Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Novo Barros | -- | 3817 | 2023-03-02 07:30:30 | | | |
| 2 | Rita Xu | -3 word(s) | 3814 | 2023-03-02 07:46:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/41805 (accessed on 14 January 2026).
Serra M, Casas A, Toubarro D, Barros AN, Teixeira JA. Microbial Hyaluronic Acid Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/41805. Accessed January 14, 2026.
Serra, Mónica, Ana Casas, Duarte Toubarro, Ana Novo Barros, José António Teixeira. "Microbial Hyaluronic Acid Production" Encyclopedia, https://encyclopedia.pub/entry/41805 (accessed January 14, 2026).
Serra, M., Casas, A., Toubarro, D., Barros, A.N., & Teixeira, J.A. (2023, March 02). Microbial Hyaluronic Acid Production. In Encyclopedia. https://encyclopedia.pub/entry/41805
Serra, Mónica, et al. "Microbial Hyaluronic Acid Production." Encyclopedia. Web. 02 March, 2023.
Copy Citation
Microbial production of hyaluronic acid (HA) is an area of research that has been gaining attention in recent years due to the increasing demand for this biopolymer for several industrial applications. Hyaluronic acid is a linear, non-sulfated glycosaminoglycan that is widely distributed in nature and is mainly composed of repeating units of N-acetylglucosamine and glucuronic acid. It has a wide and unique range of properties such as viscoelasticity, lubrication, and hydration, which makes it an attractive material for several industrial applications such as cosmetics, pharmaceuticals, and medical devices.
hyaluronic acid
fermentation
microbial production
1. Introduction
Hyaluronic acid (HA) is a naturally occurring biopolymer that is widely distributed in nature. It is a linear, non-sulfated glycosaminoglycan that is composed mainly of repeating units of N-acetylglucosamine and glucuronic acid linked by β- (1-4) and β- (1-3) glycosidic bonds making its structure energetically stable (Figure 1) [1]. Each disaccharide presents a molecular weight (MW) of around 400 Da, and a hyaluronic acid chain can be composed of 10,000 disaccharides, which means a molecular weight of around 4.0 × 103 kDa [2][3].
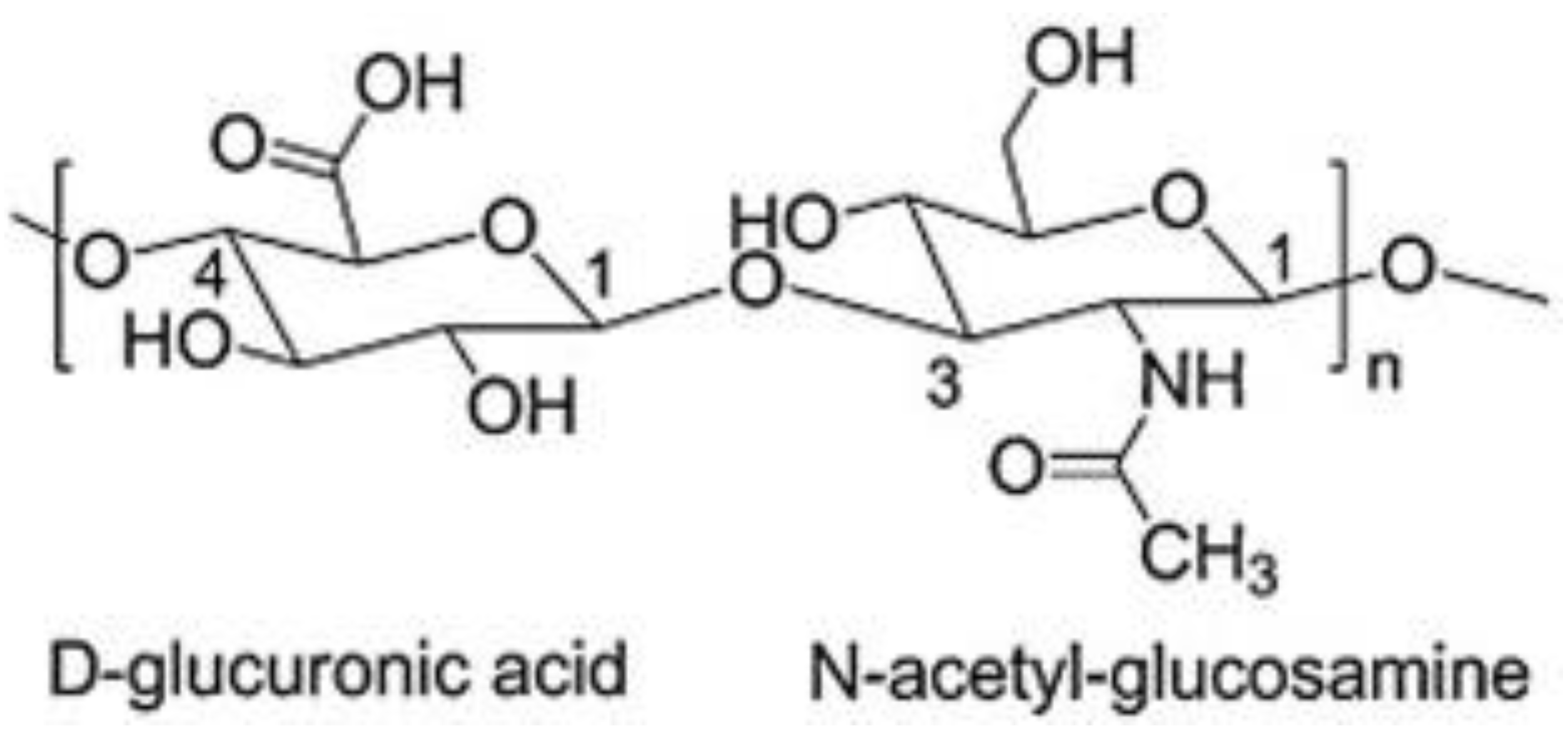
Figure 1. Chemical structure of HA.
Hyaluronic acid plays an important role in living organisms and is an attractive biomaterial for different applications due to its features, in particular moisturizing retention ability, viscoelasticity, resistance to mechanical damage, and lack of immunogenicity and toxicity. In many cases, it acts as a lubricant (joints), a structure stabilizer, an organ space filler (skin), and a shock absorber (cartilage) [4][5][6].
It is a naturally occurring polysaccharide found in the body that plays a vital role in skin health and beauty. It is a major component of the extracellular matrix (ECM) and is responsible for maintaining skin hydration, elasticity, and volume [5][7]. As we age, the levels of hyaluronic acid in our skin decrease, leading to wrinkles, dryness, and loss of firmness. Therefore, the use of products containing hyaluronic acid has become increasingly popular in the cosmetic industry to combat the signs of ageing and promote healthy, youthful-looking skin [8].
This biocompatible polymer immobilizes the water in the tissue, and it can change the dermal volume that influences cell proliferation, differentiation, and tissue repair. The biological functions depend on its molecular weight (MW), for example, mucoadherence is a property of hyaluronic acid with a high molecular weight. This type of hyaluronic acid is used as space fillers, antiangiogenic and immunosuppressive, while medium-size hyaluronic acid chains are involved in ovulation, embryogenesis, and wound repair. Small chains of hyaluronic acid have inflammatory, immuno-stimulatory, angiogenic, and anti-apoptotic properties [3][7][9].
Hyaluronic acid is a humectant, which means it attracts and retains moisture. It can hold up to 1000 times its weight in water, making it an effective ingredient in moisturizers and serums. By keeping the skin hydrated, hyaluronic acid helps to plump up the skin and reduce the appearance of fine lines and wrinkles. It also improves skin elasticity, making it appear firmer and more youthful. In addition to its ability to hydrate and plump the skin, hyaluronic acid has anti-inflammatory properties that can help to reduce redness and irritation. This makes it a great ingredient for sensitive skin types, as well as for those with conditions such as rosacea or eczema [8][10].
The applications of hyaluronic acid are diverse such as the correction of facial folds and wrinkles, body contouring, and as a marker in the diagnosis of tumours. Hyaluronic acid can also be used in the supplementation of joint fluid, in eye surgery, regeneration of surgical wounds, and as a drug delivery agent for various administration routes [2][4][11][12].
Hyaluronic acid can also be used to enhance the effects of other skincare ingredients. For example, when combined with retinoids, it can help to reduce the dryness and irritation that can be caused by these powerful anti-ageing ingredients [13]. Similarly, when used in combination with antioxidants, it can help to protect the skin from environmental damage [10][14].
While hyaluronic acid is a naturally occurring substance in the body, topical products containing hyaluronic acid are typically derived from either rooster combs or fermentation. There are different types of hyaluronic acid, such as sodium hyaluronate and hyaluronic acid. Sodium hyaluronate is a salt form of hyaluronic acid that is smaller and can penetrate deeper into the skin [15].
The demand and the value of hyaluronic acid have increased over the years. According to R. G. Ferreira et al. [16], the estimated market of hyaluronic acid was EUR 7.6 billion in 2019. It is expected to have an annual growth of 8.1% from 2016 to 2027, which means that 20 MT of hyaluronic acid produced by that year will have an average price between EUR 1500/kg and EUR 4000/kg. The main market segments are derma fillers in cosmetics, osteoarthritis, and ophthalmology. Consequently, it is necessary to understand the improvement points in the hyaluronic acid production process, including downstream processing [16].
2. Hyaluronic Acid Production Using the Fermentation Process
Microbial production of hyaluronic acid is an area of research that has been gaining attention in recent years. Hyaluronic acid is widely distributed in nature [1], and it has a wide range of properties such as viscoelasticity, lubrication, and hydration that make it an attractive material for various industrial applications [2][4][11][12].
Microorganisms such as bacteria and yeast have been used to produce HA through fermentation. Streptococcus zooepidemicus, a Gram-positive bacteria, is one of the most widely used organisms for the production of HA due to its high hyaluronic acid production rate and ease of cultivation [5][7][17].
Traditionally, hyaluronic acid is extracted from animal sources such as rooster combs and cocks’ combs. However, the difficulty in controlling animal tissue, high costs, and ethical concerns associated with animal-derived HA have led to the development of microbial production methods [5][7][9].
During the production of hyaluronic acid, there are still challenges to be overcome, such as the limited production of hyaluronic acid due to the high viscosity of the broth, causing difficulties in the mixing and mass transfer rate of oxygen; competition for the same precursors for cell growth and hyaluronic acid production; and the accumulation of lactic acid, the main by-product of HA fermentation, causing the inhibition of cell growth and hyaluronic acid production [5][7][9].
To overcome these challenges and also increase the microbial production of hyaluronic acid, several strategies have been studied: selection of producing strains and appropriate culture media, the establishment of culture methods, and determination of culture conditions, among others.
3. Selection of a Microorganism Producer and Its Cultivation Media
The selection of the appropriate microorganism to produce hyaluronic acid is an important factor in the microbial production process. Each microorganism has its own unique advantages and disadvantages in terms of production performance and profitability. When choosing a microorganism to produce hyaluronic acid, it is essential to consider the specific requirements of the final application of this molecule [5][7][9].
The microorganism must be able to produce high yields of hyaluronic acid, be easy to cultivate, and have a low cost of production. Several microorganisms have been used to produce hyaluronic acid through fermentation, including Streptococcus zooepidemicus, Bacillus subtilis, and Escherichia coli [18][19][20][21].
Streptococcus zooepidemicus produces a high molecular weight HA with excellent biocompatibility, making it suitable for medical applications such as wound healing and joint lubrication. However, Streptococcus is a slow-growing organism, and the production process is costly due to the need for expensive media and downstream processing. However, it does not require the use of toxic chemicals or solvents, resulting in a pure and safe end product [16][22].
Bacillus subtilis is another microorganism that has been used to produce hyaluronic acid. It is a Gram-positive bacterium that is known for its ability to produce high yields of HA and is also non-pathogenic. However, it is considered more difficult to cultivate than Streptococcus zooepidemicus. Bacillus produces a lower molecular weight hyaluronic acid that is suitable for cosmetic applications such as moisturizing creams and serums. Bacillus grows rapidly and is relatively cheap to produce, making it a more profitable option for cosmetic companies [18][23].
Escherichia coli is a Gram-negative bacterium that has been used for the production of hyaluronic acid. It is a well-studied organism and has a wide range of genetic tools available for use in genetic engineering. However, it produces a lower yield of hyaluronic acid compared to Streptococcus zooepidemicus and Bacillus subtilis [20][21][24].
Another microorganism that has been recently explored for hyaluronic acid production is the yeast Pichia pastoris. This organism produces a high yield of low molecular weight HA, making it suitable for use in the pharmaceutical industry. The production process is relatively cheap and can be scaled up easily, making it a promising candidate for large-scale production [25][26].
When considering the profitability and possible applications of the hyaluronic acid produced by each microorganism, it is essential to consider factors such as production costs, purity of the final product, and market demand. For example, the high-molecular-weight hyaluronic acid produced by Streptococcus has significant potential in the medical field due to its excellent biocompatibility, making it a high-value product. Meanwhile, the lower-molecular-weight hyaluronic acid produced by Bacillus has excellent potential in the cosmetic industry, with demand for hyaluronic acid-based cosmetics continuing to grow.
In conclusion, the selection of the appropriate microorganism for hyaluronic acid production is critical for determining the production performance and profitability of the process. By evaluating the advantages of the final application, producers can optimize their production process, enhance product quality, and increase profitability.
Group A and C streptococci, namely Streptococcus zooepidemicus, have been the most explored strain in the production process of microbial hyaluronic acid and have obtained the best results. Nevertheless, due to the pathogenicity of this natural HA-producer bacteria, other metabolically engineered microorganisms have been studied, such as Bacillus subtilis [18][19][23], Corynebacterium glutamicum [27][28][29], Escherichia Coli [20][21][24], Lactococcus lactis [30][31], Pichia pastoris [25], and Kluyveromyces lactis [32]. Among the cited strains, Corynebacterium glutamicum has presented the best results [28][29].
The cultivation medium to produce hyaluronic acid A is also an important factor to consider. The medium must contain a source of carbon, nitrogen, vitamins, minerals, and other growth factors for the microorganism. Additionally, the medium should be optimized to promote the production of hyaluronic acid by the microorganism [5][26].
Concerning media composition for hyaluronic acid production, an important factor is the cultivation condition. Streptococcus zooepidemicus is a nutritionally demanding microorganism, and a nitrogen source is an essential nutrient for its growth. This bacterium does not synthesize some amino acids that are favourable for its growth and hyaluronic acid production. Therefore, media supplementation with nutrients, such as amino acids, has been studied [5][26].
3.1. Alternative Sources as Substrates for the Culture Media
One of the key factors in the production of hyaluronic acid is the culture medium that is used to grow the microorganism producers.
Traditionally, the culture medium for hyaluronic acid production has been based on complex sugars such as glucose and fructose, but these can be expensive and may not be readily available [33][34]. As a result, researchers have been investigating alternative sources as substrates for the culture medium. Some of the most promising alternative sources include:
-
Agricultural waste: By-products of agriculture, such as sugar beet pulp, corn steep liquor, and wheat bran, have been found to be suitable substrates for hyaluronic acid production. These waste products are readily available and have the added benefit of being environmentally friendly [35].
-
Synthetic substrates: Some researchers have also investigated synthetic substrates, such as hydrolysates of starch and cellulose, as an alternative substrate for hyaluronic acid production. These substrates are relatively cheap and easy to produce, making them an attractive option for commercial production [37].
Overall, alternative sources as substrates for the culture media for hyaluronic acid production have been explored to reduce the costs of production and make the process more sustainable. While more research is needed to fully understand the potential of these alternative sources, they offer promising possibilities for the future of hyaluronic acid production.
Different agricultural resources and industrial wastes have been explored as alternative nutritive sources for microbial hyaluronic acid. The goal of the formulation of cost-effective culture media is to maintain low costs during microbial hyaluronic acid production and to reduce pollution problems, as well as to improve the efficiency of the fermentation processes.
Amado et al. [36] studied the optimization of a media containing cheese whey to produce hyaluronic acid by Streptococcus zooepidemicus. The major nutrients in cheese whey are lactose, soluble proteins (β-lactoglobulin, α-lactalbumin), lipids, and B-group vitamins. Using cheese whey protein and glucose as nitrogen and carbon sources, respectively, the maximum production rate was 0.87 g/L h (0.75 g/L h in control media with glucose and yeast extract), the HA production was 4.02 g/L (3.19 g/L in control media), and the HA average molecular weights (HA-MW) were 3.71 × 103 kDa.
In another study, Amado et al. [35] formulated a culture media containing corn steep liquor (CSL) instead of tryptone as a nitrogen source. The highest hyaluronic acid production in this media was 3,48 g/L (comparable to the control media of 3.60 g/L) with a molecular weight of 3.8 × 103 kDa (higher than the control media of 3.0 × 103 kDa).
Arslan & Aydogan [38] tested the effectiveness of sheep wool peptone (SWP) and molasses as nitrogen and carbon sources, respectively, in fermentation media to produce hyaluronic acid, using the same strain as Amado et al. They verified a higher HA production in media containing sheep wool peptone (3.54 g/L) than in media containing tryptone TP (2.58 g/L) and peptone PP (2.47 g/L). Sheep wool peptone has a lower protein content (70.6 g/100 g) than tryptone and peptone (83.1 and 83.3 g/100 g, respectively), and by contrast, sheep wool peptone has higher element contents (K, P, and Mg) than tryptone and peptone, and these elements promote hyaluronic acid production. Moreover, this nitrogen source contains high cystine and arginine contents, which are the main amino acids that affect hyaluronic acid production. These are possible reasons for the higher production of hyaluronic acid in sheep wool peptone than in tryptone and peptone.
Similarly, Pan et al. [39] developed a media for hyaluronic acid production which contains sugarcane molasses. After molasses treatment with activated charcoal and using it as a substrate for Streptococcus zooepidemicus, the hyaluronic acid production was 19% higher than using molasses without treatment. This suggested pre-treatment decreased the excessive metal ions content that can inhibit hyaluronic acid production. After 24 h of fermentation with pH control (pH 8), the hyaluronic acid production was 2.83 g/L.
Pires et al. [40] investigated the use of cashew apple juice in the fermentation media, and the HA production was 0.89 g/L with a molecular weight of 18.4 kDa.
Vázquez et al. [41] explored a culture media using marine by-products as substrates for Streptococcus zooepidemicus when producing microbial hyaluronic acid. Firstly, glycogen from mussel processing wastewater (MPW) and tuna peptone from viscera residue were used as a carbon source and a protein source, respectively. In this media, hyaluronic acid production and the cell growth rate were lower (2.46 g/L and 0.81 g/L/h, respectively) than in commercial media containing glucose and tryptone (3.07 g/L and 1.32 g/L/h, respectively); on the other hand, the hyaluronic acid produced in alternative media presented a higher molecular weight (2.50 × 103 kDa). Later, the same scientists explored a media formulated with peptones obtained from Scyliorhinus canicula viscera by-products. Using this alternative substrate as a nitrogen source, hyaluronic acid production was explored in fed-batch culture. Using this culture mode, the hyaluronic acid production (2.53 g/L) and molecular weight (2.11 × 103 kDa) of the polysaccharide were increased compared to production using the batch mode (2.26 g/L; 1.80 × 103 kDa). Compared with the commercial media (3.23 g/L; 0.930 g/L/h; 1.85 × 103 kDa), the hyaluronic acid production and cell growth rate were lower in the alternative media; however, the hyaluronic acid molecular weight was higher [42].
Benedini & Santana [43] studied the effect of replacing Brain Heart Infusion (BHI) with soy peptone (SP) as a nitrogen source for Streptococcus zooepidemicus. In this case, there was an increment in hyaluronic acid production and hyaluronic acid molecular weight from 0.29 g/L and 3.09 × 103 kDa to 0.30 g/L and 3.60 × 103 kDa, respectively. The increase in hyaluronic acid molecular weight was related to the diminution of lactic acid production and hyaluronic acid exposition to a higher pH.
Another study was performed in which Ghodke et al. [44] evaluated palmyra palm sugar (Pj) and soya peptone to formulate a media for hyaluronic acid production by Streptococcus zooepidemicus. Palmyra palm sugar contains sucrose and vitamins (nicotinic acid, thiamine, riboflavin, and vitamin C) that are essential to the growth of a microorganism, which led to the hyaluronic acid production and specific growth rate on palmyra palm sugar-based media (0.41 g/L; 0.54 h−1) being higher than on pure sucrose-based media (0.31 g/L; 0.32 h−1). After exploring the best initial substrate concentration (Pj, 30 g/L), the microorganism yielded a hyaluronic acid concentration of 1.22 g/L with 9.50 × 102 kDa.
Zhang et al. [37] proposed a serum-free starch media in which the cells could grow and produce hyaluronic acid. Replacing glucose with starch obtained hyaluronic acid with a yield of 6.7 g/L. The studied strain could grow in serum-free media but not in other carbohydrates such as glucose. This could be due to the reduction in lactic acid (metabolite from glucose catabolism) in the broth for decreasing glucose in the media.
In their work, Duffeck et al. [45] optimized the production of hyaluronic acid in sugarcane molasses pre-treated with active charcoal media (likewise explored by Pan et al. [39]) with nutrient supplementation (glutamine). They concluded that the hyaluronic acid production by Streptococcus zooepidemicus was higher in sugarcane molasses media (0.710 g/L) than in glucose media (0.469 g/L) due to the molasses media being rich in sugar and amino acids, and after treatment, this molasses contains fewer inhibitors to hyaluronic acid production. Regarding media supplementation, glutamine supplementation revealed positive effects on hyaluronic acid production, improving the yield of the product to 2.55 g/L, because this amino acid acts as the amino donor group to UDP-N-acetylglucosamine formation (one of hyaluronic acid precursors).
3.2. Supplementation of Culture Media
The process of media supplementation in the production of hyaluronic acid includes:
-
Carbon source supplements: Carbon sources such as glucose, fructose, and maltose can be added to the culture medium to provide an additional energy source for the microorganisms. This can help increase the rate of hyaluronic acid production [46].
-
Nitrogen source supplements: Nitrogen sources such as ammonium sulfate and yeast extract can be added to the culture medium to provide essential amino acids and other nutrients for the microorganisms. This can help increase the rate of hyaluronic acid production [33].
-
Vitamin supplements: Vitamins such as thiamine, riboflavin, and pyridoxine can be added to the culture medium to promote the growth of microorganisms and increase the rate of hyaluronic acid production [47].
-
Mineral supplements: Minerals such as sodium, potassium, and calcium can be added to the culture medium to provide essential minerals for the microorganisms and promote hyaluronic acid production [34].
-
Growth factors: Growth factors such as yeast extract, soy peptone, and tryptone can be added to the culture medium to provide additional nutrients and growth factors for the microorganisms [33]. This can help increase the rate of hyaluronic acid production.
It is important to note that the appropriate supplements and the optimal concentration of the supplement in the culture medium can vary depending on the microorganism used for hyaluronic acid production. Researchers often use a combination of different supplements and tweak the concentration to find the best condition to produce hyaluronic acid. It is also important to note that the use of supplements in the culture medium can increase production costs; hence, it is important to find a balance between costs and the enhancement of production.
The substrates used more often in hyaluronic acid production are glucose and sucrose as the primary carbon and energy sources and yeast extract and peptones as nitrogen sources [33][34][48][49][50][51][52]. Other carbon sources have been explored as substrates for hyaluronic acid production, namely, lactose, maltose, galactose, mannose, and others; however, hyaluronic acid production is not as high [18][34][53][54][55].
In their investigation, Chen et al. [56] used glucose as a carbon source and yeast extract as a nitrogen source to explore the best carbon-to-nitrogen (C/N) ratio and concluded that the C/N ratio of 2:1 maximizes hyaluronic acid synthesis at 2.45 g/L. In other C/N ratios, such as 1,3:1 and 4:1, the hyaluronic acid production was 2.20 g/L and 1.52 g/L, respectively.
Concerning media supplementation, Aroskar et al. [46] also investigated the effect of different nutrients in the culture media for Streptococcus zooepidemicus production of hyaluronic acid. The carbon source used was dextrose for producing hyaluronic acid with a higher yield (0.70 g/L) than other carbon sources used, such as sucrose (0.51 g/L), maltose (0.50 g/L), and dextrin (0.55 g/L). The researchers explored the addition of L-arginine HCl and tannic acid to the fermentation media, which resulted in an increment in the yield of HA to 1.029 g/L. L-arginine HCl is a carbon and nitrogen donor for the synthesis of nucleotides that are needed for the growth and multiplication of microorganisms, thus saving energy consumption in the organism for its production. Tannic acid represses the synthesis of the hyaluronidase enzyme that acts by depolymerizing hyaluronic acid. In light of this, the use of tannic acid showed an increase in the molecular weight of hyaluronic acid.
In the same way, Shah et al. [33] explored the supplementation media for Streptococcus zooepidemicus with glutamine and sodium iodoacetate. Iodoacetate decreases lactic acid synthesis by inhibiting the glycolysis pathway; consequently, there was a redirection of the carbon flow from lactic acid formation to UDP glucuronic acid formation, one of the precursors of hyaluronic acid. Glutamine, as mentioned above, is an amino acid involved in the formation of one of the hyaluronic acid precursors. Accordingly, the HA concentration was increased from 2 g/L in media without supplementation to 5 g/L, the specific growth rate was decreased from 0.42 h−1 to 0.25 h−1, and the hyaluronic acid molecular weight was increased from 2.4 × 103 kDa to 3.2 × 103 kDa.
Aiming to obtain HA with high yield and molecular weight, Im et al. [34] studied the optimization of media components using the strain Streptococcus sp. ID9102 (KCTC 11935BP), which is negative to hemolytic activity and hyaluronidase. Exploring the best carbon source, a higher hyaluronic acid production was reached when glucose was the carbon source (1.58 g/L) followed by lactose and sucrose, which reached similar results (≈1.3 g/L). According to the results, the investigators selected the best components media as glucose, yeast extract, casein peptone, K2HPO4, and MgCl2 and obtained a hyaluronic acid production of 5.88 g/L with a molecular weight of 3.60 × 103 kDa. After supplementing the media with amino acids (glutamine and glutamate) and organic acid (oxalic acid), the HA titer and HA-MW were higher (6.94 g/L and 5.90 × 103 kDa, respectively).
Kim et al. [47] also studied the production of HA from Streptococcus equi mutant KFCC 10830. This mutant has nonhemolytic and hyaluronidase-negative characteristics. Furthermore, the scientists explored the effect of lysozyme addition during cultivation on the fermentation media. Lysozyme can damage the cell walls of hyaluronic acid producer microorganisms, which then produce more hyaluronic acid to protect themselves from the action of lysozyme. According to this, the addition of lysozyme increased the production and molecular weight of hyaluronic acid.
References
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239.
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411.
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701.
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526.
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Fact. 2011, 10, 99.
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Sannino, A.; Madaghiele, M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019, 117, 134–147.
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351.
- Carlomagno, F.; Roveda, G.; Michelotti, A.; Ruggeri, F.; Tursi, F. Anti-Skin-Aging Effect of a Treatment with a Cosmetic Product and a Food Supplement Based on a New Hyaluronan: A Randomized Clinical Study in Healthy Women. Cosmetics 2022, 9, 54.
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek, L.A.M.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 624967.
- Tan, J.; Spada, J.; Kerscher, M.; Anfilova, M.; Abdulla, S.; Altmeyer, A.; Delva, C.; Kerob, D.; Araviiskaia, E. 26268 M89, a combination of 89% of Vichy volcanic mineralizing water and hyaluronic acid improves signs and symptoms of subjects with rosacea/sensitive/reactive skin: Results from an open-label, observational, international study. J. Am. Acad. Dermatol. 2021, 85, AB93.
- Goa, K.L.; Benfield, P. Hyaluronic Acid: A Review of its Pharmacology and Use as a Surgical Aid in Ophthalmology, and its Therapeutic Potential in Joint Disease and Wound Healing. Drugs 1994, 47, 536–566.
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25.
- Cordero, A.; Leon-Dorantes, G.; Pons-Guiraud, A.; Di Pietro, A.; Asensi, S.V.; Walkiewicz-Cyraska, B.; Litvik, R.; Turlier, V.; Mery, S.; Merial-Kieny, C. Retinaldehyde/hyaluronic acid fragments: A synergistic association for the management of skin aging. J. Cosmet. Dermatol. 2011, 10, 110–117.
- Neves, J.R.; Grether-Beck, S.; Krutmann, J.; Correia, P.; Gonçalves, J.E., Jr.; Sant’Anna, B.; Kerob, D. Efficacy of a topical serum containing L-ascorbic acid, neohesperidin, pycnogenol, tocopherol, and hyaluronic acid in relation to skin aging signs. J. Cosmet. Dermatol. 2022, 21, 4462–4469.
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final Report of the Safety Assessment of Hyaluronic Acid, Potassium Hyaluronate, and Sodium Hyaluronate. Int. J. Toxicol. 2009, 28, 5–67.
- Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Techno-economic analysis of a hyaluronic acid production process utilizing streptococcal fermentation. Processes 2021, 9, 241.
- Yatmaz, E.; Turhan, İ. Hyaluronic acid and production by fermentation. Gıda 2015, 40, 233–240.
- Rangaswamy, V.; Santosh, V.; Dharmendra, J.; Nataraj, V.; Velankar, H.; Kapat, A. Process for producing high molecular weight hyaluronic acid. European Patent EP2046969B1, 2011.
- Jia, Y.; Zhu, J.; Chen, X.; Tang, D.; Su, D.; Yao, W.; Gao, X. Metabolic engineering of Bacillus subtilis for the efficient biosynthesis of uniform hyaluronic acid with controlled molecular weights. Bioresour. Technol. 2013, 132, 427–431.
- Lai, Z.-W.; Rahim, R.A.; Ariff, A.B.; Mohamad, R. Comparison of Hyaluronic Acid Biosynthesis by the recombinant Escherichia Coli Strains in different mode of bioreactor operation. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 905–910.
- Woo, J.E.; Seong, H.J.; Lee, S.Y.; Jang, Y.S. Metabolic Engineering of Escherichia coli for the Production of Hyaluronic Acid from Glucose and Galactose. Front. Bioeng. Biotechnol. 2019, 7, 375.
- Reddy, K.J.; Karunakaran, K.T. Purification and characterization of hyaluronic acid produced by Streptococcus zooepidemicus. J. BioSci. Biotech 2013, 2, 173–179.
- Westbrook, A.W.; Ren, X.; Moo-Young, M.; Chou, C.P. Engineering of cell membrane to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Biotechnol. Bioeng. 2018, 115, 216–231.
- Yu, H.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab. Eng. 2008, 10, 24–32.
- Jeong, E.; Shim, W.Y.; Kim, J.H. Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight. J. Biotechnol. 2014, 185, 28–36.
- Armstrong, D.C.; Cooney, M.J.; Johns, M.R. Growth and amino acid requirements of hyaluronic-acid producing Streptococcus zooepidemicus. Appl. Microbiol. Biotechnol. 1997, 47, 309–312.
- Hoffmann, J.; Altenbuchner, J. Hyaluronic acid production with Corynebacterium glutamicum: Effect of media composition on yield and molecular weight. J. Appl. Microbiol. 2014, 117, 663–678.
- Cheng, F.; Gong, Q.; Yu, H.; Stephanopoulos, G. High-titer biosynthesis of hyaluronic acid by recombinant Corynebacterium glutamicum. Biotechnol. J. 2016, 11, 574–584.
- Cheng, F.; Luozhong, S.; Guo, Z.; Yu, H.; Stephanopoulos, G. Enhanced Biosynthesis of Hyaluronic Acid Using Engineered Corynebacterium glutamicum Via Metabolic Pathway Regulation. Biotechnol. J. 2017, 12, 1700191.
- Sunguroğlu, C.; Sezgin, D.E.; Çelik, P.A.; Çabuk, A. Higher titer hyaluronic acid production in recombinant Lactococcus lactis. Prep. Biochem. Biotechnol. 2018, 48, 734–742.
- Jeeva, P.; Doss, S.S.; Sundaram, V.; Jayaraman, G. Production of controlled molecular weight hyaluronic acid by glucostat strategy using recombinant Lactococcus lactis cultures. Appl. Microbiol. Biotechnol. 2019, 103, 4363–4375.
- Gomes, A.M.V.; Netto, J.H.C.M.; Carvalho, L.S.; Parachin, N.S. Heterologous hyaluronic acid production in kluyveromyces lactis. Microorganisms 2019, 7, 294.
- Shah, M.V.; Badle, S.S.; Ramachandran, K.B. Hyaluronic acid production and molecular weight improvement by redirection of carbon flux towards its biosynthesis pathway. Biochem. Eng. J. 2013, 80, 53–60.
- Im, J.H.; Song, J.M.; Kang, J.H.; Kang, D.J. Optimization of medium components for high-molecular-weight hyaluronic acid production by Streptococcus sp. ID9102 via a statistical approach. J. Ind. Microbiol. Biotechnol. 2009, 36, 1337–1344.
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Microbial production of hyaluronic acid from agro-industrial by-products: Molasses and corn steep liquor. Biochem. Eng. J. 2017, 117, 181–187.
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Cheese whey: A cost-effective alternative for hyaluronic acid production by Streptococcus zooepidemicus. Food Chem. 2016, 198, 54–61.
- Zhang, J.; Ding, X.; Yang, L.; Kong, Z. A serum-free medium for colony growth and hyaluronic acid production by Streptococcus zooepidemicus NJUST01. Appl. Microbiol. Biotechnol. 2006, 72, 168–172.
- Arslan, N.P.; Aydogan, M.N. Evaluation of Sheep Wool Protein Hydrolysate and Molasses as Low-Cost Fermentation Substrates for Hyaluronic Acid Production by Streptococcus zooepidemicus ATCC 35246. Waste Biomass Valorization 2021, 12, 925–935.
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.L.C.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293.
- Pires, A.M.B.; Macedo, A.C.; Eguchi, S.Y.; Santana, M.H.A. Microbial production of hyaluronic acid from agricultural resource derivatives. Bioresour. Technol. 2010, 101, 6506–6509.
- Vázquez, J.A.; Montemayor, M.I.; Fraguas, J.; Murado, M.A. Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microb. Cell Fact. 2010, 9, 46.
- Vázquez, J.A.; Pastrana, L.; Piñeiro, C.; Teixeira, J.A.; Pérez-Martín, R.I.; Amado, I.R. Production of hyaluronic acid by Streptococcus zooepidemicus on protein substrates obtained from Scyliorhinus canicula discards. Mar. Drugs 2015, 13, 6537–6549.
- Benedini, L.J.; Santana, M.H.A. Effects of soy peptone on the inoculum preparation of Streptococcus zooepidemicus for production of hyaluronic acid. Bioresour. Technol. 2013, 130, 798–800.
- Ghodke, R.S.; Kakati, J.P.; Tadi, S.R.R.; Mohan, N.; Silvaprakasam, S. Kinetic modeling of hyaluronic acid production in palmyra palm (Borassus flabellifer) based medium by Streptococcus zooepidemicus MTCC 3523. Biochem. Eng. J. 2018, 137, 284–293.
- Duffeck, H.C.B.P.; Pan, N.C.; Saikawa, G.I.A.; da Rocha, S.P.D.; Baldo, C.; Celligoi, M.A.P.C. Biomedical Potential of Hyaluronic Acid from Streptococcus zooepidemicus Produced in Sugarcane Molasses. Braz. J. Dev. 2020, 6, 49963–49980.
- Aroskar, V.J.; Kamat, S.D.; Kamat, D. Effect of Various Nutritional Supplements on Hyaluronic Acid Production. IIOAB Lett. 2013, 2, 16–24.
- Kim, J.H.; Yoo, S.J.; Oh, D.K.; Kweon, Y.G.; Park, D.W.; Lee, C.H.; Gil, G.H. Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzyme Microb. Technol. 1996, 19, 440–445.
- Lai, Z.W.; Rahim, R.A.; Ariff, A.B.; Mohamad, R. Biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus using oxygen vector and optimum impeller tip speed. J. Biosci. Bioeng. 2012, 114, 286–291.
- Liu, L.; Du, G.; Chen, J.; Wang, M.; Sun, J. Comparative study on the influence of dissolved oxygen control approaches on the microbial hyaluronic acid production of Streptococcus zooepidemicus. Bioprocess Biosyst. Eng. 2009, 32, 755–763.
- Armstrong, D.C.; Johns, M.R. Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus. Appl. Environ. Microbiol. 1997, 63, 2759–2764.
- Don, M.M.; Shoparwe, N.F. Kinetics of hyaluronic acid production by Streptococcus zooepidemicus considering the effect of glucose. Biochem. Eng. J. 2010, 49, 95–103.
- Zakeri, A.; Rasaee, M.J. Identification of wild type Streptococcus Zooepidemicus and optimization of culture medium and fermentation conditions for production of hyaluronic acid. Biosci. Biotechnol. Res. Asia 2016, 13, 189–198.
- Gedikli, S.; Güngör, G.; Toptaş, Y.; Sezgin, D.E.; Demirbilek, M.; Yazıhan, N.; Çelik, P.A.; Denkbaş, E.B.; Bütün, V.; Çabuk, A. Optimization of hyaluronic acid production and its cytotoxicity and degradability characteristics. Prep. Biochem. Biotechnol. 2018, 48, 610–618.
- Rangaswamy, V.; Jain, D. An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol. Lett. 2008, 30, 493–496.
- Hashimoto, M.; Kakema, T.; Fujii, K.; Ikemi, M. Method for producing hyaluronic acid. US patent 8927234B2, 2015.
- Chen, S.J.; Chen, J.L.; Huang, W.C.; Chen, H.L. Fermentation process development for hyaluronic acid production by Streptococcus zooepidemicus ATCC 39920. Korean J. Chem. Eng. 2009, 26, 428–432.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.8K
Revisions:
2 times
(View History)
Update Date:
02 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




