol. 2016, 26, 335–339.
- Tagliabue, E.; Gandini, S.; Bellocco, R.; Maisonneuve, P.; Newton-Bishop, J.; Polsky, D.; Lazovich, D.; Kanetsky, P.A.; Ghiorzo, P.; Gruis, N.A.; et al. MC1R variants as melanoma risk factors independent of at-risk phenotypic characteristics: A pooled analysis from the M-SKIP project. Cancer Manag. Res. 2018, 10, 1143–1154.
- Yamauchi, T.; Shangraw, S.; Zhai, Z.; Ravindran Menon, D.; Batta, N.; Dellavalle, R.P.; Fujita, M. Alcohol as a non-UV social-environmental risk factor for melanoma. Cancers 2022, 14, 5010.
- Rota, M.; Pasquali, E.; Bellocco, R.; Bagnardi, V.; Scotti, L.; Islami, F.; Negri, E.; Boffetta, P.; Pelucchi, C.; Corrao, G.; et al. Alcohol drinking and cutaneous melanoma risk: A systematic review and dose-risk meta-analysis. Br. J. Dermatol. 2014, 170, 1021–1028.
- Miura, K.; Zens, M.S.; Peart, T.; Holly, E.A.; Berwick, M.; Gallagher, R.P.; Mack, T.M.; Elwood, J.M.; Karagas, M.R.; Green, A.C. Alcohol consumption and risk of melanoma among women: Pooled analysis of eight case-control studies. Arch. Dermatol. Res. 2015, 307, 819–828.
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593.
- Rivera, A.; Nan, H.; Li, T.; Qureshi, A.; Cho, E. Alcohol intake and risk of incident melanoma: A pooled analysis of three prospective studies in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1550–1558.
- Gandini, S.; Masala, G.; Palli, D.; Cavicchi, B.; Saieva, C.; Ermini, I.; Baldini, F.; Gnagnarella, P.; Caini, S. Alcohol, alcoholic beverages, and melanoma risk: A systematic literature review and dose-response meta-analysis. Eur. J. Nutr. 2018, 57, 2323–2332.
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080.
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and biological mechanisms. Nutrients 2021, 13, 3173.
- Heymann, H.M.; Gardner, A.M.; Gross, E.R. Aldehyde-induced DNA and protein adducts as biomarker tools for alcohol use disorder. Trends Mol. Med. 2018, 24, 144–155.
- Cheung, C.; Smith, C.K.; Hoog, J.O.; Hotchkiss, S.A. Expression and localization of human alcohol and aldehyde dehydrogenase enzymes in skin. Biochem. Biophys. Res. Commun. 1999, 261, 100–107.
- Pargoletti, E.; Rimoldi, L.; Meroni, D.; Cappelletti, G. Photocatalytic removal of gaseous ethanol, acetaldehyde and acetic acid: From a fundamental approach to real cases. Int. Mater. Rev. 2022, 67, 864–897.
- Cheung, C.; Davies, N.G.; Hoog, J.O.; Hotchkiss, S.A.; Smith Pease, C.K. Species variations in cutaneous alcohol dehydrogenases and aldehyde dehydrogenases may impact on toxicological assessments of alcohols and aldehydes. Toxicology 2003, 184, 97–112.
- Ahn, J.; Hayes, R.B. Environmental influences on the human microbiome and implications for noncommunicable disease. Annu. Rev. Public Health 2021, 42, 277–292.
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612.
- Goh, Y.I.; Verjee, Z.; Koren, G. Alcohol content in declared non-to low alcoholic beverages: Implications to pregnancy. Can. J. Clin. Pharmacol. 2010, 17, e47–e50.
- Kelber, O.; Steinhoff, B.; Nauert, C.; Biller, A.; Adler, M.; Abdel-Aziz, H.; Okpanyi, S.N.; Kraft, K.; Nieber, K. Ethanol in herbal medicinal products for children: Data from pediatric studies and pharmacovigilance programs. Wien. Med. Wochenschr. 2017, 167, 183–188.
- Gorgus, E.; Hittinger, M.; Schrenk, D. Estimates of ethanol exposure in children from food not labeled as alcohol-containing. J. Anal. Toxicol. 2016, 40, 537–542.
- Jones, A.W. Excretion of low-molecular weight volatile substances in human breath: Focus on endogenous ethanol. J. Anal. Toxicol. 1985, 9, 246–250.
- Painter, K.; Cordell, B.J.; Sticco, K.L. Auto-brewery syndrome. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022.
- Hafez, E.M.; Hamad, M.A.; Fouad, M.; Abdel-Lateff, A. Auto-brewery syndrome: Ethanol pseudo-toxicity in diabetic and hepatic patients. Hum. Exp. Toxicol. 2017, 36, 445–450.
- Welch, B.T.; Coelho Prabhu, N.; Walkoff, L.; Trenkner, S.W. Auto-brewery syndrome in the setting of long-standing Crohn’s disease: A case report and review of the literature. J. Crohn’s Colitis 2016, 10, 1448–1450.
- Malik, F.; Wickremesinghe, P.; Saverimuttu, J. Case report and literature review of auto-brewery syndrome: Probably an underdiagnosed medical condition. BMJ Open Gastroenterol. 2019, 6, e000325.
- Vonghia, L.; Leggio, L.; Ferrulli, A.; Bertini, M.; Gasbarrini, G.; Addolorato, G.; Alcoholism Treatment Study, G. Acute alcohol intoxication. Eur. J. Intern. Med. 2008, 19, 561–567.
- Bayoumy, A.B.; Mulder, C.J.J.; Mol, J.J.; Tushuizen, M.E. Gut fermentation syndrome: A systematic review of case reports. United Eur. Gastroenterol. J. 2021, 9, 332–342.
- Dinis-Oliveira, R.J. The auto-brewery syndrome: A perfect metabolic “storm” with clinical and forensic implications. J. Clin. Med. 2021, 10, 4637.
- Pronk, J.T.; Yde Steensma, H.; Van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633.
- Homann, N.; Jousimies-Somer, H.; Jokelainen, K.; Heine, R.; Salaspuro, M. High acetaldehyde levels in saliva after ethanol consumption: Methodological aspects and pathogenetic implications. Carcinogenesis 1997, 18, 1739–1743.
- Millet, D.B.; Guenther, A.; Siegel, D.A.; Nelson, N.B.; Singh, H.B.; de Gouw, J.A.; Warneke, C.; Williams, J.; Eerdekens, G.; Sinha, V.; et al. Global atmospheric budget of acetaldehyde: 3-D model analysis and constraints from in-situ and satellite observations. Atmos. Chem. Phys. 2010, 10, 3405–3425.
- Singh, H.B.; Salas, L.J.; Chatfield, R.B.; Czech, E.; Fried, A.; Walega, J.; Evans, M.J.; Field, B.D.; Jacob, D.J.; Blake, D.; et al. Analysis of the atmospheric distribution, sources, and sinks of oxygenated volatile organic chemicals based on measurements over the Pacific during TRACE-P. J. Geophys. Res.-Atmos. 2004, 109, D15S07.
- Custer, T.; Schade, G. Methanol and acetaldehyde fluxes over ryegrass. Tellus B 2007, 59, 673–684.
- Cruz, L.P.S.; Luz, S.R.; Campos, V.P.; Santana, F.O.; Alves, R.S. Determination and risk assessment of formaldehyde and acetaldehyde in the ambient air of gas stations in Salvador, Bahia, Brazil. J. Brazil. Chem. Soc. 2020, 31, 1137–1148.
- Hadei, M.; Shahsavani, A.; Hopke, P.K.; Kermani, M.; Yarahmadi, M.; Mahmoudi, B. Comparative health risk assessment of in-vehicle exposure to formaldehyde and acetaldehyde for taxi drivers and passengers: Effects of zone, fuel, refueling, vehicle’s age and model. Environ. Pollut. 2019, 254, 112943.
- Naddafi, K.; Nabizadeh, R.; Rostami, R.; Ghaffari, H.R.; Fazlzadeh, M. Formaldehyde and acetaldehyde in the indoor air of waterpipe cafes: Measuring exposures and assessing health effects. Build. Environ. 2019, 165, 106392.
- Chang, P.T.; Hung, P.C.; Tsai, S.W. Occupational exposures of flour dust and airborne chemicals at bakeries in Taiwan. J. Occup. Environ. Hyg. 2018, 15, 580–587.
- Hadei, M.; Hopke, P.K.; Shahsavani, A.; Moradi, M.; Yarahmadi, M.; Emam, B.; Rastkari, N. Indoor concentrations of VOCs in beauty salons; association with cosmetic practices and health risk assessment. J. Occup. Med. Toxicol. 2018, 13, 30.
- Karaffa, L.S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; RSC Publishing: London, UK, 2013.
- Miyake, T.; Shibamoto, T. Quantitative analysis of acetaldehyde in foods and beverages. J. Agric. Food Chem. 1993, 41, 1968–1970.
- Uebelacker, M.; Lachenmeier, D.W. Quantitative determination of acetaldehyde in foods using automated digestion with simulated gastric fluid followed by headspace gas chromatography. J. Autom. Methods Manag. Chem. 2011, 2011, 907317.
- Nieminen, M.T.; Salaspuro, M. Local acetaldehyde-An essential role in alcohol-related upper gastrointestinal tract carcinogenesis. Cancers 2018, 10, 11.
- Talhout, R.; Opperhuizen, A.; van Amsterdam, J.G. Role of acetaldehyde in tobacco smoke addiction. Eur. Neuropsychopharmacol. 2007, 17, 627–636.
- Rabinoff, M.; Caskey, N.; Rissling, A.; Park, C. Pharmacological and chemical effects of cigarette additives. Am. J. Public Health 2007, 97, 1981–1991.
- Marttila, E.; Bowyer, P.; Sanglard, D.; Uittamo, J.; Kaihovaara, P.; Salaspuro, M.; Richardson, M.; Rautemaa, R. Fermentative 2-carbon metabolism produces carcinogenic levels of acetaldehyde in Candida albicans. Mol. Oral Microbiol. 2013, 28, 281–291.
- Kumamoto, C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391.
- Eram, M.S.; Ma, K. Decarboxylation of pyruvate to acetaldehyde for ethanol production by hyperthermophiles. Biomolecules 2013, 3, 578–596.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
09 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




