Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jean-Denis Bailly | -- | 2700 | 2023-03-06 07:32:26 | | | |
| 2 | Sirius Huang | Meta information modification | 2700 | 2023-03-07 02:00:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Al Hallak, M.; Verdier, T.; Bertron, A.; Roques, C.; Bailly, J. Aerosolization of Moulds Particles from Contaminated Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/41889 (accessed on 08 February 2026).
Al Hallak M, Verdier T, Bertron A, Roques C, Bailly J. Aerosolization of Moulds Particles from Contaminated Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/41889. Accessed February 08, 2026.
Al Hallak, Mohamad, Thomas Verdier, Alexandra Bertron, Christine Roques, Jean-Denis Bailly. "Aerosolization of Moulds Particles from Contaminated Materials" Encyclopedia, https://encyclopedia.pub/entry/41889 (accessed February 08, 2026).
Al Hallak, M., Verdier, T., Bertron, A., Roques, C., & Bailly, J. (2023, March 06). Aerosolization of Moulds Particles from Contaminated Materials. In Encyclopedia. https://encyclopedia.pub/entry/41889
Al Hallak, Mohamad, et al. "Aerosolization of Moulds Particles from Contaminated Materials." Encyclopedia. Web. 06 March, 2023.
Copy Citation
Fungi are well known as common contaminants of the indoor environment with the ability to grow on many types of building materials and to subsequently release biological particles into the indoor air. The aerosolization of allergenic compounds or mycotoxins borne by fungal particles or vehiculated by dust may have a direct impact on the occupant’s health.
indoor air quality (IAQ)
bioaerosols
fungi
building materials
aerosolization
mycotoxins
1. Introduction: Importance of Moulds as Indoor Contaminants
The biological contaminants of the indoor environments include fungi, bacteria, viruses, pollen, etc. [1]. However, the ability of fungi to grow on almost all building materials, whether natural or synthetic, especially if they are hygroscopic or wet [2], necessitate the study of their development on such substrates. Water activity (amount of free water available for microbial metabolism) is considered the most impacting factor for fungal development [3]. Researches have emphasized that many kinds of materials are susceptible to growth once there is a sufficient amount of available water: wood, gypsum boards, wallpapers, mortars, etc. [4]. In parallel, according to various intrinsic parameters, including chemical composition, pH, presence of dust, etc., building materials can present different bioreceptivities, i.e., susceptibilities, to support and favour mould growth [5][6][7][8]. Moreover, their development is governed by various environmental factors, including temperature and relative humidity, and their spread in the indoor environment can also involve other factors, including air exchange rate, air movement, building structure and location, design and ventilation system; however, this list is not exhaustive [2].
Due to their reliance to water activity, water-damaged buildings are highly sensitive sites in terms of indoor fungal development [5]. However, the increase in water content on/into building materials may also be caused by plumbing [9] and water vapour condensation on the walls in strongly insulated buildings [10]. In Northern Europe and North America, the first works on indoor contamination by fungi were published in the 1970s. This problem increased strongly with time in conjunction with changes in human activities and the increasing time spent inside buildings (more than 80% of the time in industrialized countries). Studies showed that 20% to 40% of buildings in these regions display a visible mould presence [11][12]. Moreover, various building types were reported to present moisture problems and subsequent fungal contaminations, including homes, schools, workplaces and hospitals, representing many sources of exposure to toxic compounds [13][14][15]. Indeed, in parallel with their growth, moulds are able to release biological particles (spores, mycelium fragments, etc.) and toxic compounds (allergic molecules, mycotoxins) into the air, referred as bioaerosols.
Bioaerosols correspond to aerosols involving microorganisms, such as fungi, bacteria and viruses, or organic compounds emanating from microorganisms, such as endotoxins, metabolites, toxins, etc. [16][17]. The biological part of bioaerosols forms approximately 50% of all aerosol particles [18], and their sizes usually range from 0.001 nm to 100 μm [19]. They are released from surfaces into the indoor air either by active processes that are specific to the moulds’ forms of development or by passive processes that mostly rely on air movement in buildings due to human activity or ventilation. These aerosolization processes contribute to the degradation of indoor air quality (IAQ) and may subsequently affect the health of occupants due to the inhalation of toxic particles, leading to allergies or respiratory troubles [16][17][19][20][21].
Indeed, depending on their size, particles can be inhaled by exposed individuals and infiltrate different parts of the lungs (from the trachea to the bronchioles), leading to various health problems [22][23]. Even if the direct causality between the presence of moulds and specific diseases among exposed individuals is difficult to demonstrate, strong associations have been reported in many works [24][25][26][27] and such a direct relationship is also highly suspected by health authorities [27]. In addition, it should be emphasized that different fungal genera and, more precisely, different fungal species pose different health risks for humans. For example, some species belonging to the Aspergillus genera, such as Aspergillus flavus [28][29][30][31] or Aspergillus fumigatus [32][33][34], are well known to play specific role in three different clinical settings in humans: opportunistic infections, allergic states and toxicosis [34]. Cladosporium species are rarely directly pathogenic to humans, even if they have been sometimes reported to cause infections of the skin and lungs. However, the spores of Cladosporium species are significant allergens and their presence in large amounts in the air can severely affect people with asthma and other respiratory diseases [35][36]. By contrast, Penicillium species are diverse and widely distributed in the environment, but despite their abundance and diversity, they are not commonly associated with human and animal infections [37].
2. Aerosolization of Moulds Particles from Contaminated Materials
Most aerosolization studies focused on moulds as common microorganisms that are capable of growing on building materials, reproducing and then releasing airborne particles into the indoor air under specific conditions and in various forms, such as spores or mycelial fragments, that may contain mycotoxins. The release of fungal particles from colonized sites, e.g., building materials, into the indoor air can be defined as an aerosolization process [16]. In the following section, the mechanisms of aerosolization and different factors influencing the release of particles (fungal spores, fungal fragments) from contaminated surfaces into the indoor air are presented.
2.1. The Mechanism of Aerosolization and Characteristics of Airborne Particles
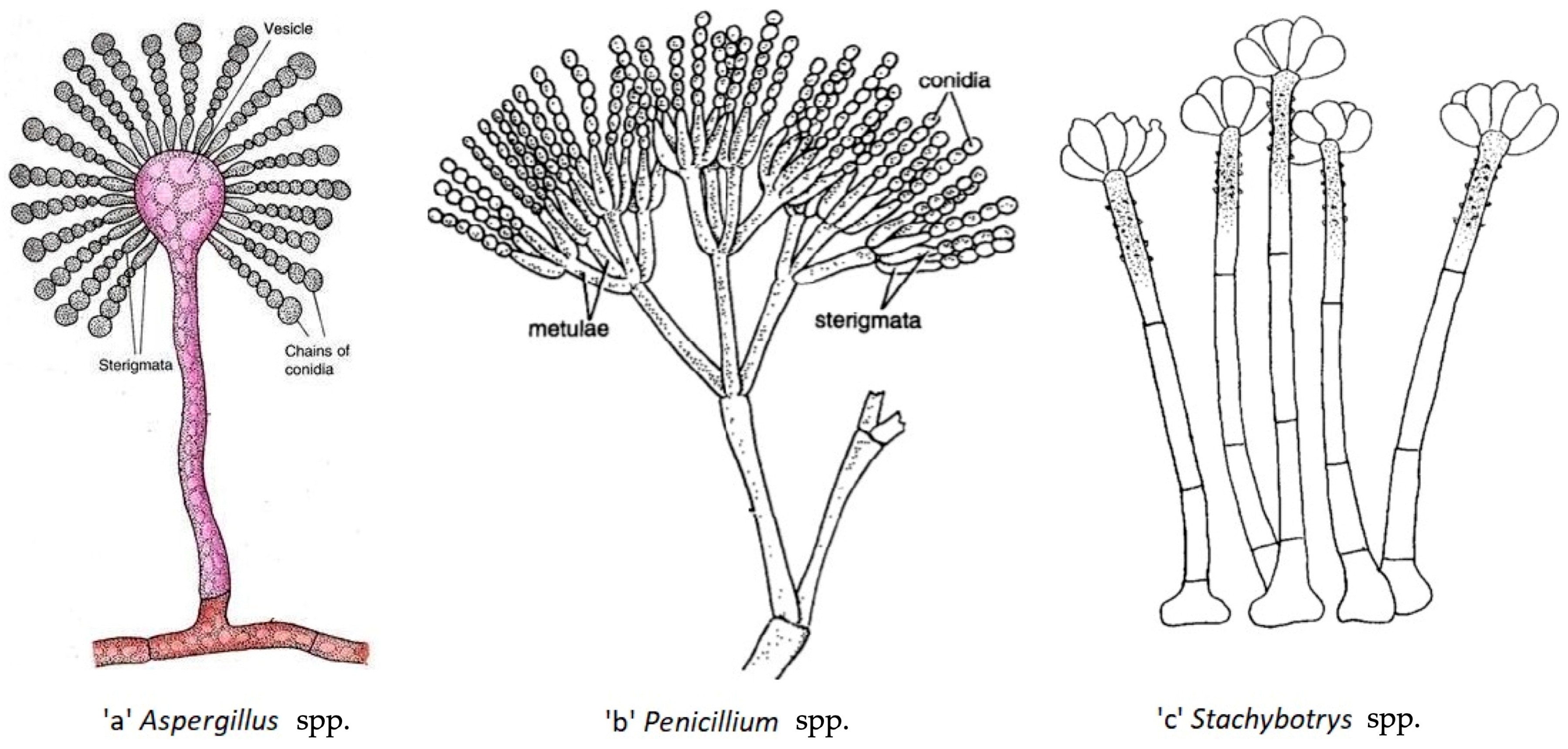
Both passive and active mechanisms may be involved in the release of fungal particles from materials [38]. Active release involves fungal spores and is based on forces arising inside the fungi and is attributable to a burst of energy by osmotic pressure and surface tension discharge [39]. Passive release can involve spores but also other particles and occurs due to energy originating from outside the fungus, such as mechanical disturbances of the colony, e.g., handling, vibrating or ventilating, which may cause release of particles from surfaces [38]. In the indoor environment, human daily activities, such as vacuuming, sweeping, walking, etc., are considered passive mechanisms for the release of fungal particles and have been shown to increase fungal spore concentrations in the indoor air [40]. In addition, the transfer of spores into air strongly depends on their shape and on the organization of conidia in fungal structures (Figure 1). For instance, Aspergillus and Penicillium spp. are characterized by spores that are organized in long chains (Figure 1a,b), which allow them to be easily released. On the other hand, spores of the Stachybotrys spp. are clusters covered with dry slime and are therefore not directly exposed to air flow, which makes it harder for them to become airborne (Figure 1c) [41][42][43]. Thus, the aerosolization processes and mechanisms are affected by the nature of involved micro-organism, some of them being designed to spread more easily in the air (Aspergillus spp., Penicillium spp., etc.) than others (Stachybotrys spp.).
Different studies reported that the release of fungal fragments from contaminated building materials may exceed that of spores. In aerosolization chamber studies, Górny et al. [44] and Cho et al. [45] reported that the release of fungal fragments is 11 to 320 times higher than the release of spores for Aspergillus versicolor, 17 to 170 higher for Cladosporium cladosporioides and 7 to 270 higher for Penicillium mellini, emphasizing that other fungal particles than spores can be aerosolized from a contaminated substrate. By evaluating the size of these particles, researchers were able to distinguish between fungal fragments and fungal spores. In that work, authors based their classification on previous studies that evaluated spore size distributions through the microscopic observations of the studied fungal species. They were 2–3.5 µm for A. versicolor (close to spheres), 2–3 µm by 4–7 µm for C. cladosporioides (ellipsoidal shape) and 5–6 µm for P. mellini (close to spheres) [46]. Based on these data, the particle size of 1.6 µm was selected by Gorny et al. as the lowest size limit separating fungal spores from fungal fragments [44]. They evaluated the air speed necessary to observe the highest ratios of fragments/spore release. The results were 5.8 m/s for A. versicolor, 1.4 m/s for C. cladosporioides and 0.3 m/s for P. mellini, and the highest ratios were obtained at 29 m/s for all species [44]. It has to be noted that air speeds used in that study are very much higher than those normally encountered in buildings.
After their release, airborne fungal spores and fragments can be considered as solid particles and will behave differently in the air and, if inhaled, in the lungs, based on various particle-related (physical, chemical) and human-related (biological) factors [15]. Physical factors include the morphological characteristics (size, shape, density, electrical charge, etc.) of particles. Chemical factors include composition and hygroscopicity [47]. Regarding physico-chemical factors, it has been reported that the inhalation of airborne particles by human is interdependent on both their size and the period they are actually suspended in the air [48]: microbial particles of 100, 10, 3, 1 and 0.5 μm require 5.8 s, 8.2 min, 1.5 h, 12 h and 41 h to be inhaled, respectively, according to Stoke’s law [49]. Biological host-related factors, such as the breathing pattern, the route of the breath and the anatomy of the airways, will also influence inhalation, as well as fungi-related factors, such as the presence of membranous proteins (e.g., adhesins). Fragments and spores can be both harmful to human health but the fact that fragments are smaller in size than spores provides them a stronger ability to go deeper through the respiratory system when inhaled by humans (Figure 2) [38][44].
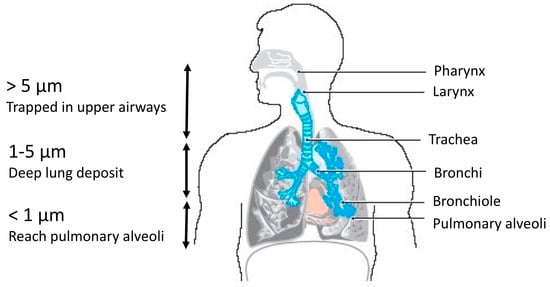
Figure 2. Relation between the size of particulate matters (PM) and their ability to penetrate the respiratory tract of human (adapted from Costa et al. [50]).
For instance, according to their size (5 to 6 µm), Penicillium mellini spores may penetrate the respiratory system only to pharynx level, whereas the spores of Aspergillus versicolor of 2 to 3.5 µm may penetrate up to bronchi levels. The fragments of different species may penetrate in all the respiratory system as their sizes are <1.6 µm (Figure 2) [51]. These data are obviously to be re-evaluated according to the physiological state of the subject’s bronchi.
2.2. External Factors Impacting the Release of Bioaerosols
There are numerous environmental factors that may impact directly or indirectly the release and spread of bioaerosols in the indoor air. They are related either to the involved microorganisms, to the colonized material, to the occupants’ activities and to the environment that impact both of the growth of microorganisms as well as their aerosolization.
Microbial-related factors include diversity, age of colonization, spreading and stage of development, etc. As presented previously, certain fungal species are more likely to be aerosolized than others (e.g., Stachybotrys spp. vs. Aspergillus spp.). It has to be highlighted that there are no data on the impact of multi-species colonization on the release of the different involved species (additivity, synergy or even antagonism towards aerosolization process).
Material factors include hydroscopy, smoothness, roughness, composition, etc. [41][46][52]. Lee et al. [5] studied the release of different fungal particles from different flood-damaged building materials in vitro: linoleum, rugs, carpets and pillows. They observed that, at an air velocity of 0.9 m/s, the total amount of particles released over 10 min was the highest for linoleum (25,503 particles/cm2), followed by rugs (1562 particles/cm2), carpets (508 particles/cm2) and pillows (24 particles/cm2), which was correlated with the highest particle concentration observed in linoleum and the lowest observed in the pillow samples. While comparing the duration required for release, Lee et al. detected that time required to aerosolize 90% of the total released particles was shortest for linoleum (<6 s), followed by pillows (<12 s), carpets (24 s) and rugs (78 s), which correlates with the hardness and smoothness of the surfaces [5]. Another study focused on the nature of substrate as an important factor affecting the release of mycotoxins. From different substrates maintained at a constant temperature, relative humidity and luminosity conditions, Moularat and Robine [53] observed clear variations in the release of sterigmatocystin (ST) from A. versicolor. The percentage of toxin released compared to the total amount produced on the substrates was 4% in the case of traditional wallpaper, while only 1% of ST was aerosolized from fibre glass and vinyl wallpaper due to the chemical and physical effects of substrate on the aerosolization process [53]. The chemical composition of different substrates provides diverse nutrients and thus different energy levels for the microorganisms to grow. It may therefore directly influence the quantity of toxins produced during fungal development. Considering its further aerosolization, microbial adhesion on the material, microbial development and water retention in the material depend on the structure of the substrate, which would, in this situation, influence the percentage of the toxin to be aerosolized [53].
Environmental factors mainly include turbulence and air velocity, temperature and relative humidity [40][48][54]. The indoor air velocity may be affected by natural ventilation that varies according to the weather and climate and also to the air exchange rates. It can also be influenced by mechanical ventilation due to the presence of ventilators inside the rooms or by heating or air-handling systems. Górny et al. [44] and Aleksic et al. [41] investigated the effect of increasing air velocities on the aerosolization of particles in laboratory conditions. They studied the release of fungal spores and fungal fragments from agar surfaces and ceiling tiles [44] or from wallpapers [41], using specific aerosolization devices. As expected, higher air velocities implied the higher release rates of fungal particles from surfaces into the air in both studies. It was found by Aleksic et al. [41] that air velocities of 0.3 m/s (movement in a room), 2 m/s (mechanical ventilation) and 6 m/s (strong mechanical ventilation or strong draft while opening a window) were able to aerosolize particles of Penicillium brevicompactum, Aspergillus versicolor and Stachybotrys chartarum, respectively [41]. Moreover, an air velocity of 0.3 m/s was sufficient to release different numbers of particles for A. versicolor, Cladosporium cladosporioides and Penicillium mellini [44].
Relative humidity is also one of the most studied environmental factors that impact aerosolization. Frankel et al. [54] tested the effect of relative humidity (RH) on the release of particulate matters (PM1) (0.54–1.037 µm) and inhalable fractions (1–20 µm) from gypsum boards colonized with Penicillium spp. in laboratory conditions. They found that the percentages of gypsum board surface area colonized with fungi correlated positively and significantly with the number of aerosolized particles. In addition, a significantly higher number of PM1 were aerosolized from low RH (22.5 to 27.7%) surfaces (median: 433, range 41–1764 particles/min/cm2) compared to those with high RH (94.3 to 96.7%) (median: 83, range 9–303 particles/min/cm2). In general, the literature shows that the lower the RH, the higher the concentration of fungal particle in the air [55][56][57]. However, it was demonstrated that particles released under wet conditions have a higher total inflammatory potential (TIP) than those released under dry conditions [54]. It is worth noting that the total inflammatory potential is obtained by using Granulocytes assay (an assay used to assess the microbial contamination of medical drugs) [58]. HL-60 cells are exposed to the particles collected by GSP sampler and react by producing reactive oxygen species (ROS) when exposed to microbial compounds. The total inflammatory potential of a particle correlates positively with the ROS produced [54].
Moreover, meteorological factors have also been shown to impact indoor airborne concentrations of bioaerosols. Frankel et al. [59] compared the quantities of airborne particles of fungi and bacteria in the indoor environment according to different seasons. They concluded that: (1) in winter and spring, the main sources of airborne fungi present in the indoor air are originated from indoor environments, while in other seasons, the main sources of fungal airborne particles have outdoor sources. (2) In all seasons, as the outdoor temperature, indoor temperature and AER (air exchange rate) increase, the concentration of indoor airborne particles of fungi increases [59].
In summary, this text highlights that air velocity, material type and relative humidity appear to be, if not the main, the most studied factors in different aerosolization building-related studies. It appears that microbial-related factors are less studied in building environments, thus it is difficult to quantify their impact on particles aerosolization and to compare it with environmental or material factors. In addition, it should be noted that, among the reviewed studies, there is no indication of the relative importance of one of these factors or another on the release of particles.
These findings provide perspective on the aerosolization of microbial particles from materials, which is a well-known phenomenon in the field of microbiology but surprisingly unstudied in the context of building contamination. This section also emphasizes that the aerosolization mechanisms seems a key parameter in the degradation of IAQ by material-colonizing mould.
References
- Ayanbimpe, G.M.; Danjuma, W.S.; Okolo, M.O. Relationship between Fungal Contamination of Indoor Air and Health Problems of Some Residents in Jos; IntechOpen: London, UK, 2012.
- Haleem Khan, A.A.; Mohan Karuppayil, S. Fungal Pollution of Indoor Environments and Its Management. Saudi J. Biol. Sci. 2012, 19, 405–426.
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A Review of Indoor Microbial Growth across Building Materials and Sampling and Analysis Methods. Build. Environ. 2014, 80, 136–149.
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS Laboratory Manual Series 2; CBS-Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010.
- Lee, J.E.; Lee, B.U.; Bae, G.N.; Jung, J.H. Evaluation of Aerosolization Characteristics of Biocontaminated Particles from Flood-Damaged Housing Materials. J. Aerosol Sci. 2017, 106, 93–99.
- Abdel Hameed, A.A.; Khoder, M.I.; Ibrahim, Y.H.; Saeed, Y.; Osman, M.E.; Ghanem, S. Study on Some Factors Affecting Survivability of Airborne Fungi. Sci. Total Environ. 2012, 414, 696–700.
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and Reanalysing the Concept of Bioreceptivity 25 Years On. Sci. Total Environ. 2021, 770, 145314.
- Shirakawa, M.A.; Selmo, S.M.; Cincotto, M.A.; Gaylarde, C.C.; Brazolin, S.; Gambale, W. Susceptibility of Phosphogypsum to Fungal Growth and the Effect of Various Biocides. Int. Biodeterior. Biodegrad. 2002, 49, 293–298.
- Shoemaker, R.C.; House, D.E. A Time-Series Study of Sick Building Syndrome: Chronic, Biotoxin-Associated Illness from Exposure to Water-Damaged Buildings. Neurotoxicol. Teratol. 2005, 27, 29–46.
- Soulios, V.; Jan de Place Hansen, E.; Peuhkuri, R. Hygrothermal Performance of Hydrophobized and Internally Insulated Masonry Walls—Simulating the Impact of Hydrophobization Based on Experimental Results. Build. Environ. 2021, 187, 107410.
- Nielsen, K.F. Mycotoxin Production by Indoor Molds. Fungal Genet. Biol. 2003, 39, 103–117.
- Platt, S.D.; Martin, C.J.; Hunt, S.M.; Lewis, C.W. Damp Housing, Mould Growth, and Symptomatic Health State. BMJ 1989, 298, 1673–1678.
- Gollogly, L. World Health Statistics 2009; World Health Organization: Geneva, Switzerland, 2009; ISBN 92-4-156381-8.
- Bernstein, J.A.; Alexis, N.; Bacchus, H.; Bernstein, I.L.; Fritz, P.; Horner, E.; Li, N.; Mason, S.; Nel, A.; Oullette, J.; et al. The Health Effects of Nonindustrial Indoor Air Pollution. J. Allergy Clin. Immunol. 2008, 121, 585–591.
- World Health Organization. WHO Guidelines for Indoor Air Quality: Dampness and Mould; WHO Regional Office Europe: Copenhagen, Denmark, 2009; ISBN 978-92-890-4168-3.
- Srikanth, P.; Sudharsanam, S.; Steinberg, R. Bio-aerosols in indoor environment: Composition, health effects and analysis. Indian J. Med. Microbiol. 2008, 26, 302–312.
- Douwes, J.; Thorne, P.; Pearce, N.; Heederick, D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200.
- Jaenicke, R. Abundance of Cellular Material and Proteins in the Atmosphere. Science 2005, 308, 73.
- Humbal, C.; Gautam, S.; Trivedi, U. A Review on Recent Progress in Observations, and Health Effects of Bioaerosols. Environ. Int. 2018, 118, 189–193.
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Airborne Bioaerosols and Their Impact on Human Health. J. Environ. Sci. 2018, 67, 23–35.
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14.
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71.
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakul, W.; Barnes, C.; Baxi, S.; Grimes, C.; Horner, W.E.; Kennedy, K.; Larenas-Linnemann, D.; et al. Exposure and Health Effects of Fungi on Humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404.
- Dales, R.E.; Zwanenburg, H.; Burnett, R.; Franklin, C.A. Respiratory Health Effects of Home Dampness and Molds among Canadian Children. Am. J. Epidemiol. 1991, 134, 196–203.
- Cooley, J.D.; Wong, W.C.; Jumper, C.A.; Straus, D.C. Correlation between the Prevalence of Certain Fungi and Sick Building Syndrome. Occup. Environ. Med. 1998, 55, 579–584.
- Bush, R.K.; Portnoy, J.M.; Saxon, A.; Terr, A.I.; Wood, R.A. The Medical Effects of Mold Exposure. J. Allergy Clin. Immunol. 2006, 117, 326–333.
- ANSES. Moisissures dans le Bâti; ANSES: Paris, France, 2016.
- Mokobi, F. Aspergillus flavus—An Overview. Available online: https://microbenotes.com/aspergillus-flavus/ (accessed on 10 February 2023).
- Krishnan, S.; Manavathu, E.K.; Chandrasekar, P.H. Aspergillus flavus: An Emerging Non-Fumigatus Aspergillus Species of Significance. Mycoses 2009, 52, 206–222.
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human Pathogen, Allergen and Mycotoxin Producer. Microbiology 2007, 153, 1677–1692.
- Mokobi, F. Aspergillus fumigatus—An Overview. Available online: https://microbenotes.com/aspergillus-fumigatus/ (accessed on 10 February 2023).
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus—What Makes the Species a Ubiquitous Human Fungal Pathogen? PLoS Pathog. 2013, 9, e1003743.
- Latgé, J.-P. Aspergillus fumigatus, a Saprotrophic Pathogenic Fungus. Mycologist 2003, 17, 56–61.
- Howard, D.H. Pathogenic Fungi in Humans and Animals; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-203-90910-2.
- Weryszko-Chmielewska, E.; Kasprzyk, I.; Nowak, M.; Sulborska, A.; Kaczmarek, J.; Szymanska, A.; Haratym, W.; Gilski, M.; Jedryczka, M. Health Hazards Related to Conidia of Cladosporium—Biological Air Pollutants in Poland, Central Europe. J. Environ. Sci. 2018, 65, 271–281.
- AlMatar, M.; Makky, E.A. Cladosporium cladosporioides from the Perspectives of Medical and Biotechnological Approaches. 3 Biotech 2016, 6, 4.
- Mok, T.; Koehler, A.P.; Yu, M.Y.; Ellis, D.H.; Johnson, P.J.; Wickham, N.W. Fatal Penicillium citrinum Pneumonia with Pericarditis in a Patient with Acute Leukemia. J. Clin. Microbiol. 1997, 35, 2654–2656.
- Mensah-Attipoe, J.; Toyinbo, O. Fungal Growth and Aerosolization from Various Conditions and Materials. In Fungal Infection; IntechOpen: London, UK, 2019.
- Paul, B.; Spooner, B. Soil Fungi: Diversity and Detection. Plant Soil 2001, 232, 147–154.
- Corsi, R.L.; Siegel, J.A.; Chiang, C. Particle Resuspension During the Use of Vacuum Cleaners on Residential Carpet. J. Occup. Environ. Hyg. 2008, 5, 232–238.
- Aleksic, B.; Draghi, M.; Ritoux, S.; Bailly, S.; Lacroix, M.; Oswald, I.P.; Bailly, J.-D.; Robine, E. Aerosolization of Mycotoxins after Growth of Toxinogenic Fungi on Wallpaper. Appl. Environ. Microbiol. 2017, 83, e01001-17.
- Morey, P.R. Microbiological Sampling Strategies in Indoor Environments. In Sampling and Analysis of Indoor Microorganisms; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 51–74. ISBN 978-0-470-11243-4.
- Santa Izabel, T.; Cruz, A.; Barbosa, F.; Leão-Ferreira, S.; Marques, M.; Gusmao, L. The Genus Stachybotrys (Anamorphic Fungi) in the Semi-Arid Region of Brazil. Rev. Bras. Bot. 2010, 33, 479–487.
- Górny, R.L.; Reponen, T.; Willeke, K.; Schmechel, D.; Robine, E.; Boissier, M.; Grinshpun, S.A. Fungal Fragments as Indoor Air Biocontaminants. Appl. Environ. Microbiol. 2002, 68, 3522–3531.
- Cho, S.-H.; Seo, S.-C.; Schmechel, D.; Grinshpun, S.A.; Reponen, T. Aerodynamic Characteristics and Respiratory Deposition of Fungal Fragments. Atmos. Environ. 2005, 39, 5454–5465.
- Reponen, T.; Seo, S.-C.; Grimsley, F.; Lee, T.; Crawford, C.; Grinshpun, S.A. Fungal Fragments in Moldy Houses: A Field Study in Homes in New Orleans and Southern Ohio. Atmos. Environ. 2007, 41, 8140–8149.
- World Health Organization. Guidelines for Concentration and Exposure-Response Measurement of Fine and Ultra Fine Particulate Matter for Use in Epidemiological Studies; World Health Organization: Geneva, Switzerland, 2002.
- Górny, R.L. Microbial Aerosols: Sources, Properties, Health Effects, Exposure Assessment—A Review. KONA Powder Part. J. 2020, 37, 64–84.
- Chen, B.T.; Fletcher, R.A.; Cheng, Y.-S.; Kulkarni, P.; Baron, A.P.; Willeke, K. Calibration of Aerosol Instruments. In Aerosol Measurements: Principles, Techniques, and Applications, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 449–478.
- Costa, A.; Pinheiro, M.; Magalhaes, J.; Ribeiro, R.; Seabra, V.; Reis, S.; Sarmento, B. The Formulation of Nanomedicines for Treating Tuberculosis. Adv. Drug Deliv. Rev. 2016, 102, 102–115.
- Kim, K.-H.; Kabir, E.; Kabir, S. A Review on the Human Health Impact of Airborne Particulate Matter. Environ. Int. 2015, 74, 136–143.
- Górny, R.; Dutkiewicz, J. Bacterial and Fungal Aerosols in Indoor Environment in Central and Eastern European Countries. Ann. Agric. Environ. Med. 2003, 9, 17–23.
- Moularat, S.; Robine, E. A Method to Determine the Transfer of Mycotoxins from Materials to Air. CLEAN Soil Air Water 2008, 36, 578–583.
- Frankel, M.; Hansen, E.W.; Madsen, A.M. Effect of Relative Humidity on the Aerosolization and Total Inflammatory Potential of Fungal Particles from Dust-Inoculated Gypsum Boards. Indoor Air 2014, 24, 16–28.
- Money, N.P. Chapter 3—Spore Production, Discharge, and Dispersal. In The Fungi, 3rd ed.; Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 67–97. ISBN 978-0-12-382034-1.
- Madsen, A.M. Effects of Airflow and Changing Humidity on the Aerosolization of Respirable Fungal Fragments and Conidia of Botrytis cinerea. Appl. Environ. Microbiol. 2012, 78, 3999–4007.
- Pasanen, A.-L.; Pasanen, P.; Jantunen, M.J.; Kalliokoski, P. Significance of Air Humidity and Air Velocity for Fungal Spore Release into the Air. Atmos. Environ. Part A Gen. Top. 1991, 25, 459–462.
- Timm, M.; Hansen, E.W.; Moesby, L.; Christensen, J.D. Utilization of the Human Cell Line HL-60 for Chemiluminescence Based Detection of Microorganisms and Related Substances. Eur. J. Pharm. Sci. 2006, 27, 252–258.
- Frankel, M.; Bekö, G.; Timm, M.; Gustavsen, S.; Hansen, E.W.; Madsen, A.M. Seasonal Variations of Indoor Microbial Exposures and Their Relation to Temperature, Relative Humidity, and Air Exchange Rate. Appl. Environ. Microbiol. 2012, 78, 8289–8297.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
874
Revisions:
2 times
(View History)
Update Date:
07 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




