You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Won Wong | -- | 1477 | 2023-03-07 03:27:18 | | | |
| 2 | Conner Chen | + 10 word(s) | 1487 | 2023-03-08 02:56:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chan, Y.T.; Cheok, Y.Y.; Cheong, H.C.; Tang, T.F.; Sulaiman, S.; Hassan, J.; Looi, C.Y.; Tan, K.; Abubakar, S.; Wong, W.F. Zika Virus Properties. Encyclopedia. Available online: https://encyclopedia.pub/entry/41916 (accessed on 25 December 2025).
Chan YT, Cheok YY, Cheong HC, Tang TF, Sulaiman S, Hassan J, et al. Zika Virus Properties. Encyclopedia. Available at: https://encyclopedia.pub/entry/41916. Accessed December 25, 2025.
Chan, Yee Teng, Yi Ying Cheok, Heng Choon Cheong, Ting Fang Tang, Sofiah Sulaiman, Jamiyah Hassan, Chung Yeng Looi, Kim-Kee Tan, Sazaly Abubakar, Won Fen Wong. "Zika Virus Properties" Encyclopedia, https://encyclopedia.pub/entry/41916 (accessed December 25, 2025).
Chan, Y.T., Cheok, Y.Y., Cheong, H.C., Tang, T.F., Sulaiman, S., Hassan, J., Looi, C.Y., Tan, K., Abubakar, S., & Wong, W.F. (2023, March 07). Zika Virus Properties. In Encyclopedia. https://encyclopedia.pub/entry/41916
Chan, Yee Teng, et al. "Zika Virus Properties." Encyclopedia. Web. 07 March, 2023.
Copy Citation
ZIKV belongs to the genus of Flavivirus in the Flaviviridae family that comprises multiple deadly human pathogens, including the dengue virus (DENV), Japanese encephalitis (JEV), the yellow fever virus (YFV), and the West Nile virus (WNV). ZIKV infection is known to result in severe manifestations including neurological complications in adults and congenital abnormalities in newborns.
Zika virus
neurological symptoms
gene and structure
1. Gene and Structure
The zika virus genome is made up of 10.8 kb positive-sense, single-stranded RNA flanked by the 5′ and 3′ untranslated regions (UTRs) with a single open reading frame (ORF) [1][2]. The ORF region encodes a single polypeptide, which is processed into three structural proteins, including a capsid (C), precursor membrane (prM), and envelope (E), as well as seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The C proteins construct the icosahedral viral capsid, which encapsulates the viral genomic RNA, while the prM and E proteins are anchored on the outer membrane. prM is cleaved by host cell furin protease to generate mature virion, whereas the E protein is involved in binding and membrane fusion, which permit viral entry into the host cells [3].
NS1 is a glycoprotein of approximately 60 kDa that serves as an RNA replication complex in flaviviruses. Due to the importance of its replicative function in flaviviruses, the NS1 sequence is highly conserved, whereby ZIKV shares a >50% sequence homology with DENV2 ( dengue virus (DENV)) and West Nile virus (WNV) NS1 [4]. The protein is found in the following different forms in various locations in the host cells: (i) dimers in membrane-bound vesicles in the lumen of the endoplasmic reticulum, (ii) dimers in association with the membranes of flavivirus-infected cells, and (iii) highly immunogenic hexamers that are secreted into extracellular fluid [5][6].
NS2A is a 22 kDa transmembrane protein located in the endoplasmic reticulum, and it plays a critical role in the viral replication process [7]. It also interacts with NS2B and NS3 to recruit viral RNA, prM, and E to the virion assembly site for virus morphogenesis [8][9]. It has been suggested that the NS2A protein participates in ZIKV-induced neurological damage, as it interacted with multiple adherent junctions in an embryonic mouse cortex and impaired radial glial cell proliferation in human forebrain organoids [10].
NS2B/NS3 forms the viral protease complex that is involved in genome replication and cleavage of the viral polypeptide [11]. NS3 carries the protease domain at the N-terminus and the RNA helicase domain at the C-terminus, while NS2B acts as the membrane-bound domain that positions NS3 to its substrate and forms part of the NS3 catalytic domain for substrate binding [12][13].
NS4A/NS4B cause neurological impairment via manipulating the cellular survival and autophagy signaling pathways [14]. The introduction of NS4A or NS4B in human fetal neural stem cells (NSCs) resulted in impaired neurosphere formation, likely through inhibiting Akt kinase phosphorylation at Thr308 and Ser473 and mammalian target of rapamycin (mTOR) phosphorylation at Ser2448, which disrupted autophagy [15]. NS5, on the other hand, comprises methyltransferase with a short linker to the RNA-dependent RNA polymerase (RdRP) that is vital for RNA replication. It performs guanylyl transferase activity to catalyze the de novo formation of a methylated RNA cap structure using a triphosphorylated RNA transcript [16].
2. African and Asian ZIKV Lineages
Phylogenetic analysis has classified the ZIKV into two major genotypes, namely, the African and Asian lineages; the latter is further subdivided into the local Asian or contemporary American subclades [17][18]. The African and Asian ZIKV lineages display differences in virulence, transmissibility, and replication kinetics [19][20][21], despite sharing a high degree of similarity (>88.9%) in their genomic sequences [1]. The African ZIKV strain demonstrates a higher rate of transmissibility in the mosquito vector Aedes aegypti compared to its Asian counterpart [22]. Its infection results in a higher rate of lethality and can lead to cases of fetal death [23]. In contrast, the low-virulence Asian lineage does not induce early cell death, but it may lead to chronic infections in the fetal central nervous system [24]. The reemergence of ZIKV epidemics in 2015 were dominated by a strain of Asian ZIKV lineage that is commonly named the American strain [25]. Preceding the outbreak, ZIKV Asian lineage had been associated with an evolutionary mutation in the viral E gene (V473M) during replication and transmission between mosquito and host [26]. This mutation increases its virulence and viremia generation, hence enhancing transmission, which could be a critical determinant in the epidemics. Intriguingly, an effort to inverse the V473M substitution in the epidemic ZIKV strain isolated in Puerto Rico in 2015 reversed the pathogenic phenotypes of the virus [26]. Recent ZIKV outbreaks of the local Asian lineage have been reported in different states of India in 2018 and 2021 [27][28][29].
3. Transmission and Life Cycle
Similar to other flaviviruses, ZIKV is vector-borne and can be disseminated by infected female Ae. aegypti and Ae. albopictus mosquitoes. However, it differs from DENV in that it can be transmitted vertically from a pregnant mother to a baby [30][31], via blood transfusions [32], and via sexual intercourse [33][34]. Vertical transmission is observed in the mosquito vectors, Ae. aegypti and Ae. albopictus, to the larvae of infected mosquitoes [30][35].
The life cycle of ZIKV is highly similar to other members of the Flavivirus family; it begins with the entry of a viral particle into a host cell via clathrin-mediated endocytosis modulated by the binding of viral protein E. Viral entry is facilitated by the rolling and accumulation of viral particles along a host cell surface. The differential expression of various binding factors in a host cell surface dictates the viral tropism. The presence of the transmembrane receptor tyrosine kinase protein anexelekto (AXL), which is highly expressed by neural cells, dendritic cell-specific intracellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN), tyrosine-protein kinase receptor (TYRO3,) and T-cell immunoglobulin and mucin domain 1 (TIM-1) on host cells is vital for the viral endocytic event [36][37].
When ZIKV reaches a clathrin-expressing surface, the host cell membrane invaginates and fuses with the viral membrane in the presence of acidic host cell cytoplasm, allowing the viral genome to be released into the cytoplasm (Figure 1). Following the release, protein translation occurs, and the newly synthesized viral proteins will be recruited into the endoplasmic reticulum for assembly [38]. With help from the NS proteins, new and immature viral particles migrate to the Golgi body, where precursor prM proteins are cleaved. Mature virions are subsequently released from the cell and are ready for a new cycle of infection. Occasionally, an immature viral particle carrying the uncleaved prM can be released [39].
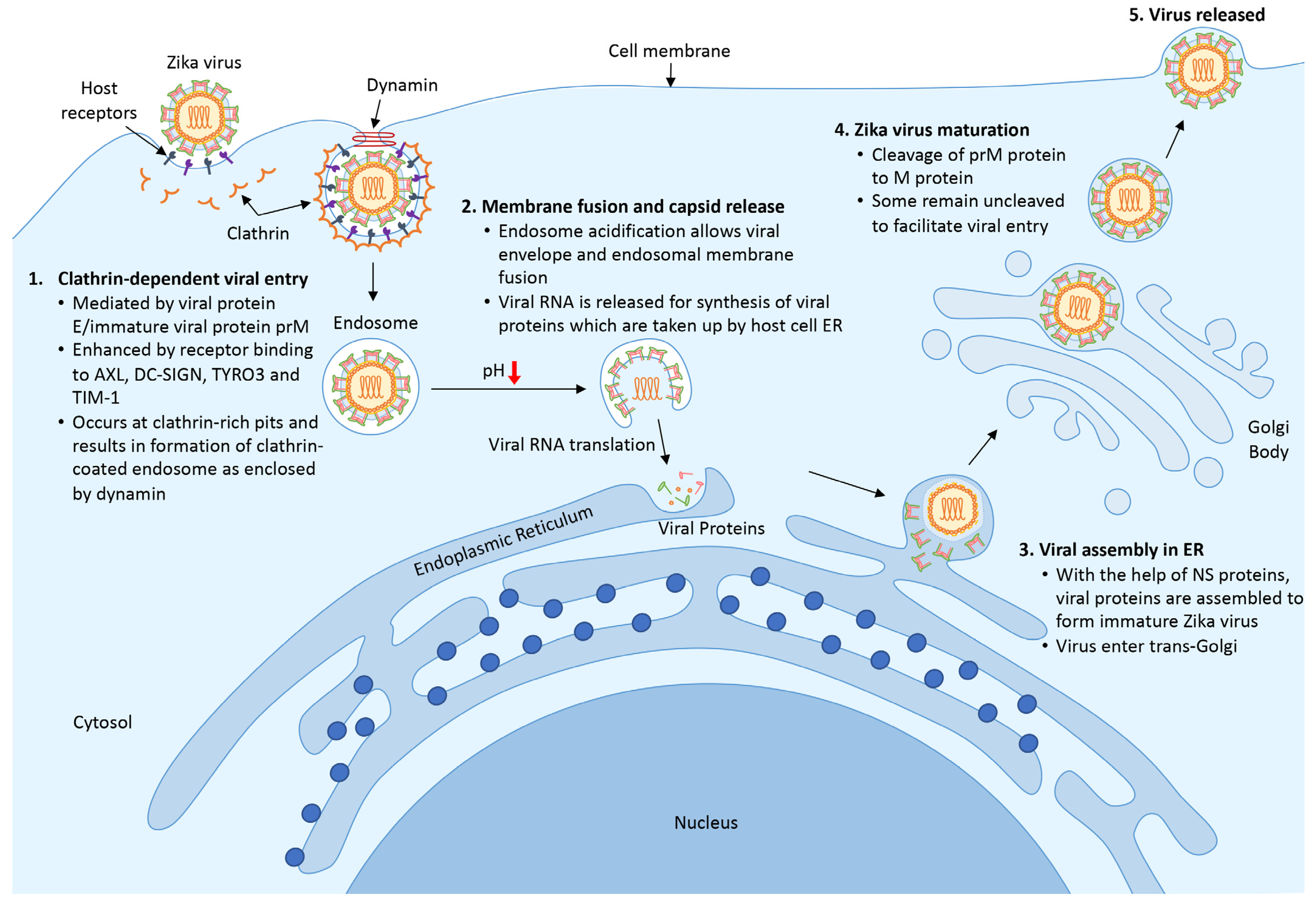
Figure 1. Life cycle of ZIKV. (1) ZIKV encounters host cells and binds to host receptors (AXL, DC-SIGN, TYRO3, and TIM-1) via viral protein E and prM and initiates clathrin-dependent viral entry. (2) Upon entering the cell, the endosome matures and acidifies, resulting in the release of viral RNA and the translational process to synthesize viral proteins. (3) New viral proteins are assembled into an immature viral particle within the endoplasmic reticulum. (4) Immature viral particles enter the trans-Golgi network where prM protein is cleaved into a mature virus. Finally, the newly formed virus is released to the surrounding areas and is ready for subsequent infection.
4. Symptoms Caused by ZIKV Infection
During the Yap Island outbreak in 2007, a majority of the cases were mild, with clinical symptoms that included low-grade fever, maculopapular rash, arthralgia, and conjunctivitis [40]. Severe neurological complications of ZIKV infection were observed in a small number of cases during the French Polynesian outbreak. This was highlighted by the increased prevalence of an autoimmune disease causing acute or subacute flaccid paralysis, known as Guillain–Barre syndrome, to approximately a 20-fold higher rate than was expected (1/2 in 100,000 people per year) in adults, approximately 3 weeks following the ZIKV outbreak [41]. Trends of microcephaly among newborns of infected mothers were reported during the outbreak in Brazil from 2015 to 2016 [42]. Other forms of neurological deficits, including meningoencephalitis [43][44], transverse myelitis [45], ophthalmic manifestation with optic nerve and retina complications [46][47], and other neuronal developmental defects [48], were identified among infants. Subsequent studies using human brain organoids [49], as well as animal models using macaques, mice, or fruit flies [31][50][51], have confirmed the viral neurotropism and developmental impact. Early neurological impairments, including severe intellectual disability, spastic tetraparesis, dysphagia, and failure to thrive [52], as well as severe motor impairment, were recently described in congenital ZIKV-infected children [53].
ZIKV causes neurological deficits through damaging neuronal development and proliferation. Li, et al. [54] showed that in human neural progenitor cells (NPCs), ZIKV infection caused cell-cycle arrest, apoptosis, and the inhibition of cell differentiation, which eventually gave rise to cortical thinning and microcephaly. Gabriel, et al. [55] reported that ZIKV infection resulted in the premature differentiation of NPCs, which was associated with centrosome perturbation, progenitor depletion, disrupted ventricular zone proliferation, impaired neurogenesis, and cortical thinning. In addition, Onorati, et al. [56] utilized a single-cell RNA-sequencing technique to investigate the effects of ZIKV on the neuropathogenesis of neocortical and spinal cord neuroepithelial stem cells, and they demonstrated that ZIKV infection caused disrupted cell mitoses, supernumerary centrosomes, structural disorganization, and cell death. Treatment with nucleoside analogs inhibited ZIKV replication and ZIKV-mediated death in neuroepithelial stem cells [56].
References
- Wang, A.; Thurmond, S.; Islas, L.; Hui, K.; Hai, R. Zika virus genome biology and molecular pathogenesis. Emerg. Microbes Infect. 2017, 6, e13.
- Enfissi, A.; Codrington, J.; Roosblad, J.; Kazanji, M.; Rousset, D. Zika virus genome from the Americas. Lancet 2016, 387, 227–228.
- Liu, Z.-Y.; Shi, W.-F.; Qin, C.-F. The evolution of Zika virus from Asia to the Americas. Nat. Rev. Microbiol. 2019, 17, 131–139.
- Akey, D.L.; Brown, W.C.; Dutta, S.; Konwerski, J.; Jose, J.; Jurkiw, T.J.; DelProposto, J.; Ogata, C.M.; Skiniotis, G.; Kuhn, R.J.; et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 2014, 343, 881–885.
- Youn, S.; Li, T.; McCune, B.T.; Edeling, M.A.; Fremont, D.H.; Cristea, I.M.; Diamond, M.S. Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulates replication of West Nile virus. J. Virol. 2012, 86, 7360–7371.
- Hilgenfeld, R. Zika virus NS1, a pathogenicity factor with many faces. EMBO J. 2016, 35, 2631–2633.
- Marquez-Jurado, S.; Nogales, A.; Avila-Perez, G.; Iborra, F.J.; Martinez-Sobrido, L.; Almazan, F. An Alanine-to-Valine Substitution in the Residue 175 of Zika Virus NS2A Protein Affects Viral RNA Synthesis and Attenuates the Virus In Vivo. Viruses 2018, 10, 547.
- Zhang, X.; Xie, X.; Xia, H.; Zou, J.; Huang, L.; Popov, V.L.; Chen, X.; Shi, P.-Y. Zika Virus NS2A-Mediated Virion Assembly. mBio 2019, 10, e02375-02319.
- Zhang, X.; Xie, X.; Zou, J.; Xia, H.; Shan, C.; Chen, X.; Shi, P.-Y. Genetic and biochemical characterizations of Zika virus NS2A protein. Emerg. Microbes Infect. 2019, 8, 585–602.
- Yoon, K.-J.; Song, G.; Qian, X.; Pan, J.; Xu, D.; Rho, H.-S.; Kim, N.-S.; Habela, C.; Zheng, L.; Jacob, F.; et al. Zika-Virus-Encoded NS2A Disrupts Mammalian Cortical Neurogenesis by Degrading Adherens Junction Proteins. Cell Stem Cell 2017, 21, 349–358.
- Sampath, A.; Padmanabhan, R. Molecular targets for flavivirus drug discovery. Antivir. Res. 2009, 81, 6–15.
- Noble, C.G.; Seh, C.C.; Chao, A.T.; Shi, P.Y. Ligand-Bound Structures of the Dengue Virus Protease Reveal the Active Conformation. J. Virol. 2012, 86, 438–446.
- Aleshin, A.E.; Shiryaev, S.A.; Strongin, A.Y.; Liddington, R.C. Structural evidence for regulation and specificity of flaviviral proteases and evolution of the Flaviviridae fold. Protein Sci. 2007, 16, 795–806.
- McLean, J.E.; Wudzinska, A.; Datan, E.; Quaglino, D.; Zakeri, Z. Flavivirus NS4A-induced Autophagy Protects Cells against Death and Enhances Virus Replication. J. Biol. Chem. 2011, 286, 22147–22159.
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.-S.; Lee, S.-A.; Ge, J.; Wang, S.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 2016, 19, 663–671.
- Issur, M.; Geiss, B.J.; Bougie, I.; Picard-Jean, F.; Despins, S.; Mayette, J.; Hobdey, S.E.; Bisaillon, M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA 2009, 15, 2340–2350.
- Faye, O.; Freire, C.C.; Iamarino, A.; Faye, O.; de Oliveira, J.V.; Diallo, M.; Zanotto, P.M.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014, 8, e2636.
- Haddow, A.D.; Nasar, F.; Guzman, H.; Ponlawat, A.; Jarman, R.G.; Tesh, R.B.; Weaver, S.C. Genetic Characterization of Spondweni and Zika Viruses and Susceptibility of Geographically Distinct Strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni Virus. PLoS Negl. Trop. Dis. 2016, 10, e0005083.
- Calvez, E.; O'Connor, O.; Pol, M.; Rousset, D.; Faye, O.; Richard, V.; Tarantola, A.; Dupont-Rouzeyrol, M. Differential transmission of Asian and African Zika virus lineages by Aedes aegypti from New Caledonia. Emerg. Microbes. Infect. 2018, 7, 159.
- Shi, H.; Yin, J. Kinetics of Asian and African Zika virus lineages over single-cycle and multi-cycle growth in culture: Gene expression, cell killing, virus production, and mathematical modeling. Biotechnol. Bioeng. 2021, 118, 4231–4245.
- Simonin, Y.; van Riel, D.; Van de Perre, P.; Rockx, B.; Salinas, S. Differential virulence between Asian and African lineages of Zika virus. PLoS Negl. Trop. Dis. 2017, 11, e0005821.
- Aubry, F.; Jacobs, S.; Darmuzey, M.; Lequime, S.; Delang, L.; Fontaine, A.; Jupatanakul, N.; Miot, E.F.; Dabo, S.; Manet, C.; et al. Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nat. Commun. 2021, 12, 916.
- Sheridan, M.A.; Balaraman, V.; Schust, D.J.; Ezashi, T.; Roberts, R.M.; Franz, A.W.E. African and Asian strains of Zika virus differ in their ability to infect and lyse primitive human placental trophoblast. PLoS ONE 2018, 13, e0200086.
- Anfasa, F.; Siegers, J.Y.; van der Kroeg, M.; Mumtaz, N.; Stalin Raj, V.; de Vrij, F.M.S.; Widagdo, W.; Gabriel, G.; Salinas, S.; Simonin, Y.; et al. Phenotypic Differences between Asian and African Lineage Zika Viruses in Human Neural Progenitor Cells. mSphere 2017, 2, e00292-17.
- WHO. Zika Epidemiology Update; World Health Organization: Geneva, Switzerland, 2019.
- Shan, C.; Xia, H.; Haller, S.L.; Azar, S.R.; Liu, Y.; Liu, J.; Muruato, A.E.; Chen, R.; Rossi, S.L.; Wakamiya, M.; et al. A Zika virus envelope mutation preceding the 2015 epidemic enhances virulence and fitness for transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 20190–20197.
- Khan, E.; Jindal, H.; Mishra, P.; Suvvari, T.K.; Jonna, S. The 2021 Zika outbreak in Uttar Pradesh state of India: Tackling the emerging public health threat. Trop. Doct. 2022, 52, 474–478.
- Bardhan, M.; Pramanik, D.; Riyaz, R.; Hasan, M.M.; Essar, M.Y. Dual burden of Zika and COVID-19 in India: Challenges, opportunities and recommendations. Trop. Med. Health 2021, 49, 83.
- Yadav, P.D.; Malhotra, B.; Sapkal, G.; Nyayanit, D.A.; Deshpande, G.; Gupta, N.; Padinjaremattathil, U.T.; Sharma, H.; Sahay, R.R.; Sharma, P.; et al. Zika virus outbreak in Rajasthan, India in 2018 was caused by a virus endemic to Asia. Infect. Genet. Evol. 2019, 69, 199–202.
- Thangamani, S.; Huang, J.; Hart, C.E.; Guzman, H.; Tesh, R.B. Vertical Transmission of Zika Virus in Aedes aegypti Mosquitoes. Am. J. Trop. Med. Hyg. 2016, 95, 1169–1173.
- Yockey, L.J.; Varela, L.; Rakib, T.; Khoury-Hanold, W.; Fink, S.L.; Stutz, B.; Szigeti-Buck, K.; Van den Pol, A.; Lindenbach, B.D.; Horvath, T.L.; et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 2016, 166, 1247–1256.
- Musso, D.; Nhan, T.; Robin, E.; Roche, C.; Bierlaire, D.; Zisou, K.; Shan Yan, A.; Cao-Lormeau, V.M.; Broult, J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. = Eur. Commun. Dis. Bull. 2014, 19, 20761.
- Moreira, J.; Peixoto, T.M.; Siqueira, A.M.; Lamas, C.C. Sexually acquired Zika virus: A systematic review. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2017, 23, 296–305.
- Prisant, N.; Bujan, L.; Benichou, H.; Hayot, P.-H.; Pavili, L.; Lurel, S.; Herrmann, C.; Janky, E.; Joguet, G. Zika virus in the female genital tract. Lancet Infect. Dis. 2016, 16, 1000–1001.
- Ciota, A.T.; Bialosuknia, S.M.; Ehrbar, D.J.; Kramer, L.D. Vertical Transmission of Zika Virus by Aedes aegypti and Ae. albopictus Mosquitoes. Emerg. Infect. Dis. 2017, 23, 880–882.
- Persaud, M.; Martinez-Lopez, A.; Buffone, C.; Porcelli, S.A.; Diaz-Griffero, F. Infection by Zika viruses requires the transmembrane protein AXL, endocytosis and low pH. Virology 2018, 518, 301–312.
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896.
- Agrelli, A.; de Moura, R.R.; Crovella, S.; Brandao, L.A.C. ZIKA virus entry mechanisms in human cells. Infect. Genet. Evol. 2019, 69, 22–29.
- Rey, F.A.; Stiasny, K.; Heinz, F.X. Flavivirus structural heterogeneity: Implications for cell entry. Curr. Opin. Virol. 2017, 24, 132–139.
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Eng. J. Med. 2009, 360, 2536–2543.
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539.
- de Oliveira, W.K.; Carmo, E.H.; Henriques, C.M.; Coelho, G.; Vazquez, E.; Cortez-Escalante, J.; Molina, J.; Aldighieri, S.; Espinal, M.A.; Dye, C. Zika Virus Infection and Associated Neurologic Disorders in Brazil. N. Engl. J. Med. 2017, 376, 1591–1593.
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugières, P.; Fourati, S.; Cleret de Langavant, L.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596.
- Schwartzmann, P.V.; Ramalho, L.N.Z.; Neder, L.; Vilar, F.C.; Ayub-Ferreira, S.M.; Romeiro, M.F.; Takayanagui, O.M.; dos Santos, A.C.; Schmidt, A.; Figueiredo, L.T.M.; et al. Zika Virus Meningoencephalitis in an Immunocompromised Patient. Mayo Clin. Proc. 2017, 92, 460–466.
- Mécharles, S.; Herrmann, C.; Poullain, P.; Tran, T.-H.; Deschamps, N.; Mathon, G.; Landais, A.; Breurec, S.; Lannuzel, A.J.T.L. Acute myelitis due to Zika virus infection. Lancet 2016, 387, 1481.
- Yepez, J.B.; Murati, F.A.; Pettito, M.; Peñaranda, C.F.; de Yepez, J.; Maestre, G.; Arevalo, J.F.; for The Johns Hopkins Zika, C. Ophthalmic Manifestations of Congenital Zika Syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017, 135, 440–445.
- Tsui, I.; Moreira, M.E.L.; Rossetto, J.D.; Vasconcelos, Z.; Gaw, S.L.; Neves, L.M.; Zin, O.A.; Haefeli, L.; Silveira Filho, J.C.B.; Gomes, S.C.; et al. Eye Findings in Infants with Suspected or Confirmed Antenatal Zika Virus Exposure. Pediatrics 2018, 142, e20181104.
- Lopes Moreira, M.E.; Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Damasceno, L.; Pone, M.; Carvalho, L.M.A.; Pone, S.M.; Vasconcelos, Z.; Ribeiro, I.P.; et al. Neurodevelopment in Infants Exposed to Zika Virus in Utero. N. Engl. J. Med. 2018, 379, 2377–2379.
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818.
- Liu, Y.; Gordesky-Gold, B.; Leney-Greene, M.; Weinbren, N.L.; Tudor, M.; Cherry, S. Inflammation-Induced, STING-Dependent Autophagy Restricts Zika Virus Infection in the Drosophila Brain. Cell Host Microbe 2018, 24, 57–68.
- Raper, J.; Kovacs-Balint, Z.; Mavigner, M.; Gumber, S.; Burke, M.W.; Habib, J.; Mattingly, C.; Fair, D.; Earl, E.; Feczko, E. Long-term alterations in brain and behavior after postnatal Zika virus infection in infant macaques. Nat. Commun. 2020, 11, 2534.
- Romani, N.; Pieras, M.; Frick, M.A.; Sulleiro, E.; Rodo, C.; Silgado, A.; Suy, A.; Espiau, M.; Thorne, C.; Giaquinto, C.; et al. Neurological Short-Term Outcomes of a Cohort of Children Born to Zika Virus-Infected Mothers in Barcelona. Children 2022, 9, 537.
- Ribeiro, C.T.M.; Hamanaka, T.; Pone, S.; Aibe, M.S.; Gomes, S.C.; Nielsen-Saines, K.; Brickley, E.B.; Moreira, M.E.; Pone, M. Gross motor function in children with Congenital Zika Syndrome from Rio de Janeiro, Brazil. Eur. J. Pediatr. 2022, 181, 783–788.
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–126.
- Gabriel, E.; Ramani, A.; Karow, U.; Gottardo, M.; Natarajan, K.; Gooi, L.M.; Goranci-Buzhala, G.; Krut, O.; Peters, F.; Nikolic, M.; et al. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell 2017, 20, 397–406.
- Onorati, M.; Li, Z.; Liu, F.; Sousa, A.M.M.; Nakagawa, N.; Li, M.; Dell'Anno, M.T.; Gulden, F.O.; Pochareddy, S.; Tebbenkamp, A.T.N.; et al. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 2016, 16, 2576–2592.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
839
Revisions:
2 times
(View History)
Update Date:
08 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




