
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leandro Israel da Silva | -- | 3844 | 2023-03-03 19:50:32 | | | |
| 2 | Jason Zhu | -22 word(s) | 3822 | 2023-03-06 06:34:23 | | |
Video Upload Options
Phosphorus (P) is one of the essential macronutrients for plant growth, being a highly required resource to improve the productive performance of several crops, especially in highly weathered soils. However, a large part of the nutrients applied in the form of fertilizers becomes “inert” in the medium term and cannot be assimilated by plants. Rationalizing the use of phosphorus is a matter of extreme importance for environmental sustainability and socioeconomic development. Therefore, alternatives to the management of this nutrient are needed, and the use of P-solubilizing microorganisms is an option to optimize its use by crops, allowing the exploration of less available fractions of the nutrient in soils and reducing the demand for phosphate fertilizers.
1. Phosphate-Solubilizing Microorganisms
2. Phosphate Solubilization Mechanisms
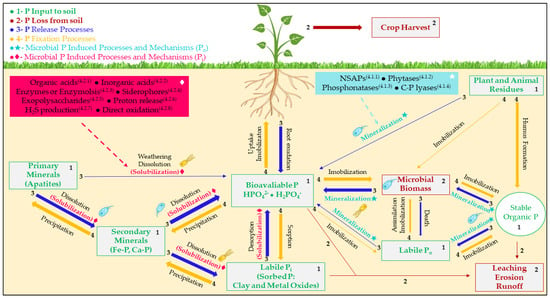
2.1. Organic Phosphate
2.1.1. Non-Specific Acid Phosphatases (NSAPs)
2.1.2. Phytases
2.1.3. Phosphonatases
2.1.4. Carbon–Phosphorus Lyases
2.2. Inorganic Phosphate
2.2.1. Organic Acids
2.2.2. Inorganic Acids
2.2.3. Enzymes or Enzymolysis
2.2.4. Siderophores
2.2.5. Exopolysaccharides
2.2.6. Proton Release
2.2.7. H2S Production
2.2.8. Direct Oxidation of Glucose
3. Applications of Phosphate-Solubilizing Microorganisms as Plant Growth Promoters
4. Market and Agricultural Practices with Phosphate-Solubilizing Microorganisms
References
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68.
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256.
- Shrivastava, M.; Srivastava, P.C.; D’Souza, S.F. Phosphate-Solubilizing Microbes: Diversity and Phosphates Solubilization Mechanism. In Rhizospheric Microbes in Soil; Meena, V., Ed.; Springer: Singapore, 2018; pp. 137–165.
- Djuuna, I.A.F.; Prabawardani, S.; Massora, M. Population Distribution of Phosphate-solubilizing Microorganisms in Agricultural Soil. Microbes Environ. 2022, 37, ME21041.
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Diversity, mechanism and biotechnology of phosphate solubilizing microorganism in mangrove—A review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110.
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587.
- Zhang, L.; Feng, G.; Declerck, S. Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018, 12, 2339–2351.
- Dastager, S.G.; Deepa, C.K.; Pandey, A. Isolation and characterization of novel plant growth promoting Micrococcus sp. NII-0909 and its interaction with cowpea. Plant Physiol. Biochem. 2010, 48, 987–992.
- Franco-Correa, M.; Quintana, A.; Duque, C.; Suarez, C.; Rodríguez, M.X.; Barea, J.M. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl. Soil Ecol. 2010, 45, 209–217.
- Madhaiyan, M.; Poonguzhali, S.; Lee, J.S.; Lee, K.C.; Saravanan, V.S.; Santhanakrishnan, P. Microbacterium azadirachtae sp. nov., a plant-growth-promoting actinobacterium isolated from the rhizoplane of neem seedlings. Int. J. Syst. Evol. Microbiol. 2010, 60, 1687–1692.
- Saif, S.; Khan, M.S.; Zaidi, A.; Ahmad, E. Role of Phosphate-Solubilizing Actinomycetes in Plant Growth Promotion: Current Perspective. In Phosphate Solubilizing Microorganisms; Khan, M., Zaidi, A., Musarrat, J., Eds.; Springer: Cham, Switzerland, 2014; pp. 137–156.
- McGill, W.B.; Cole, C.V. Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 1981, 26, 267–268.
- Schnepf, A.; Jones, D.; Roose, T. Modelling nutrient uptake by individual hyphae of arbuscular mycorrhizal fungi: Temporal and spatial scales for an experimental design. Bull. Math. Biol. 2011, 73, 2175–2200.
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068.
- Bünemann, E.K. Assessment of gross and net mineralization rates of soil organic phosphorus—A review. Soil Biol. Biochem. 2015, 89, 82–98.
- Tate, K.R. The biological transformation of P in soil. Plant Soil 1984, 76, 245–256.
- Tamburini, F.; Pfahler, V.; Bunemann, E.K.; Guelland, K.; Bernasconi, S.M.; Frossard, E. Oxygen isotopes unravel the role of microorganisms in phosphate cycling in soils. Environ. Sci. Technol. 2012, 46, 5956–5962.
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158.
- Nygren, C.M.R.; Rosling, A. Localisation of phosphomonoesterase activity in ectomycorrhizal fungi grown on different phosphorus sources. Mycorrhiza 2009, 19, 197–204.
- Gandhi, N.U.; Chandra, S.B. A comparative analysis of three classes of bacterial non-specific Acid phosphatases and archaeal phosphoesterases: Evolutionary perspective. Acta Inform. Med. 2012, 20, 167–173.
- Rejsek, K.; Vranova, V.; Formanek, P. Determination of the Proportion of Total Soil Extracellular Acid Phosphomonoesterase (E.C. 3.1.3.2) Activity Represented by Roots in the Soil of Different Forest Ecosystems. Sci. World J. 2012, 2012, 250805.
- Liang, Y.; Li, M.; Pan, F.; Ma, J.; Yang, Z.; Ling, T.; Song, Z. Alkaline Phosphomonoesterase-Harboring Microorganisms Mediate Soil Phosphorus Transformation With Stand Age in Chinese Pinus massoniana Plantations. Front. Microbiol. 2020, 11, 67–76.
- Gaiero, J.R.; Bent, E.; Fraser, T.D. Validating novel oligonucleotide primers targeting three classes of bacterial non-specific acid phosphatase genes in grassland soils. Plant Soil 2018, 427, 39–51.
- Neal, A.L.; Blackwell, M.; Akkari, E. Phylogenetic distribution, biogeography and the effects of land management upon bacterial non-specific Acid phosphatase Gene diversity and abundance. Plant Soil 2018, 427, 175–189.
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl. Soil Ecol. 2017, 111, 48–56.
- Singh, B.; Satyanarayana, T. Microbial phytases in phosphorus acquisition and plant growth promotion. Physiol. Mol. Biol. Plants 2011, 17, 93–103.
- Azeem, M.; Riaz, A.; Chaudhary, A.N.; Hayat, R.; Hussain, Q.; Tahir, M.I.; Imran, M. Microbial phytase activity and their role in organic P mineralization. Arch. Agron. Soil Sci. 2014, 61, 751–766.
- Kour, D.; Kaur, T.; Yadav, N.; Rastegari, A.A.; Singh, B.; Kumar, V.; Yadav, A.N. Chapter 10—Phytases from microbes in phosphorus acquisition for plant growth promotion and soil health. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rastegari, A.A., Yadav, A.N., Yadav, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–176.
- Sadaf, N.; Muhammad, Z.H.; Naeem, I.; Muyassar, H.A.; Aishah, A. Harnessing the Phytase Production Potential of Soil-Borne Fungi from Wastewater Irrigated Fields Based on Eco-Cultural Optimization under Shake Flask Method. Agriculture 2022, 12, 103.
- Wang, X.X.; Hoffland, E.; Feng, G.; Kuyper, T.W. Phosphate Uptake from Phytate Due to Hyphae-Mediated Phytase Activity by Arbuscular Mycorrhizal Maize. Front. Plant Sci. 2017, 8, 684.
- Kamat, S.S.; Raushel, F.M. The enzymatic conversion of phosphonates to phosphate by bacteria. Curr. Opin. Chem. Biol. 2013, 17, 589–596.
- Kafarski, P. Phosphonates: Their Natural Occurrence and Physiological Role. In Contemporary Topics about Phosphorus in Biology and Materials; Churchill, D.G., Sikirić, M.D., Čolović, B., Milhofer, H.F., Eds.; IntechOpen: London, UK, 2020.
- Horsak, R.D.; Bedient, P.B.; Hamilton, M.C.; Thomas, F.B. Pesticides. Environ. Forensics 1964, 143–165.
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471.
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499.
- Hagner, M.; Mikola, J.; Saloniemi, I. Effects of a glyphosate-based herbicide on soil animal trophic groups and associated ecosystem functioning in a northern agricultural field. Sci. Rep. 2019, 9, 8540.
- Helander, M.; Pauna, A.; Saikkonen, K. Glyphosate residues in soil affect crop plant germination and growth. Sci. Rep. 2019, 9, 19653.
- Kryuchkova, Y.V.; Burygin, G.L.; Gogoleva, N.E.; Gogolev, Y.V.; Chernyshova, M.P.; Makarov, O.E.; Turkovskaya, O.V. Isolation and characterization of a glyphosate-degrading rhizosphere strain, Enterobacter cloacae K7. Microbiol. Res. 2014, 169, 99–105.
- Chávez-Ortiz, P.; Tapia-Torres, Y.; Larsen, J.; García-Oliva, F. Glyphosate-based herbicides alter soil carbon and phosphorus dynamics and microbial activity. Appl. Soil Ecol. 2022, 169, 104256.
- Stosiek, N.; Talma, M.; Klimek-Ochab, M. Carbon-Phosphorus Lyase—The State of the Art. Appl. Biochem. Biotechnol. 2020, 190, 1525–1552.
- Seweryn, P.; Van, L.B.; Kjeldgaard, M.; Russo, C.J.; Passmore, L.A.; Hove-Jensen, B.; Jochimsen, B.; Brodersen, D.E. Structural insights into the bacterial carbon-phosphorus lyase machinery. Nature 2015, 525, 68–72.
- Villarreal-Chiu, J.F. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front. Microbiol. 2012, 3, 19.
- Manav, M.C.; Sofos, N.; Hove-Jensen, B.; Brodersen, D.E. The Abc of Phosphonate Breakdown: A Mechanism for Bacterial Survival. BioEssays 2018, 40, 800091.
- Smeck, N.E. Phosphorus dynamics in soils and landscapes. Geoderma 1985, 36, 185–199.
- Kome, G.; Enang, R.; Tabi, F.; Yerima, B. Influence of Clay Minerals on Some Soil Fertility Attributes: A Review. Open J. Soil Sci. 2019, 9, 155–188.
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971.
- Marra, L.M.; de Oliveira-Longatti, S.M.; Soares, C.R.F.S.; Olivares, F.L.; Moreira, F.M.S. The Amount of Phosphate Solubilization Depends on the Strain, C-Source, Organic Acids and Type of Phosphate. Geomicrobiol. J. 2019, 36, 232–242.
- Duebel, A.; Gransee, A.; Merbach, W. Transformation of organic rhizodeposits by rhizoplane bacteria and its influence on the availability of tertiary calcium phosphate. J. Plant Nutr. Soil Sci. 2000, 163, 387–392.
- Satyaprakash, M.; Nikitha, T.; Reddi, E.U.B.; Sadhana, B.; Vani, S.S. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144.
- Kumar, A.; Kumar, A.; Patel, H. Role of microbes in phosphorus availability and acquisition by plants. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1344–1347.
- Mander, C.; Wakelin, S.; Young, S.; Condron, L.; O’Callaghan, M. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol. Biochem. 2012, 44, 93–101.
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199.
- Kishore, N.; Pindi, P.K.; Reddy, S.R. Phosphate-solubilizing microorganisms: A critical review. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K., Eds.; Springer: New Delhi, India, 2015; pp. 307–333.
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate solubilization by microorganisms. Adv. Biol. Res. 2019, 161–176.
- Mendes, G.O.; Murta, H.M.; Valadares, R.V.; Silveira, W.B.; Silva, I.R.; Costa, M.D. Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner. Eng. 2020, 155, 106458.
- Patel, D.K.; Archana, G.; Kumar, G.N. Variation in the Nature of Organic Acid Secretion and Mineral Phosphate Solubilization by Citrobacter sp. DHRSS in the Presence of Different Sugars. Curr Microbiol. 2008, 56, 168–174.
- Kim, K.; McDonald, G.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352.
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43.
- Siddique, R.; Gul, A.; Ozturk, M.; Altay, V. Phosphate Solubilizing Bacteria for Soil Sustainability. In Handbook of Assisted and Amendment-Enhanced Sustainable Remediation Technology; Prasad, M.N., Ed.; Willey: Telangana, India, 2021; pp. 425–432.
- Cantin, P.; Karam, A.; Guay, R. Solubilization of Phosphorus from Apatite by Sulfuric Acid Produced from the Microbiological Oxidation of Sulfur. In Effect of Mineral-Organic-Microorganism Interactions on Soil and Freshwater Environments; Berthelin, J., Huang, P.M., Bollag, J.M., Andreux, F., Eds.; Springer: Boston, MA, USA, 1999; pp. 247–252.
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid. Based Complement. Altern. Med. 2011, 2011, 615032.
- Verma, V.; Joshi, K.; Mazumdar, B. Study of Siderophore Formation in Nodule-Forming Bacterial Species. Res. J. Chem. Sci. 2012, 2, 26–29.
- Rizvi, A.; Ahmed, B.; Khan, M.S.; Umar, S.; Lee, J. Sorghum-Phosphate Solubilizers Interactions: Crop Nutrition, Biotic Stress Alleviation, and Yield Optimization. Front. Plant Sci. 2021, 12, 746780.
- Cooper, S.R.; McArdle, J.V.; Raymond, K.N. Siderophore electrochemistry: Relation to intracellular iron release mechanism. Proc. Natl. Acad. Sci. USA 1978, 75, 3551–3554.
- Jyothi, V.; Sowmya, H.V.; Thippeswamy, B. Siderophore production by phosphate solubilizing fungi from rhizospheric soil of medicinal plants. Int. J. Biol. Biotechnol. 2020, 17, 599–606.
- Cui, K.; Xu, T.; Chen, J.; Yang, H.; Liu, X.; Zhuo, R.; Peng, Y.; Tang, W.; Wang, R.; Chen, L. Siderophores, a potential phosphate solubilizer from the endophyte Streptomyces sp. CoT10, improved phosphorus mobilization for host plant growth and rhizosphere modulation. J. Clean. Prod. 2022, 367, 133110.
- Yi, Y.; Huang, W.; Ge, Y. Exopolysaccharide: A novel important factor in the microbial dissolution of tricalcium phosphate. World J. Microbiol. Biotechnol. 2007, 24, 1059–1065.
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate Solubilization Mechanisms in Alkaliphilic Bacterium Bacillus marisflavi FA7. Curr. Sci. 2018, 114, 845–853.
- Kailasam, S.; Arumugam, S.; Balaji, K.; Kanth, S.V. Adsorption of chromium by exopolysaccharides extracted from lignolytic phosphate solubilizing bacteria. Int. J. Biol. Macromol. 2022, 206, 788–798.
- Ochoa-Loza, F.J.; Artiola, J.F.; Maier, R.M. Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J. Environ. Qual. 2001, 30, 479–485.
- Mohite, B.V.; Koli, S.H.; Narkhede, C.P. Prospective of Microbial Exopolysaccharide for Heavy Metal Exclusion. Appl. Biochem. Biotechnol. 2017, 183, 582–600.
- Gaind, S. Phosphate dissolving fungi: Mechanism and application in alleviation of salt stress in wheat. Microbiol. Res. 2016, 193, 94–102.
- Matos, A.D.M.; Gomes, I.C.P.; Nietsche, S.; Xavier, A.A.; Gomes, W.S.; dos Santos Neto, J.A.; Pereira, M.C.T. Phosphate solubilization by endophytic bacteria isolated from banana trees. An. Acad. Bras. Cienc. 2017, 89, 2945–2954.
- Arcand, M.M.; Schneider, K.D. Plant- and microbial-based mechanisms to improve the agronomic effectiveness of phosphate rock: A review. An. Acad. Bras. Cienc. 2006, 78, 791–807.
- Öğüt, M.; Er, F.; Neumann, G. Increased proton extrusion of wheat roots by inoculation with phosphorus solubilising microorganims. Plant Soil 2011, 339, 285–297.
- Habte, M.; Osorio, N.W. Effect of Nitrogen Form on the Effectiveness of a Phosphate-Solubilizing Fungus to Dissolve Rock Phosphate. J Biofertil. Biopestic. 2012, 3, 127.
- Florentino, A.P.; Weijma, J.; Stams, A.J.; Sanchez-Andrea, I. Ecophysiology and application of acidophilic sulfur-reducing microorganisms. In Biotechnology of Extremophiles: Grand Challenges in Biology and Biotechnology; Rampelotto, P., Ed.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 141–175.
- Sperber, J. Release of Phosphate from Soil Minerals by Hydrogen Sulphide. Nature 1958, 181, 934.
- Wilfert, P.; Meerdink, J.; Degaga, B.; Temmink, H.; Korving, L.; Witkamp, G.J.; van Loosdrecht, M.C.M. Sulfide induced phosphate release from iron phosphates and its potential for phosphate recovery. Water Res. 2020, 171, 115389.
- Chen, J.; Zhao, G.; Wei, Y. Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth-promoting effect on Chinese fir seedlings. Sci Rep. 2021, 11, 9081.
- Zutter, N.; Maarten, A.; Pieter, V.; Verwaeren, J.; Leen, D.G.; Kris, A. Innovative Rhizosphere-Based Enrichment under P-Limitation Selects for Bacterial Isolates with High-Performance P-Solubilizing Traits. Microbiol. Spectr. 2022, 10, e02052-22.
- Singh, T.B.; Sahai, V.; Ali, A.; Prasad, M.; Yadav, A.; Shrivastav, P.; Goyal, D.; Dantu, P.K. Screening and evaluation of PGPR strains having multiple PGP traits from the hilly terrain. J. Appl. Biol. Biotechnol. 2020, 8, 38–44.
- Song, O.R.; Lee, S.J.; Lee, Y.S.; Lee, S.C.; Kim, K.K.; Choi, Y.L. Solubilization of insoluble inorganic phosphate by Burkholderia cepacia DA23 isolated from cultivated soil. Braz. J. Microbiol. 2008, 39, 151–156.
- Sashidhar, B.; Podile, A.R. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J. Appl. Microbiol. 2010, 109, 1–12.
- Mei, C.; Chretien, R.L.; Amaradasa, B.S.; He, Y.; Turner, A.; Lowman, S. Characterization of Phosphate Solubilizing Bacterial Endophytes and Plant Growth Promotion In Vitro and in Greenhouse. Microorganisms 2021, 9, 1935.
- Rasul, M.; Yasmin, S.; Suleman, M.; Zaheer, A.; Reitz, T.; Tarkka, M.T.; Islam, E.; Sajjad, M.M. Glucose dehydrogenase gene containing phosphobacteria for biofortification of Phosphorus with growth promotion of rice. Microbiol. Res. 2019, 223–225, 1–12.
- Bharwad, K.; Rajkumar, S. Modulation of PQQ-dependent glucose dehydrogenase (mGDH and sGDH) activity by succinate in phosphate solubilizing plant growth promoting Acinetobacter sp. SK2. 3 Biotech 2020, 10, 5.
- Iyer, B.; Rajkumar, S. Succinate irrepressible periplasmic glucose dehydrogenase of Rhizobium sp. Td3 and SN1 contributes to its phosphate solubilization ability. Arch. Microbiol. 2019, 201, 649–659.
- Singh, R.K.; Singh, P.; Li, H.B. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane: A comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220.
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions Between Plants and Non-Symbiotic Growth Promoting Bacteria Under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368.
- Nadal, M.C.; Ferreira, G.M.R.; Andrade, G.V.S.; Buttrós, V.H.; Rodrigues, F.A.; Silva, C.M.; Martins, A.D.; Rufato, L.; Luz, J.M.Q.; Dória, J.; et al. Endophytic Bacteria Can Replace the Need for Synthetic Auxin during In Vitro Rooting of Pyrus communis. Agronomy 2022, 12, 1226.
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of Plant Growth and Drought Tolerance by Native Microorganisms (AM Fungi and Bacteria) from Dry Environments: Mechanisms Related to Bacterial. J. Plant Growth Regul. 2009, 28, 115–124.
- Zaheer, A.; Mirza, B.S.; Mclean, J.E.; Yasmin, S.; Shah, T.M.; Malik, K.A.; Mirza, M.S. Association of plant growth-promoting Serratia spp. with the root nodules of chickpea. Res. Microbiol. 2016, 167, 510–520.
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The Ecological Role of Volatile and Soluble Secondary Metabolites Produced by Soil Bacteria. Trends Microbiol. 2017, 25, 280–292.
- Moura, G.G.D.; Barros, A.V.; Machado, F.; Martins, A.D.; Silva, C.M.; Durango, L.G.C.; Forim, M.; Alves, E.; Pasqual, M.; Dória, J. Endophytic bacteria from strawberry plants control gray mold in fruits via production of antifungal compounds against Botrytis cinerea L. Microbiol. Res. 2021, 251, 126793.
- Rani, M.; Weadge, J.T.; Jabaji, S. Isolation and Characterization of Biosurfactant-Producing Bacteria From Oil Well Batteries With Antimicrobial Activities Against Food-Borne and Plant Pathogens. Front. Microbiol. 2020, 11, 64.
- Buttrós, V.H.; Araújo, N.A.F.; D’Ávila, V.A.; Pereira, M.M.A.; Melo, D.S.; Pasqual, M.; Dória, J. A Little Helper: Beneficial Bacteria with Growth-Promoting Mechanisms Can Reduce Asian Soybean Rust Severity in a Cell-Free Formulation. Agronomy 2022, 12, 2635.
- Araújo, R.C.; Ribeiro, M.S.; Rodrigues, F.A.; Silva, B.S.; Dória, J.; Pasqual, M. Association of growth-promoting bacteria and hydroponic system aiming at reducing the time of production of banana seedlings. Arch. Agron. Soil Sci. 2022, 1–14.
- Silva, L.I.; Oliveira, I.P.; Jesus, E.C.; Pereira, M.C.; Pasqual, M.; Araújo, R.C.; Dória, J. Fertilizer of the Future: Beneficial Bacteria Promote Strawberry Growth and Yield and May Reduce the Need for Chemical Fertilizer. Agronomy 2022, 12, 2465.
- Moura, G.G.D.; Barros, A.V.; Machado, F.; Silva, C.M.; Lienke, C.; Petters-Vandresen, D.A.L.; Alves, E.; Schwan, R.F.; Pasqual, M.; Dória, J. The Friend Within: Endophytic Bacteria as a Tool for Sustainability in Strawberry Crops. Microorganisms 2022, 10, 2341.
- Joshi, S.K.; Guaraha, A.K. Global biofertilizer market: Emerging trends and opportunities. In Developments in Applied Microbiology and Biotechnology, Trends of Applied Microbiology for Sustainable Economy; Soni, R., Suyal, D.P., Yadav, A.N., Goel, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 689–697.
- Pirttilä, A.M.; Mohammad Parast Tabas, H.; Baruah, N.; Koskimäki, J.J. Biofertilizers and Biocontrol Agents for Agriculture: How to Identify and Develop New Potent Microbial Strains and Traits. Microorganisms 2021, 9, 817.
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54.
- Mącik, M.; Agata, G.; Magdalena, F. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 162, pp. 31–87.
- Silva, U.C.; Cuadros-Orellana, S.; Silva, D.R.C.; Freitas-Júnior, L.F.; Fernandes, A.C.; Leite, L.R.; Oliveira, C.A.; dos Santos, V.L. Genomic and Phenotypic Insights Into the Potential of Rock Phosphate Solubilizing Bacteria to Promote Millet Growth in vivo. Front. Microbiol. 2021, 11, 574550.
- Abdelgalil, S.A.; Kaddah, M.M.Y.; Duab, M.E.A. A sustainable and effective bioprocessing approach for improvement of acid phosphatase production and rock phosphate solubilization by Bacillus haynesii strain ACP1. Sci. Rep. 2022, 12.
- Jokkaew, S.; Jantharadej, K.; Pokhum, C.; Chawengkijwanich, C.; Suwannasilp, B.B. Free and Encapsulated Phosphate-Solubilizing Bacteria for the Enhanced Dissolution of Swine Wastewater-Derived Struvite—An Attractive Approach for Green Phosphorus Fertilizer. Sustainability 2022, 14, 12627.
- Cheng, Y.; Wan, W. Alkaline phosphomonoesterase-harboring bacteria facilitate phosphorus availability during winter composting with different animal manures. J. Clean. Prod. 2022, 376, 134299.




