Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zheng, J.; Wu, F.; Wang, F.; Cheng, J.; Zou, H.; Li, Y.; Du, J.; Kan, J. Mineral Nutrient Biomarkers and Its Applications in Epidemiology. Encyclopedia. Available online: https://encyclopedia.pub/entry/41814 (accessed on 07 February 2026).
Zheng J, Wu F, Wang F, Cheng J, Zou H, Li Y, et al. Mineral Nutrient Biomarkers and Its Applications in Epidemiology. Encyclopedia. Available at: https://encyclopedia.pub/entry/41814. Accessed February 07, 2026.
Zheng, Jianheng, Feng Wu, Feijie Wang, Junrui Cheng, Hong Zou, Yuan Li, Jun Du, Juntao Kan. "Mineral Nutrient Biomarkers and Its Applications in Epidemiology" Encyclopedia, https://encyclopedia.pub/entry/41814 (accessed February 07, 2026).
Zheng, J., Wu, F., Wang, F., Cheng, J., Zou, H., Li, Y., Du, J., & Kan, J. (2023, March 02). Mineral Nutrient Biomarkers and Its Applications in Epidemiology. In Encyclopedia. https://encyclopedia.pub/entry/41814
Zheng, Jianheng, et al. "Mineral Nutrient Biomarkers and Its Applications in Epidemiology." Encyclopedia. Web. 02 March, 2023.
Copy Citation
Minerals are dietary supplements that are essential for preserving healthy physiology and function. Mineral elements such as iron (Fe), zinc (Zn), iodine (I), and selenium (Se) are highly valued in modern healthy diets as they have special roles in cellular metabolism. In addition, the oxidative or antioxidant properties of certain metals may affect cardiovascular health, and reduce the risk of anemia, cancer and so on. Therefore, adequate intake of essential minerals through diet and/or supplements is recommended to promote health.
dietary
minerals
biomarkers
1. Iodine Biomarker
Iodine is a crucial trace element participated in the generation of the thyroid hormones triiodothyronine (T3) and thyroxine (T4) [1]. According to the recommendations of WHO, most countries set the daily iodine intake for adult at 150 μg and increase it to 250 μg during pregnancy for its critical involvement in the growth of the embryo’s nervous system [2][3]. The frequency of iodine deficient disorders has decreased over the past three decades owing to international efforts and initiatives including “salt” iodization [4]. However, iodine deficiency is still common in some few places, though. Regularly monitoring the population’s iodine status will be critical in maintaining appropriate intakes and the absence of excessive intakes. The recommended biomarkers, based on the current evaluation system, include urine iodine concentration (UIC), thyroid-stimulating hormone (TSH), thyroglobulin (Tg), T4 and T3, and goiter. These biomarkers may directly reflect dietary iodine consumption or may be linked to thyroid hormones metabolism, which can accurately reflect the body’s iodine levels [5].
In general, the population median of the UIC survey is based on a single spot sample, while causes an inaccurate assessing of iodine status. According to Bertinato’s study, iodine intake distribution based on two UIC samples, combined with an estimated mean requirement or tolerable higher intake level, cut-point method is developed and proved to be a promising approach that could be employed in population iodine status monitoring [6]. Cui et al. examined the effectiveness of serum iodine to assess iodine consumption in children, finding that serum iodine level was favorably linked with daily iodine consumption [7]. In Hlucny’s study, iodine levels were examined following a 3-day iodine-titrated diet for 10 participants. Serum iodine, Tg, and 24-hour UIC levels were measured after each diet. At baseline, a 24-hour UIC and an iodine-specific food frequency questionnaire (FFQ) were completed. UIC elevated on average by 19.3 µg/L for every gram of iodized salt ingested. These findings imply that serum iodine and 24 h UIC can represent a person’s iodine level and act as iodine status biomarkers [8]. Further research and development of more sensitive and specific biomarkers to assess individual iodine status for routine patient care is highly needed. The WHO recommends that a median UIC of more than 100 μg/L to determinate population iodine sufficiency, but some authorities suggest 60–70 μg/L [5]. The current opinion is that urine iodine concentration (UIC) is a proven biomarker for determining the iodine status of the population and will facilitate the ability to continuously monitor in nutrition programs [6][9]. An overview of the iodine biomarkers and related metabolism pathway are shown in Figure 1.
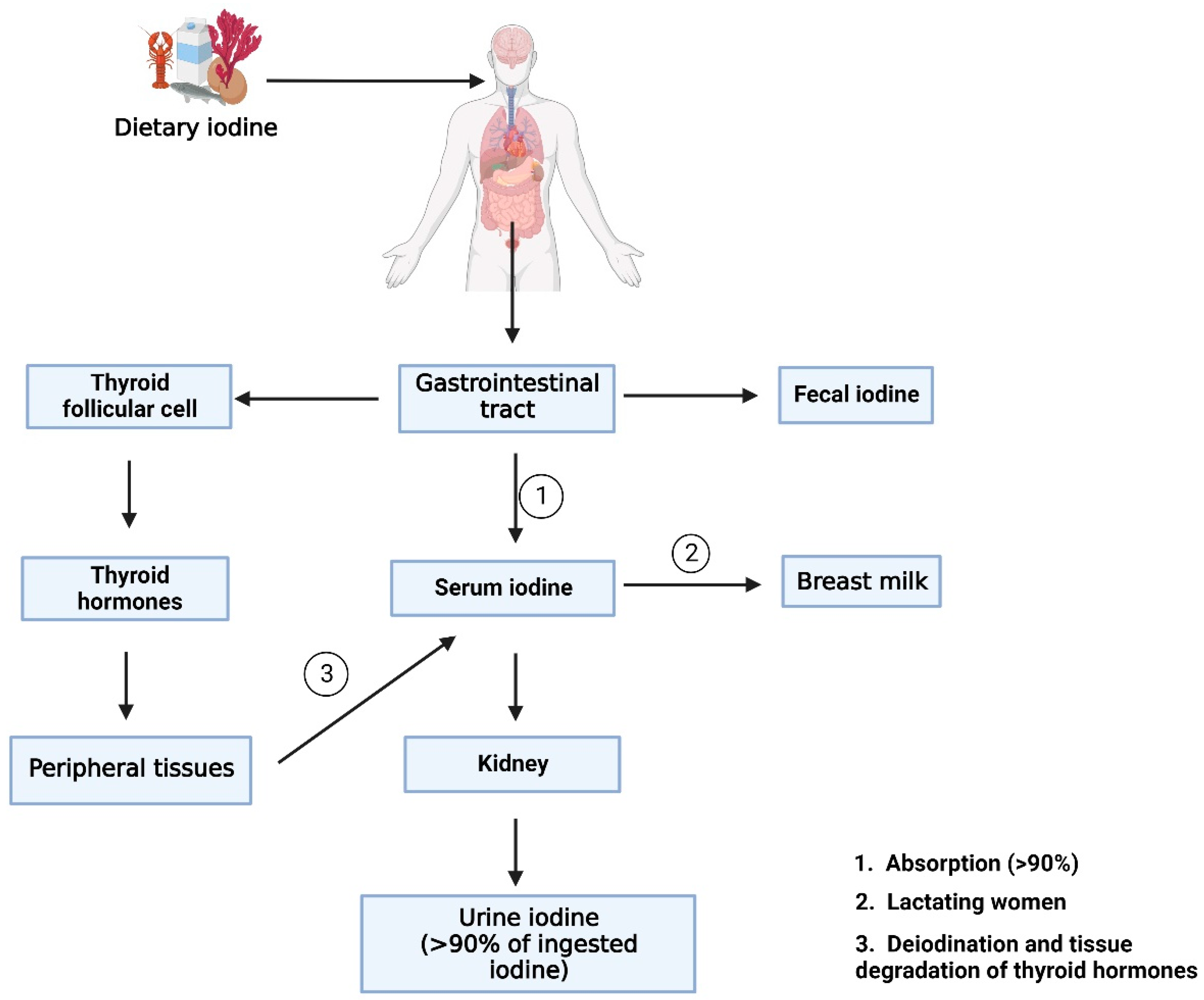
Figure 1. An overview of the iodine biomarkers and related metabolism pathway. (Created with BioRender.com).
After ingestion, dietary iodine is digested by the gastrointestinal tract, and the majority of the iodine taken into the body is dispersed in the blood as serum iodine. Some of the serum iodine is converted to breast milk in lactating mothers. The other part of the iodine absorbed in the body is involved in the synthesis of thyroid hormones. After entering the thyroid follicular cell, iodine incorporated into thyroglobulin to participate in the synthesis of thyroid hormones. Then thyroid hormone is deiodination and degradation by peripheral tissues and converted back into serum iodine. The portions of serum iodine that are not absorbed and utilized by the human body are transformed into urine iodine and eliminated from the body via kidney metabolism. The remaining unabsorbed iodine is excreted to the body as fecal iodine. Serum iodine, thyroid hormone, fecal iodine, and urinary iodine produced during these metabolic processes are useful biomarkers for measuring iodine levels in the body.
Application of Iodine Biomarker
During pregnancy, dietary requirements for iodine increase by 50% to 250 µg/d due to the increased production of thyroid hormones required by the mother and her fetus [10], low thyroid hormone levels may result in a variety of negative effects, notably on brain growth and development, making it worth considering specific assessment for pregnant women iodine status. Postulated pathways for how iodine shortage affects growth may involve decreased levels of insulin-like growth factor 1 (IGF-1) and IGF binding protein 3 (IGFBP-3). Kanike et al. evaluated urine iodine concentrations and thyroid function in 50 mothers and infants at birth, one week, one, two, three, and four months, as well as close discharge. Their findings revealed that iodine deficit was prevalent in pregnancies and neonates born at exceptionally low gestational age compared to full term, particularly in pregnant mothers at risk of hypothyroidism. To minimize iodine shortage, iodine supplementation should be explored throughout pregnancy and postpartum [11].
Placental concentrations may better represent the pregnancy’s long-term iodine status. Gestational diabetes mellitus (GDM) is a disorder that causes glucose intolerance throughout pregnancy and subsequently subsides after birth. Adult’s iodine deficiency has been associated with altered insulin and glucose homeostasis, primarily through thyroid hormones. To investigate the correlation between placental iodine concentrations and diabetes risk in pregnant women. A study evaluated the prevalence of gestational diabetes mellitus (GDM) in 471 mother-infant pairs in a birth cohort of 24 to 28 weeks gestation. Neven et al. measured iodine concentrations in placenta, insulin levels in maternal and cord blood, and the Homeostasis Model Assessment (HOMA) for insulin resistance (IR) index and β-cell activity to investigate the association between placenta iodine concentration and GDM. Higher placental iodine levels were shown to reduce the incidence of GDM. Plasma insulin concentrations in cord blood were shown to be inversely linked to placental iodine concentration. Furthermore, changes in plasma insulin level, HOMA-IR score, and β-cell activity were connected to reduced placental iodine load. These results support the hypothesis that mild to moderate iodine deficiency in healthy pregnant women is related to subclinical and early-onset alterations in normal insulin homeostasis [12].
2. Iron Biomarker
Iron is a crucial trace element for human health as it regulates a broad range of biological reactions. Many metabolic processes, including energy generation, DNA synthesis, and oxygen transport, are depending on iron’s ability to flip between ferric and ferrous forms. Red meat, green leafy vegetables, nuts, and fortified morning cereals are among foods with a reasonably high iron content; however, the absorption of iron is quite varied. As the most prevalent micronutrient deficit, iron deficiency affects around one-third of the population. Anemia brought on by iron deficiency owing to malnutrition affects roughly 30% of non-pregnant women, 40% of pregnant women, and children under the age of five [13]. Plasma iron levels are regulated by the hepcidin/ferroportin system, and iron enters the circulation via ferroproteins for transportation to the liver and bone marrow, respectively, for red blood cell production and storage. Systemic iron levels are closely regulated since the body has no other way to eliminate iron than through blood loss or cell turnover [14].
As iron depletion progresses, declines in serum ferritin levels are correlated with drops in bodily iron storage. Since the onset of iron-deficient erythropoiesis, body iron stores are no longer sufficient to produce the quantities of iron necessary for the generation of hemoglobin and other functioning iron compounds. In the US National Health and Nutrition Examination Survey, circulating hemoglobin concentrations and soluble transferrin receptor (sTfR) concentrations were investigated as two physiological indicators of the beginning of iron-deficient erythropoiesis. When iron-deficient erythropoiesis occurs in an otherwise healthy person, the amount of accessible iron is inadequate to enable hemoglobin synthesis, resulting in a reduction in hemoglobin concentration. Independent of hemoglobin concentration, sTfR concentration is a sign of iron-deficient erythropoiesis. When iron-deficient erythropoiesis occurs, erythroid progenitors and other iron-requiring cells in the body increase the expression of cellular transferrin receptor 1 to acquire more iron. An extracellular portion of the cellular transferrin receptor called plasma sTfR, which is shed into the plasma and serves as a gauge for the mass of the cellular transferrin receptor. The erythroid marrow is where around 80% of plasma sTfR is generated, and when there is no iron deficiency, the concentrations are related to the volume of the erythroid marrow. Plasma sTfR concentrations are proportional to cellular iron demand in the absence of erythroid hyperplasia and are a sensitive, quantitative biomarker of the early stage of iron insufficiency: iron-deficient erythropoiesis. A physiological foundation for determining the onset of iron-deficient erythropoiesis is the serum ferritin level at which hemoglobin level starts to fall and sTfR commences to increase. Systemic iron metabolism and potential iron biomarkers are shown in Figure 2. Nutritional iron biomarkers can be measured in circulating red blood cell mass, serum ferritin (SF), percentage transferrin saturation (TSAT), RBC protoporphyrin and serum (soluble, plasma) transferrin receptor (sTfR), and hemoglobin. Among these biomarkers, hemoglobin is the most widely utilized indicator for iron deficiency diagnosis, however it is not sensitive or specific. Currently, SF is more often used as an indicator of iron storage and deficiency risk, although some studies suggest excluding inflammation status [15].
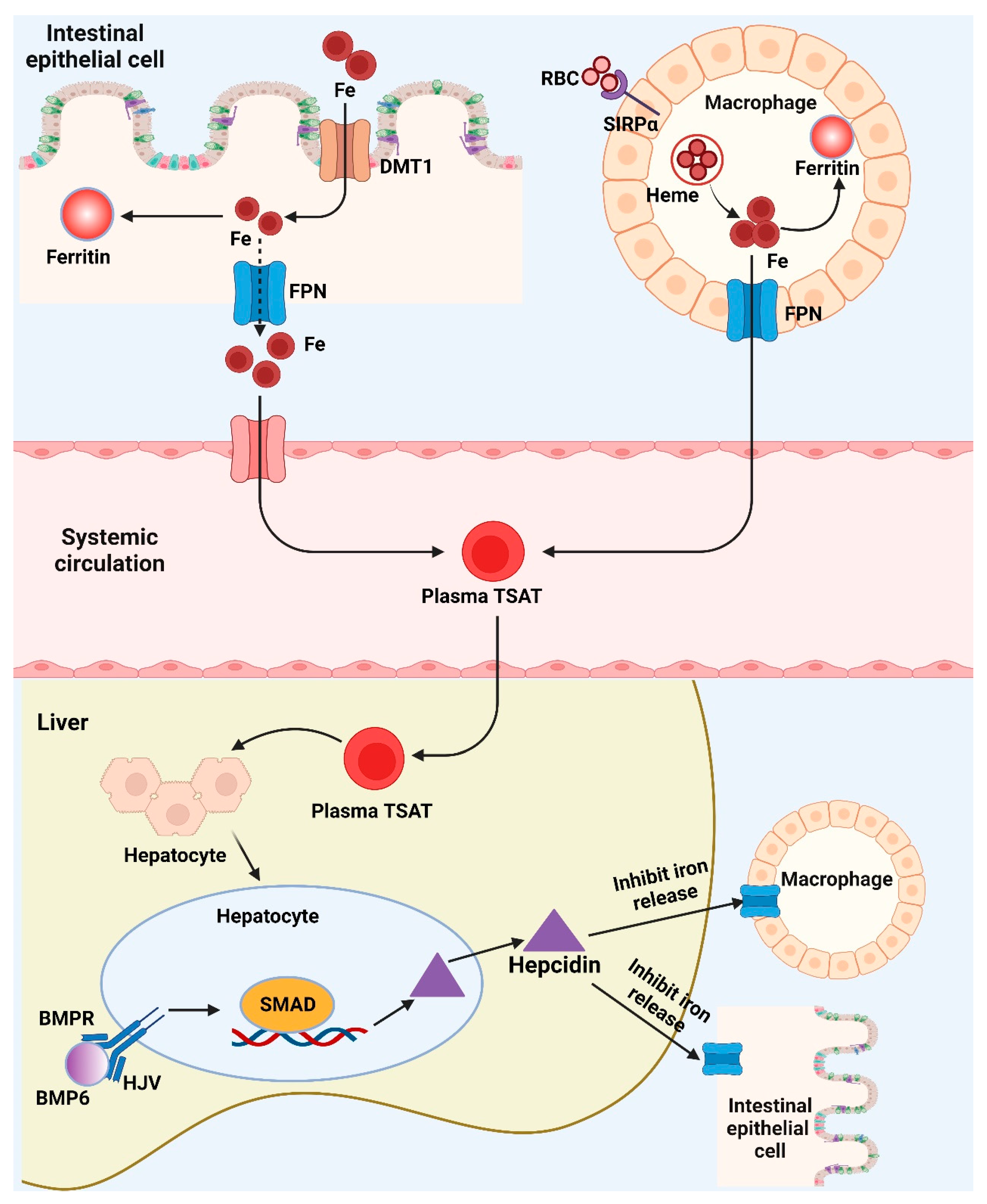
Figure 2. Brief depiction of the systemic iron metabolism and potential iron biomarkers. (Created with BioRender.com). BMP6: Bone morphogenetic protein 6; BMPR: Bone morphogenetic protein receptor; DMT1: Divalent metal transporter 1; Fe: Iron/Ferrum; FPN: Ferroportin; HJV: Hemojuvelin; RBC: Red blood cell; SIRPα: Signal regulatory protein alpha; SMAD: Small-body-size mothers against decapentaplegic homolog 1; TSAT: Percentage transferrin saturation.
The iron transporter DMT1 transfers dietary iron over the border membrane and into the enterocyte. After entering the cell, the iron has two possible storage locations: the intracellular iron storage protein-ferritin or the iron export protein-ferropotin, which allows the iron to leave the cell and enter the circulatory system. In the meantime, macrophages recognize RBCs via SIRPα, recycle RBC iron, which may subsequently be transformed into heme and exported by FPN. BMP6 will bind to the BMP receptor and its co-receptor HJV, activating SMAD signaling and promoting the transcription of hepcidin when the body’s iron stores are suitably loaded. The liver produces hepcidin, which binds to FPN on macrophages and enterocytes and stimulates its degradation. This inhibits the release of iron from macrophages and the intestines into the circulation. Fe, ferritin, and plasma TSAT can serve as useful biomarkers for iron status by reflecting the body’s iron levels in numerous metabolic pathways.
Application of Iron Biomarker
Children and women are specific populations who require efficient biomarkers to monitor their iron states. There is evidence showed that decreased serum ferritin concentrations resulted in a decrease in median hemoglobin concentration, while sTfR concentration rose, with both changing in a curvilinear pattern in children and non-pregnant women. The relationship between two independent iron-deficiency indicators, hemoglobin and sTfR, provided a threshold for serum ferritin concentrations of around 20 ng/mL in children and 25 ng/mL in non-pregnant women, which may be more clinically and epidemiologically relevant [16].
It’s interesting to note that iron influences myocardial exercise tolerance and cardiac performance in addition to anemia. Iron also plays an important part in the metabolism of the heart and skeletal muscles through mitochondrial activity. Chronic heart failure (HF) is frequently associated with iron deficiency, which can be detected from blood test results. Ueda et al. investigated the impact of serum iron levels at discharge on the prognosis of 615 patients admitted to the hospital with acute decompensated heart failure (ADHF). According to their findings, the complication rate was much greater in the low iron group. Low serum iron levels could be an independent predictor of the composite outcome after controlling for confounders such as hemoglobin and ferritin concentrations, indicating that iron may play a role in the pathogenesis of ADHF through non-hematopoietic activities [17]. Gabriele et al. detected different definitions of iron deficiency, finding that patients with TSAT <20% and serum iron ≤13 mmol/L resulting higher mortality which was independent of HF phenotype [18].
Since iron plays a crucial role in physiological activities, sports population are particularly susceptible to iron disorder. Among adolescent female athletes, 52% is considered iron deficiency according to a related study. Zhang et al. comprehensively interpret the iron status of Chinese athletes using a multi-index evaluation system and proposed specific Chinese athlete thresholds of SF for 12 ng/mL in female and 30 ng/mL in male [19].
3. Zinc Biomarker
As a dietary essential trace element, zinc plays a key role in several metabolic processes as an intracellular metal [20][21], especially at the beginning of life. Zinc deficit is a serious health problem in many regions because of inadequate dietary intake, particularly in young children. Lack of zinc can damage the immune system and increase the prevalence of infectious disorders such as pneumonia, malaria, and diarrhea worldwide [22]. It also results in a variety of non-specific general alterations in metabolism and function, such as delayed development, an increase in infections, and appearance of skin lesions [23].
It is challenging to identify whether there is an excess or deficiency of zinc since cellular, tissue, and whole-body zinc homeostasis are tightly controlled to sustain metabolic processes across a wide range of intakes. The BOND Zinc Expert Panel suggests three parameters for evaluating zinc levels: dietary zinc intake, plasma zinc concentration (PZC), and height for age of growing babies and children [24]. Free zinc is the exchangeable and bioactive form of zinc found in serum. Multiple expert committees have approved plasma zinc concentrations (PZCs) or serum zinc concentrations (SZCs) as reliable indicators of zinc status and are used to estimate the risk of insufficient zinc in the population, even though zinc found in blood accounts for only one percent of total zinc in the body. Studies found PZC fluctuate in response to changes in the overall body’s zinc balance as well as clinical symptoms of zinc deficit, it also responded to severe dietary zinc restriction and zinc supplementation. In addition, urinary zinc is also proposed to be a good biomarker of increases in zinc exposure, but not sensitive for dietary zinc lacking unless acute lack of dietary zinc intake (<1 mg/day) [25]. These biomarkers are related to zinc homeostasis can well reflect zinc concentrations in human body, which can be applied in epidemiological studies (Figure 3).
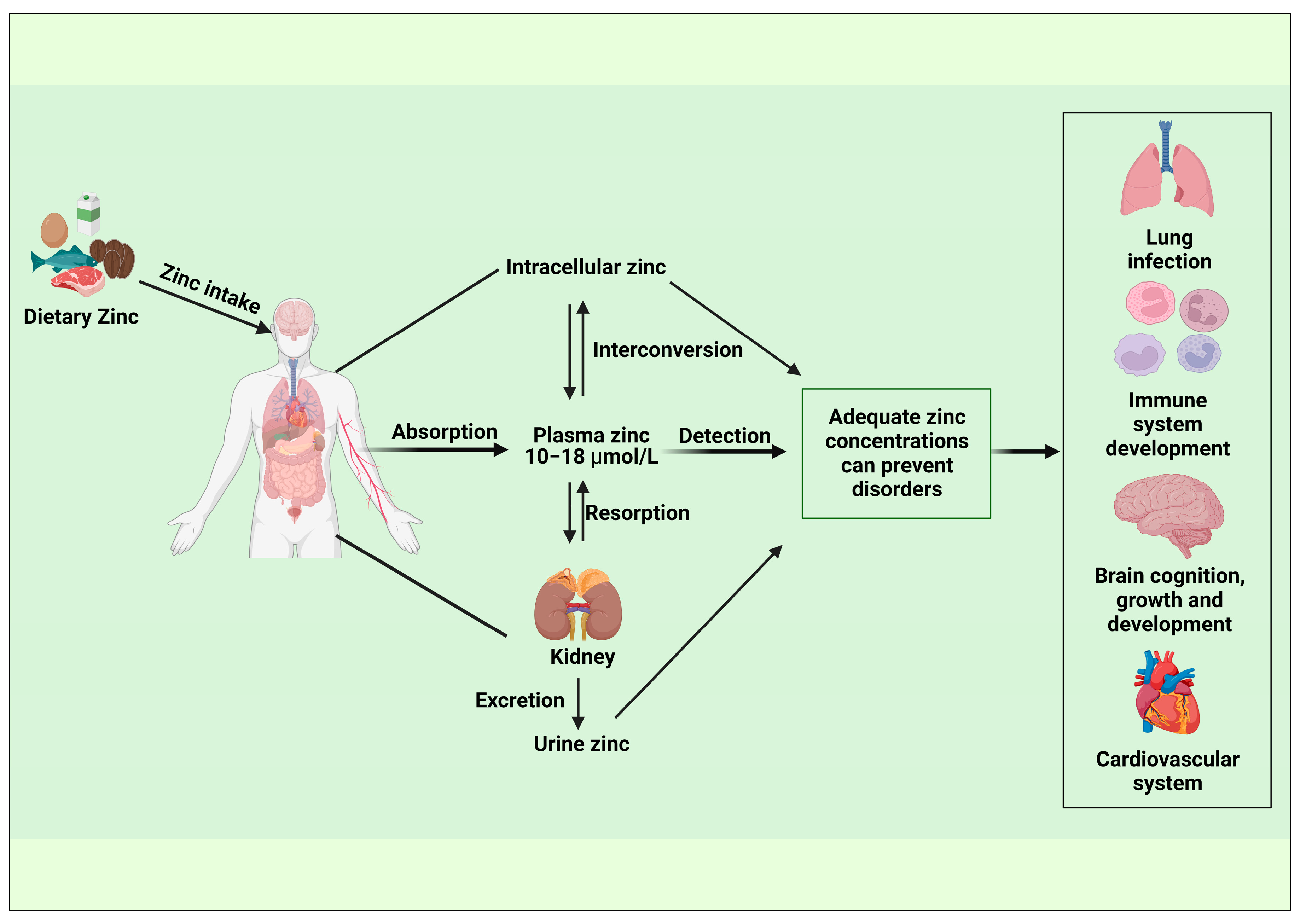
Figure 3. Epidemiological application of biomarkers associated with zinc homeostasis. (Created with BioRender.com).
Application of Zinc Biomarker
Nutritional anemia, a kind of anemia, is particularly prevalent in children and pregnant women since the synthesis of hemoglobin is associated with not only but a range of micronutrients, including iron, folate and vitamin B12. However, zinc deficiency, which is a typical symptom in children, pregnant women and patients with chronic diseases, may also lead to the development of anemia and therefore should be taken into consideration. It is typical for children, older people and chronically ill individuals to have zinc deficiency. Atasoy et al. investigated 349 primary school students aged 6.5 to 14.8 years in a case-control study. Using the receiver operating curve, the cutoff value of serum zinc level for the prediction of anemia was determined to be 71.5 μg/dL. Zinc levels were strongly correlated with hemoglobin levels, which showed that low zinc levels were primarily responsible for the observed anemia in youngsters [26].
Moreover, zinc is essential for both innate and adaptive antiviral immunity, and the antiviral response’s underlying mechanisms have been revealed to be virus specific. A study by Marina et al. showed an association between serum zinc levels and COVID-19 outcomes. Serum zinc concentrations below 50 µg/dL on admission were linked to worse clinical outcomes and increased mortality, and low SZC was a risk factor for COVID-19 outcome. Randomized clinical trials are therefore encouraged to investigate zinc supplementation as a potential method of preventing and treating people at risk of zinc deficiency [27]. Another study used a fluorescence micro-assay to examine free zinc levels in serum samples from COVID-19 survivors and non-survivors. Parallel to the previously observed total serum zinc deficit evaluated by total reflection X-ray fluorescence, free serum zinc in COVID-19 patients was much lower than in controls, while free zinc levels in survivors were significantly higher than in non-survivors. Compared with female patients, free serum zinc levels were especially low in males. This is noteworthy since being male have been identified as a risk factor for serious COVID-19. Overall, the findings indicate that lower serum free zinc levels are correlated with an increased possibility of mortality from COVID-19, implying that free zinc may be a novel prognostic biomarker for the serious and course of COVID-19 [28].
Furthermore, zinc plays crucial parts in the hepatic lipid metabolism as well as scavenging free radical oxygen species in humans. For example, in the liver, zinc functions as a prominent activator of autophagy mediated lipophagy, which lowers lipid accumulation and stimulates lipolysis. Kim et al. recruited 300 patients with nonalcoholic fatty liver disease (NAFLD) for the investigation of the relationship between serum zinc level and the NAFLD. The results showed that the mean serum zinc concentration was 139.8 ± 29.9 μg/dL and the fibrosis-4 index increased substantially with decreasing serum zinc levels. On multivariate analysis, severe liver fibrosis in NAFLD was linked to diabetes, male gender, and zinc levels <140 μg/dL. Low serum zinc levels are independent risk factors for severe liver fibrosis in NAFLD [29].
References
- Bilek, R.; Dvorakova, M.; Grimmichova, T.; Jiskra, J. Iodine, thyroglobulin and thyroid gland. Physiol. Res. 2020, 69, S225–S236.
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine status and supplementation before, during, and after pregnancy. Best Pr. Res. Clin. Endocrinol. Metab. 2020, 34, 101430.
- Monaghan, A.M.; Mulhern, M.S.; McSorley, E.M.; Strain, J.J.; Dyer, M.; van Wijngaarden, E.; Yeates, A.J. Associations between maternal urinary iodine assessment, dietary iodine intakes and neurodevelopmental outcomes in the child: A systematic review. Thyroid Res. 2021, 14, 14.
- Madar, A.A.; Heen, E.; Hopstock, L.A.; Carlsen, M.H.; Meyer, H.E. Iodine Intake in Norwegian Women and Men: The Population-Based Tromso Study 2015–2016. Nutrients 2020, 12, 3246.
- Rohner, F.; Zimmermann, M.; Jooste, P.; Pandav, C.; Caldwell, K.; Raghavan, R.; Raiten, D.J. Biomarkers of nutrition for development--iodine review. J. Nutr. 2014, 144, 1322S–1342S.
- Bertinato, J. Iodine nutrition: Disorders, monitoring and policies. Adv. Food Nutr. Res. 2021, 96, 365–415.
- Cui, T.; Wang, W.; Chen, W.; Pan, Z.; Gao, S.; Tan, L.; Pearce, E.N.; Zimmermann, M.B.; Shen, J.; Zhang, W. Serum Iodine Is Correlated with Iodine Intake and Thyroid Function in School-Age Children from a Sufficient-to-Excessive Iodine Intake Area. J. Nutr. 2019, 149, 1012–1018.
- Hlucny, K.; Alexander, B.M.; Gerow, K.; Larson-Meyer, D.E. Reflection of Dietary Iodine in the 24 h Urinary Iodine Concentration, Serum Iodine and Thyroglobulin as Biomarkers of Iodine Status: A Pilot Study. Nutrients 2021, 13, 2520.
- Pinheiro, C.; Xavier Moreira, N.; Ferreira, P.; Matta Coelho, C.; Guimaraes, J.; Pereira, G.; Cortez, A.; Bracchi, I.; Pestana, D.; Barreiros Mota, I.; et al. Iodine knowledge is associated with iodine status in Portuguese pregnant women: Results from the IoMum cohort study. Br. J. Nutr. 2021, 126, 1331–1339.
- Liu, S.; Sharp, A.; Villanueva, E.; Ma, Z.F. Breast Milk Iodine Concentration (BMIC) as a Biomarker of Iodine Status in Lactating Women and Children <2 Years of Age: A Systematic Review. Nutrients 2022, 14, 1691.
- Kanike, N.; Groh-Wargo, S.; Thomas, M.; Chien, E.K.; Mhanna, M.; Kumar, D.; Worley, S.; Singh, R.J.; Shekhawat, P.S. Risk of Iodine Deficiency in Extremely Low Gestational Age Newborns on Parenteral Nutrition. Nutrients 2020, 12, 1636.
- Neven, K.Y.; Cox, B.; Cosemans, C.; Gyselaers, W.; Penders, J.; Plusquin, M.; Roels, H.A.; Vrijens, K.; Ruttens, A.; Nawrot, T.S. Lower iodine storage in the placenta is associated with gestational diabetes mellitus. BMC Med. 2021, 19, 47.
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. The Evaluation of Iron Deficiency and Iron Overload. Dtsch. Arztebl. Int. 2021, 118, 847–856.
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obs. Gynecol. 2020, 223, 516–524.
- Lynch, S.; Pfeiffer, C.M.; Georgieff, M.K.; Brittenham, G.; Fairweather-Tait, S.; Hurrell, R.F.; McArdle, H.J.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Iron Review. J. Nutr. 2018, 148 (Suppl. S1), 1001S–1067S.
- Mei, Z.; Addo, O.Y.; Jefferds, M.E.; Sharma, A.J.; Flores-Ayala, R.C.; Brittenham, G.M. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: A US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021, 8, e572–e582.
- Ueda, T.; Kawakami, R.; Nogi, K.; Nogi, M.; Ishihara, S.; Nakada, Y.; Nakano, T.; Hashimoto, Y.; Nakagawa, H.; Nishida, T.; et al. Serum iron: A new predictor of adverse outcomes independently from serum hemoglobin levels in patients with acute decompensated heart failure. Sci. Rep. 2021, 11, 2395.
- Masini, G.; Graham, F.J.; Pellicori, P.; Cleland, J.G.F.; Cuthbert, J.J.; Kazmi, S.; Inciardi, R.M.; Clark, A.L. Criteria for Iron Deficiency in Patients With Heart Failure. J. Am. Coll. Cardiol. 2022, 79, 341–351.
- Zhang, Q.; Zheng, J.; Wu, X.; Chen, S.; Qiu, J.; Sun, M. Multi-index evaluation system and relevant critical values for monitoring iron status in Chinese athletes. Med. Sport 2022, 75, 59–68.
- Joo, Y.S.; Kim, H.W.; Lee, S.; Nam, K.H.; Yun, H.R.; Jhee, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Dietary zinc intake and incident chronic kidney disease. Clin. Nutr. 2021, 40, 1039–1045.
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc Metabolism and Metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31.
- Ackland, M.L.; Michalczyk, A.A. Zinc and infant nutrition. Arch. Biochem. Biophys. 2016, 611, 51–57.
- Brown, K.H.; Engle-Stone, R.; Krebs, N.F.; Peerson, J.M. Dietary intervention strategies to enhance zinc nutrition: Promotion and support of breastfeeding for infants and young children. Food Nutr. Bull. 2009, 30 (Suppl. S1), S144–S171.
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858S–885S.
- Lowe, N.M.; Medina, M.W.; Stammers, A.L.; Patel, S.; Souverein, O.W.; Dullemeijer, C.; Serra-Majem, L.; Nissensohn, M.; Hall Moran, V. The relationship between zinc intake and serum/plasma zinc concentration in adults: A systematic review and dose-response meta-analysis by the EURRECA Network. Br. J. Nutr. 2012, 108, 1962–1971.
- Atasoy, H.I.; Bugdayci, G. Zinc deficiency and its predictive capacity for anemia: Unique model in school children. Pediatr. Int. 2018, 60, 703–709.
- Vogel-Gonzalez, M.; Tallo-Parra, M.; Herrera-Fernandez, V.; Perez-Vilaro, G.; Chillon, M.; Nogues, X.; Gómez-Zorrilla, S.; López-Montesinos, I.; Arnau-Barrés, I.; Sorli-Redó, M.L.; et al. Low Zinc Levels at Admission Associates with Poor Clinical Outcomes in SARS-CoV-2 Infection. Nutrients 2021, 13, 562.
- Maares, M.; Hackler, J.; Haupt, A.; Heller, R.A.; Bachmann, M.; Diegmann, J.; Moghaddam, A.; Schomburg, L.; Haase, H. Free Zinc as a Predictive Marker for COVID-19 Mortality Risk. Nutrients 2022, 14, 1407.
- Kim, M.C.; Lee, J.I.; Kim, J.H.; Kim, H.J.; Cho, Y.K.; Jeon, W.K.; Kim, B.I.; Sohn, W. Serum zinc level and hepatic fibrosis in patients with nonalcoholic fatty liver disease. PLoS ONE 2020, 15, e0240195.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
03 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




