Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberta Cassano | -- | 2924 | 2023-03-01 19:22:05 | | | |
| 2 | Camila Xu | Meta information modification | 2924 | 2023-03-02 02:18:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Trombino, S.; Sole, R.; Di Gioia, M.L.; Procopio, D.; Curcio, F.; Cassano, R. Green Chemistry Principles for Cellulose-Based Hydrogels. Encyclopedia. Available online: https://encyclopedia.pub/entry/41789 (accessed on 01 March 2026).
Trombino S, Sole R, Di Gioia ML, Procopio D, Curcio F, Cassano R. Green Chemistry Principles for Cellulose-Based Hydrogels. Encyclopedia. Available at: https://encyclopedia.pub/entry/41789. Accessed March 01, 2026.
Trombino, Sonia, Roberta Sole, Maria Luisa Di Gioia, Debora Procopio, Federica Curcio, Roberta Cassano. "Green Chemistry Principles for Cellulose-Based Hydrogels" Encyclopedia, https://encyclopedia.pub/entry/41789 (accessed March 01, 2026).
Trombino, S., Sole, R., Di Gioia, M.L., Procopio, D., Curcio, F., & Cassano, R. (2023, March 01). Green Chemistry Principles for Cellulose-Based Hydrogels. In Encyclopedia. https://encyclopedia.pub/entry/41789
Trombino, Sonia, et al. "Green Chemistry Principles for Cellulose-Based Hydrogels." Encyclopedia. Web. 01 March, 2023.
Copy Citation
Hydrogels are 3D structures consisting of hydrophilic polymer networks. They can adsorb water and biological fluids in high quantities (up to 1000 times their dry weight). Cellulose is a hydrophilic material. It has inter- and intramolecular hydrogen bonds and van der Waals forces that cause many problems regarding its dissolution.
hydrogels
green chemistry
biopolymers
1. Hydrogels
Hydrogels are 3D structures consisting of hydrophilic polymer networks. They can adsorb water and biological fluids in high quantities (up to 1000 times their dry weight) [1].
With respect to the type of crosslinking, hydrogels can be classified into “physical” and “chemical” gels (Figure 1).
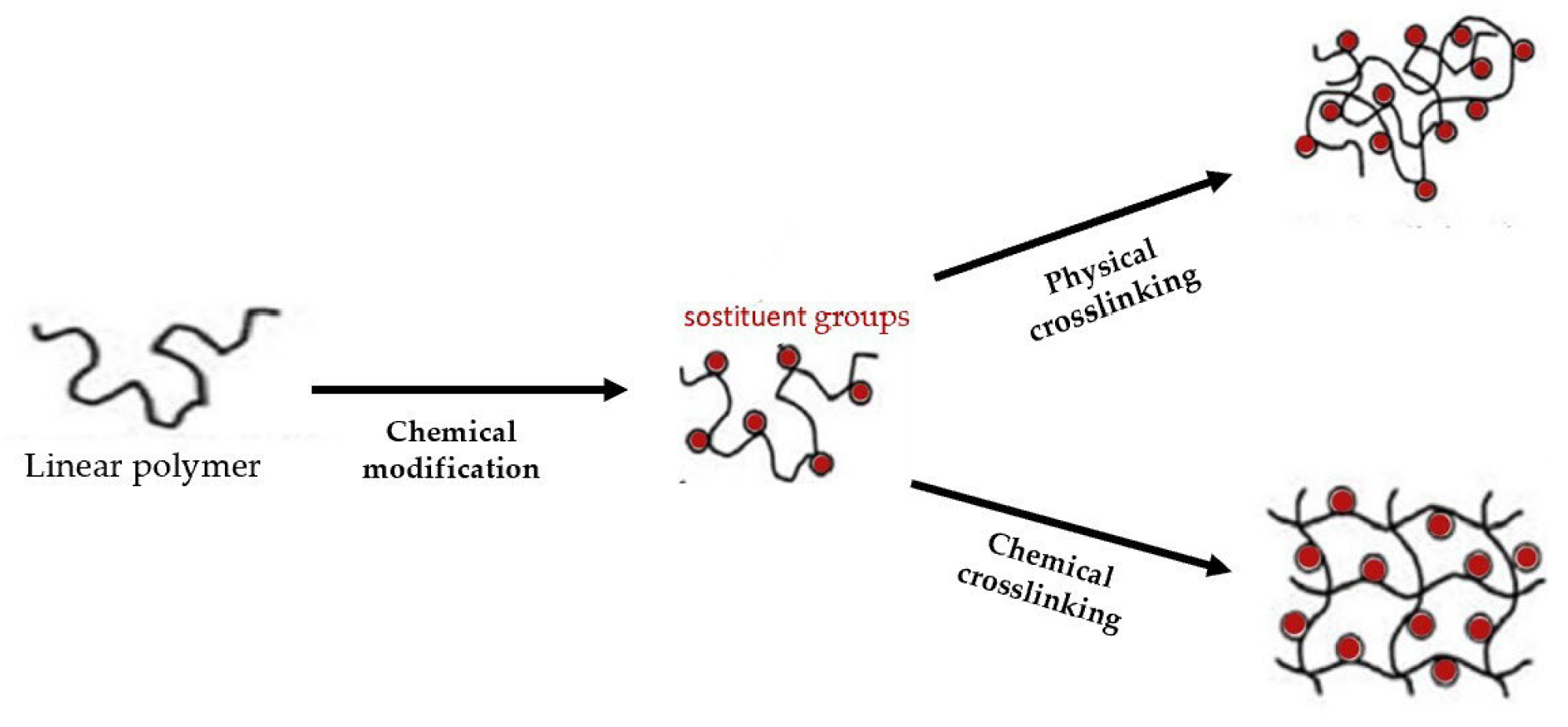
Figure 1. Scheme of chemical and physical hydrogel formation from a linear polymer.
They are classified as “reversible” or “physical” if crosslinking occurs through the establishment of weak physical interactions between the main chains: hydrogen bond, ionic interactions and Van der Waals [2]. These materials are unstable and reversible and retain their integral structure with difficulty and dissolve easily when environmental factors such as temperature, pH, etc., change.
Hydrogels are classified as “permanent” or “chemical” when they form covalent bonds between the polymer chains [3]. As a result, these types of hydrogels retain their structure even after swelling. Chemical hydrogels can be generated through crosslinking polymers using radiation, polyfunctional compounds, free-radical-generating compounds and, recently, enzymatic crosslinking [4]. They have better chemical, mechanical and thermal stability than physical hydrogels. Moreover, they can be formed from either synthetic or natural polymers [5]. The latter are biocompatible, biodegradable, bacteriostatic and even have healing properties. Synthetic hydrogels do not have these inherent bioactive properties (Figure 2).
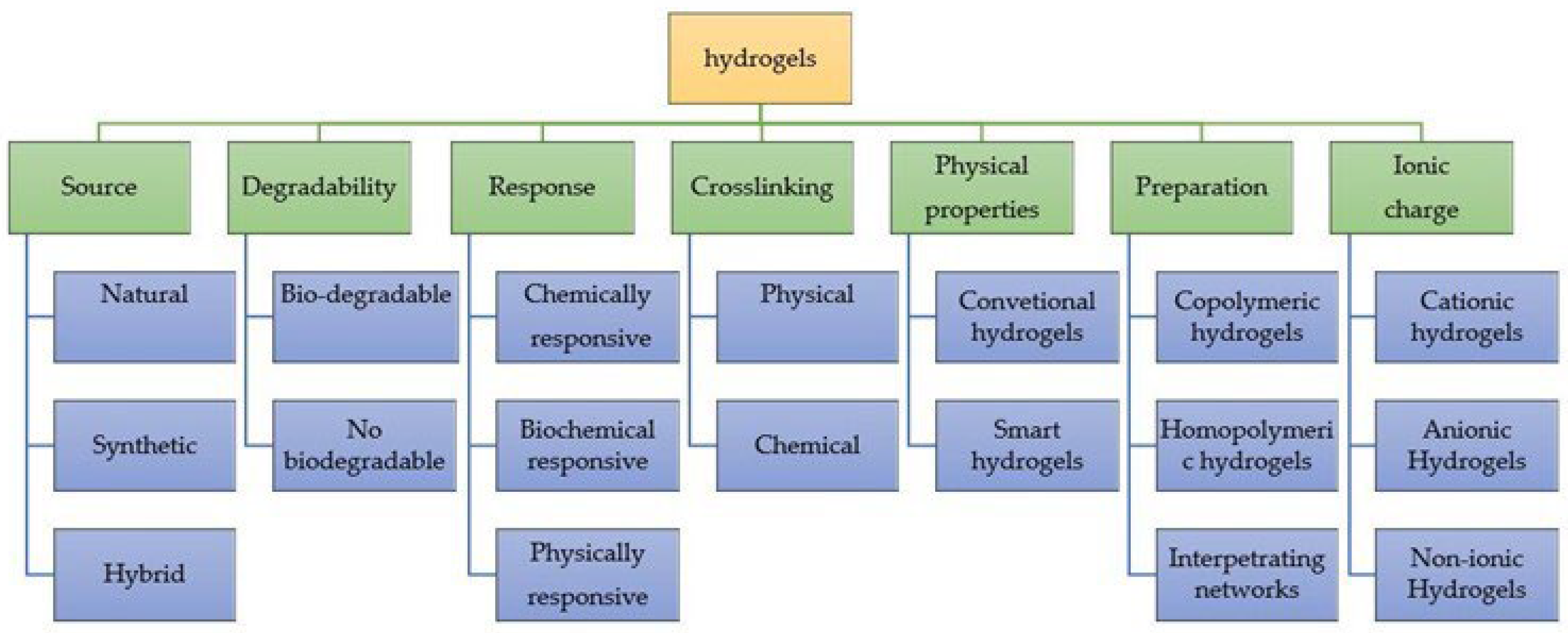
Figure 2. Representative scheme of the classification of hydrogels.
The drugs can be loaded into hydrogels in two different ways [4]. In the first method, the drug is added to the precursor polymer solution and the gelation of the drug within the matrix is then allowed to take place. The second approach involves the hydrogel swelling in a drug solution to swelling equilibrium. Next, it is important to calculate the encapsulation capacity of the drug by the hydrogel before starting drug release.
2. Cellulose-Based Hydrogels
The versatility of cellulose-based hydrogels (CBHYs) has recently prompted researchers around the world to develop new materials to be exploited in healthcare. CBHYs possess biocompatibility and similarity to living tissue, which provides wide opportunities for drug release, healing of wounds and tissue repair. Cellulose is the most abundant natural material as it can be found in trees, plants, fruits, vegetables and organic waste. Therefore, it is readily available and renewable, and this is an important aspect of any research to be developed and applied. It is a polysaccharide consisting of a linear chain of β-linked D-glucose units (1/4). Cellulose is a hydrophilic material. It has inter- and intramolecular hydrogen bonds and van der Waals forces that cause many problems regarding its dissolution [6]. Research has followed several directions to obtain methods for extracting cellulose from organic waste. In the extracted material, cellulose is structured into fibrils, surrounded by a matrix of lignin and hemicellulose. Among these methods, the steam explosion, chlorine-free and ionic liquid methods are notable. Each method has its limitations concerning the high costs involved in its large-scale implementation.
2.1. Extraction of Cellulose for the Production of Sustainable Hydrogels
The production of sustainable and green cellulose hydrogels requires a great deal of research work [7]. As an example, the efforts that have been made to extract cellulose from agricultural biomass can be highlighted [8][9][10] (Figure 3), to engineer hydrogels based on sustainable cellulose acetate [11] and to produce monolithic cellulose/alginate hydrogels for environmental applications [12].
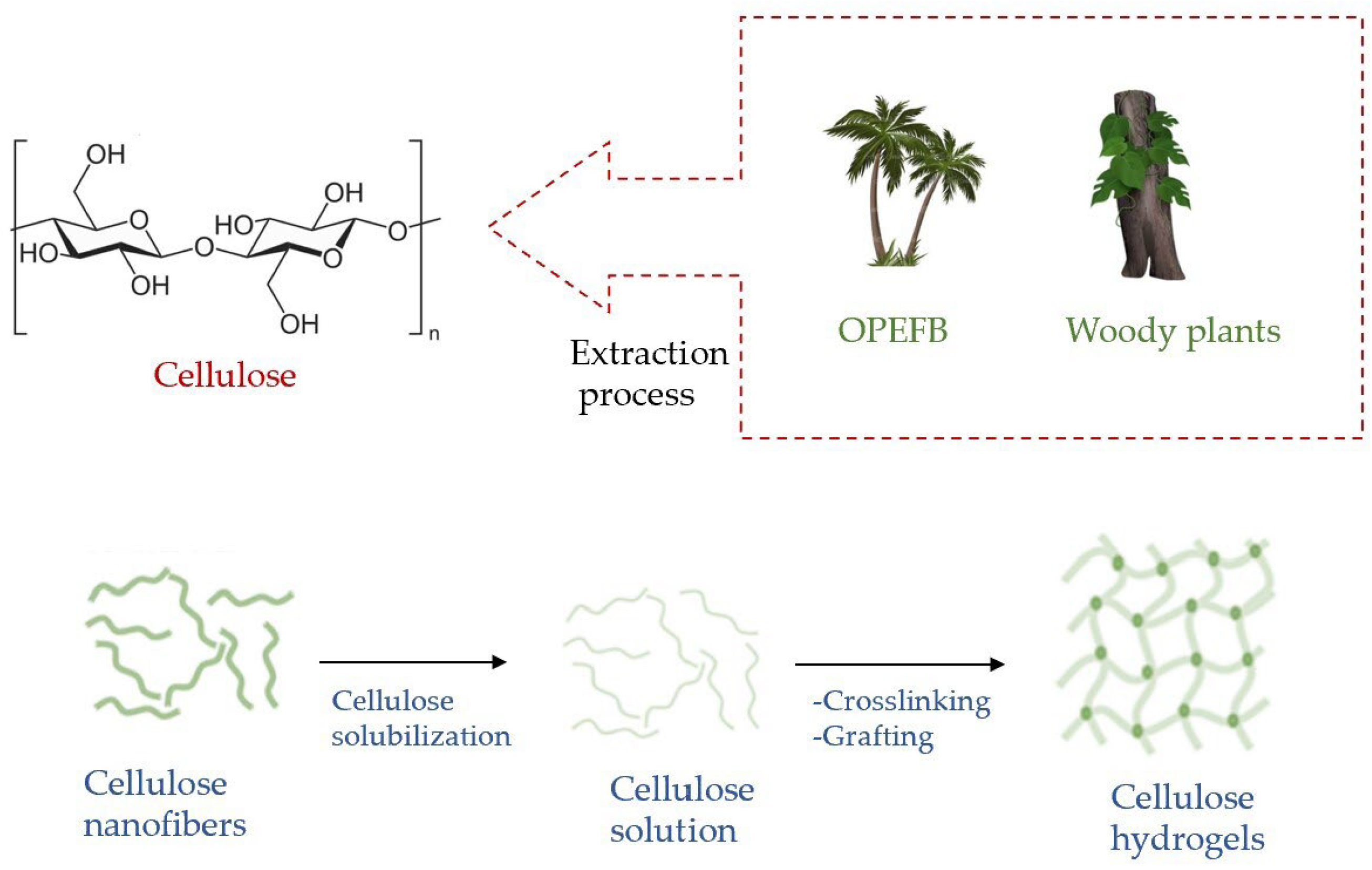
Figure 3. Sources of the extraction of cellulose and the formation of hydrogels.
Lignocellulosic biomass materials (LBMs), present both in plants with woody stems and in non-woody-stemmed plants, are mainly formed by cellulose, hemicellulose and lignin. The palm oil industry is one of the main manufacturing industries in Malaysia [13][14][15][16] and is an important font of LBMs. As a residue of this processing, Oil Palm Empty Fruit Bunches (OPEFBs) is abundantly produced. Being non-woody, OPEFBs are cheap, so it is presented as a raw material with interesting and potentially useful properties for various industrial processes, representing a valid alternative to woody plants, which are expensive [17]. OPEFBs are composed of 37.3–46.5% cellulose, 25.3–33.8% hemicellulose and 27.6–32.5% lignin [18]. This high cellulose amount makes OPEFBs an excellent source to be exploited to produce a polymer’s hydrogels in a sustainable way.
The cellulose fibres are entrapped in amorphous hemicellulose and lignin. Their extraction can occur through a procedure adapted from a study conducted by Nazir et al. [19], in which the depolymerization of hemicellulose and delignification are performed through the use of solvents such as formic acid and hydrogen peroxide at low concentrations.
Thermoresponsive cellulose hydrogels were prepared through a cold method by the incorporation of cellulose extracted from OPEFBs and Pluronic F127 polymer (PF127). Their performances were evaluated. The hydrogel with PF127 content of 20 w/v% and OPEFB cellulose content of 3 w/v% showed better performance in terms of degradation percentage ratio, swelling and in vitro release of incorporated silver sulfadiazine (SSD) [17]. The developed procedure to obtain thermos-responsive cellulose hydrogel from OPEFBs has supplied a model to exploit abundantly available agricultural biomass to produce sustainable drug-delivery systems. Podzil et al., in their review [15], described how cellulose extracted from OPEFBs can be exploited for various applications. Moreover, they have illustrated how nanocellulose produced by raw materials is introduced thanks to the hydrogen bonds that are created among different polymeric matrices and functional groups present in the same cellulose chain.
2.2. Nanocellulose
A single cell of natural plant fibres is formed by cellulose microfibrils. Microfibrils are formed by elementary fibrils (nanofibrils). The surface free energy reduction is at the basis of the process that causes the fibrils to group [18]. The extraction procedure is somewhat equivalent to a disaggregation, resulting in nanometre-sized cellulose. The term “nanocellulose” refers to three types of nanosized cellulosic extracts: cellulose nanofibers (CNFs), cellulose nanocrystals (CNCs) and bacterial nanocellulose (BNC), each having different dimensions and properties [20]. CNFs and CNCs can be extracted from OPEFB.
In 1951, Ranby Bengt published the first experimental work about the production of colloidal cellulose suspensions by sulfuric acid degradation of cellulose fibres. This pioneering study, along with other subsequent works, led to the discovery of a new nanomaterial called nanocrystalline cellulose (CNC) [21]. Since then, many works concerning the characteristics and the processing of nanocellulose have been published [22][23][24][25][26].
Isogai et al. [27] extracted cellulose nanofibers (CNFs) from wood pulp. Isogai’s method produces nanofibers exploiting TEMPO-mediated oxidation. These fibres have diameters that are from 5 to 60 nm long and lengths of many microns.
CNC has been derived by treating cellulose pulp with strong acids. In this way, the unstructured aggregates are dissolved, whereas the crystalline parts of the fibrils remain intact [28]. The extraction product, CNC, has a diameter of 5 nm and 20–100 nm length. The source of cellulose and extraction procedures influence the dimensions of both CNFs and CNCs.
Peyre et al. [29] produced cellulose nanocrystals (fine fraction) and two different-length-scale microparticles (medium and coarse fraction).
The method consists of the oxidation of microgranular cellulose by TEMPO oxidants. A pH = 8 environment and a bromide-free route were applied. The finest fraction was obtained by the centrifugation and separation of the supernatant. An ordinary filtration allowed the middle fraction (smaller microcrystals in micrometre-length scales) to be separated from the coarse fraction.
BNC does not have the same quantity of impurities that lignin and hemicellulose present. Compared to the nanocellulose obtained from wood [30], it has a higher crystallinity (80%). It is secreted by bacterial fermentation of a glucose-based culture medium [31]. The bacteria strains involved are Agrobacterium, Salmonella, Aerobacter, Escherichia, Sarcina Rhodobactor, Komagataeibacter and Gluconacetobacter xylinus [32][33][34]. A washing procedure is applied to bacterial cellulose (NaOH 1 M). In this way, BNC is extracted and the unwanted residues of proteins are removed. BNC can form hydrogels independently of the source of nanocellulose fibrils, which have an average diameter of 100 nm and length of the order of micrometres.
2.3. Cellulose Solubilization
The main limitations of cellulose are related to its dissolution in water and more organic solvents. Researchers are being forced to use other solvent systems for cellulose dissolution, such as ionic liquids (ILs), NaOH/urea and NaOH/thiourea. ILs are handled with some complication and difficulty. By far the most widely used solvent system is NaOH/urea because it is easily obtainable, inexpensive and increases the dissolution rate of cellulose at low temperatures [35]. To improve the solubilization of poorly soluble natural polysaccharides (such as cellulose, chitin and chitosan) in alkaline urea systems, a considerable amount of work has been performed since 2008, also exploiting their derivatization. Quaternary ammonium cellulose (QAC) with a degree of substitution (SD) of 0.20–0.63 was homogeneously produced, for the first time, by the reaction of cellulose with trimethylammonium chloride (CHPTAC) in an aqueous solution of NaOH and urea [36]. This work confirms alkali/urea solvent as a homogeneous reaction system for the chemical modification of poorly soluble polysaccharides, giving indications on the path for future work.
2.4. Crosslinking
Cellulose gels, depending on their crosslinking density, form a network with meshes of different diameter and stiffness [37][38][39][40].
Thanks to properties such as biodegradability, non-toxicity, flexibility and biocompatibility, nanocellulose hydrogels are employed in multiple biomedical applications.
On the other hand, to promote crosslinking and to produce hydrogels, it is necessary to ensure the efficient dissolution of cellulose; this involves the breaking of both intra- and intermolecular hydrogen bonds [34]. Several chemical protocols have been studied to dissolve cellulose in water, organic solvents or IL [41]. Cai et al. developed a procedure in aqueous solutions of NaOH/urea and LiOH/urea to dissolve cellulose at temperatures as low as −10 °C [42]. The best results were obtained with urea at 12% concentration. After dissolution, an optically transparent hydrogel crosslinked by epichlorohydrin crosslinker (ECH) was formed at −3 °C.
The hydrogel’s preparation determines its network structure. The swelling of cellulose fibres depends on ion diffusion from the solution toward the gel centre [43]. Swelling power is an important parameter because it allows not only the control of the pore size and its distribution, but also the gel’s mechanical properties. It is important to control the pore size of the gel because it is connected to the diffusion of biomolecules within the matrix [42]. If the mesh size is smaller than the diameter of the biomolecule, the diffusion of biomolecules is blocked by filtration.
Hydrogels usually appear translucid; more turbidity is evident when there is a higher solid content, and they become coloured if additives such as ions, proteins and polymers are present. The final application can guide the choice of the most appropriate technique for processing the hydrogel. The pH and the ionic strength of the aqueous solution can be tuned, and additives (salts, sugars and amino acids) can be introduced inside the gel to balance the osmolality. The cellulose backbone can be functionalized with substituent groups (such as methyl, carboxylate or amine) and modified to trap biomolecules (enzymes, antibodies and peptides) [44][45][46][47].
Cellulose derivatives, such as methylcellulose and carboxymethyl cellulose are water-soluble; they have been widely applied for the preparation of nanocomposite hydrogels as drug carriers, promoters of wound healing and other pharmaceutical applications.
Seera et al. [48] evaluated the properties of hydrogels obtained by microcrystalline cellulose (CMC) and polyvinyl alcohol (PVA), comparing the properties of physically crosslinked DMSO-NaOH/urea systems with those of hydrogels chemically crosslinked in water-NaOH/urea, and concluding that the latter had better rheological properties. Rheological analyses showed that CMC underwent a successful crosslinking with PVA, evidencing that storage modulus dominated the loss modulus until the crossover point was achieved. Drugs and water are loaded thanks to the porous structure. The swelling studies and the rheological results are linked. In fact, when crosslinking level lowers, the swelling level increases and vice versa. Release studies performed with 5-fluorouracil have shown promising results for the use of chemical crosslinking of PVA with CMC to overcome the water solubility issues presented by pure microcrystalline cellulose, which hinder its use in drug delivery.
Peng et al. [49] produced a cellulose/clay hydrogel by crosslinking cellulose, carboxymethyl cellulose and clay nanosheets with epichlorohydrin in a NaOH/urea aqueous solution. The clays (epoxidized montmorillonite) were homogeneously dispersed in the cellulose matrix. This hydrogel incorporated with the intercalated clays exhibited better absorbent and mechanical properties compared to the hydrogel containing the unmodified clay.
Jun Liu et al. proposed two strategies to produce hydrogels: pre-adsorption and in situ adsorption of hemicellulose. The first method is performed in two steps: (a) hemicellulose is adsorbed onto CNF and the film is obtained using hot mixing; (b) the film swells during immersion in water at room temperature for a day. The second method is a process in which the adsorption of hemicellulose and addition of water (for swelling) occur simultaneously [45][50][51]. The highest adsorption capacity on CNF was governed by the preadsorption method using xyloglucan as a crosslinker.
Among the different types of cellulose, bacterial nanocellulose is the most promising and suitable biomaterial [30][31][32][33][52] for producing hydrogels. This is because BNC is produced as a 3D nanostructure with high tensile strength and elastic modulus [30][32]. Moreover, BNC-based hydrogels still maintain this network structure at a high level of hydration. Even if BNC has production costs that are more disadvantageous than cellulose nanofibers (CNF) and cellulose nanocrystals (CNC), several studies reported that its hydrogels can be applied for the controlled and targeted delivery of antibodies, enzymes and drugs [37].
2.5. Grafting
Graft polymerization is a technique used to improve the physical and chemical characteristics of natural fibres, introducing some modifications. The method chosen to obtain grafting is radical polymerization [47][53][54]. This method does not allow complete control of the reaction process. As a result, the molecular weight of the product is widely distributed, and the grafting chains have variable lengths [55]. To solve these problems, reversible-deactivation radical polymerization is applied. Anin et al. made and characterized a hydrogel of bacterial-derived nanocellulose and acrylic acid (AA) for drug delivery. Acrylic acid monomers were implanted onto cellulose fibres, and the subsequent irradiation by electron beams accelerated the gelation. This hydrogel is pH- and temperature-sensitive due to the presence of acrylic acid [56]. The water absorption capacity was evaluated, and it was observed that BNC-(AA) hydrogels at pH 7 reach swelling after 48 h. At pH 10, equilibrium is reached in 24 h. The structure that is created enables this swelling mechanism, and, in particular, image analysis has evidenced that acrylic acid influences the pore size of the gel.
The gel porosity is important because it determines the kinetics of the drug release. Badshah et al. published a work concerning BNC matrices loaded with two different drugs: famotidine and tizanidine. The first drug is slightly soluble in water; the second is very soluble. The loading inside the matrixes of the two drugs was uniform. The release percentage in 15 min was 80%. Drugs underwent a faster release thanks to the porosity of BC network and its hydrophilicity [38].
The Diels–Alder reaction is promising for the design of advanced materials from cellulose nanocrystals (CNCs). It involves the accurate grafting of reactive Diels–Alder moieties under various conditions without affecting the nanocrystal structure. Garcìa-Astrain et al. [57] performed a grafting of maleimide to the surface of CNCs. Then, they employed the Diels–Alder reaction to crosslink functionalized CNCs with furan-modified gelatine. The hydrogel was stabilized by a second crosslinking performed by coupling on the amide between CS and furane-modified gelatine, obtaining a completely renewable bio-nanocomposite formulation. The authors studied the role of CNCs as a stabilizer and their effect on the swelling and viscoelastic properties.
2.6. Cellulose Hydrogels with Metal Nanoparticles or Cations
Gulsonbi et al. [58] used a green chemical synthesis process for the preparation of a biodegradable semi-interpenetrating nanocomposite hydrogel of silver/carboxymethyl cellulose-poly(acrylamide). The poly(acrylamide)/carboxymethyl cellulose hydrogel network was obtained by performing a fast-redox polymerization of N, N-methylenebisacrylamide (MBA) with CMC. The silver nanoparticles AgNPs within the hydrogel network were successively synthesized by in situ bioreduction of AgNO3 using Azadirachta indica (neem) plant extract under atmospheric conditions.
In 2018, Basu et al. [59] proposed the setup of a Ca2+ crosslinked wood-based nanofibrillated cellulose (CNF) hydrogel, thereby achieving increased knowledge on the use of nanocellulose aimed for wound healing. The obtained hydrogel, through a soaking procedure, can carry proteins of various sizes and isoelectric points. The release of the proteins was supervised, and their determining parameters were evaluated. The results showed that the electrostatic interactions between proteins and the structure of the negatively charged CNF hydrogel play a central role in the loading process, and that protein release is controlled by Fickian diffusion. An increase in protein size, as well as the presence of the positive protein charge, induced a slower release process. Positively charged proteins have also been found to improve the stability of the hydrogel. The hydrogel composed of CNF and the crosslinking agent Ca2+ has a structure that allows the transport of proteins and their release, maintaining their structural stability without compromising their activity. It is expected that by using the charge-adjustable properties of the hydrogel, CNF release profiles can be tailored for specific therapeutic needs.
F. Lin et al. [60] reported an easy strategy for the development of a hydrogel drug delivery system, employing a simple addition of Fe3O4 nanoparticles to a mixed β-cyclodextrin (β-CD)/cellulose solution. By dispersing this dropwise within a coagulant bath of CaCl2, magnetic nanocellulose nanoparticles were obtained. It has been hypothesized that β-CD grafting confers high drug-loading capacity to the hydrogel. The incorporation of Fe3O4 nanoparticles, through the fast and reversible deformation of a 3D network subjected to an external magnetic field EMF, can improve the drug delivery modes. Thus, through an environmentally sustainable methodology, it was possible to design a smart cellulose hydrogel that combines rapid response and remote control of remote drug release under the effect of EMFs by switching the “on-off” mode.
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267.
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408.
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952.
- Luo, X.; Zhang, L. New solvents and functional materials prepared from cellulose solutions in alkali/urea aqueous system. Food Res. Int. 2010, 52, 387–400.
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255.
- Rasli, S.R.A.M.; Ahmad, I.; Lazim, A.M.; Hamzah, A. Extraction and characterization of cellulose from agriculture residue- Oil palm fronds. Malays. J. Anal. Sci. 2017, 21, 1065–1073.
- Al-Rajabi, M.M.; Teow, Y.H. Green Synthesis of Thermo-Responsive Hydrogel from Oil Palm Empty Fruit Bunches Cellulose for Sustained Drug Delivery. Polymers 2021, 13, 2153.
- Chen, X.; Song, Z.; Li, S.; Tat Thang, N.; Gao, X.; Gong, X.; Guo, M. Facile one-pot synthesis of self-assembled nitrogendoped carbon dots/cellulose nanofibril hydrogel with enhanced fluorescence and mechanical properties. Green Chem. 2020, 22, 3296–3308.
- Alammar, A.; Park, S.H.; Ibrahim, I.; Deepak, A.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878.
- Yuan, J.; Yi, C.; Jiang, H.; Liu, F.; Cheng, G.J. Direct ink writing of hierarchically porous cellulose/alginate monolithic hydrogel as a highly effective adsorbent for environmental applications. ACS Appl. Polym. Mater. 2021, 3, 699–709.
- Haan, T.Y.; Ghani, M.S.H.; Mohammad, A.W. Physical and Chemical Cleaning for Nanofiltration/Reverse Osmosis (NF/RO). J. Kejuruter. 2018, 1, 51–58.
- Haan, T.Y.; Takriff, M.S. Zero waste technologies for sustainable development in palm oil mills. J. Oil Palm Environ. Health 2021, 12, 55–68.
- Padzil, F.N.M.; Lee, S.H.; Ainun, Z.M.A.; Lee, C.H.; Abdullah, L.C. Potential of Oil Palm Empty Fruit Bunch Resources in Nanocellulose Hydrogel Production for Versatile Applications: A Review. Materials 2020, 13, 1245.
- Mehanny, S.; Abu-El Magd, E.E.; Ibrahim, M.; Farag, M.; Gil-San-Millan, R.; Navarro, J.; El Habbak, A.E.H.; El-Kashif, E. Extraction and characterization of nanocellulose from three types of palm residues. J. Mater. Res. Technol. 2021, 10, 526–537.
- Teow, Y.H.; Amirudin, S.N.; Ho, K.C. Sustainable approach to the synthesis of cellulose membrane from oil palm empty fruit bunch for dye wastewater treatment. J. Water Process Eng. 2020, 34, 101182.
- Chinga-Carrasco, G. Cellulose fibres, nanofibrils and microfibrils: The morphological sequence of MFC components from a plant physiology and fibre technology point of view. Nanoscale Res. Lett. 2011, 6, 417.
- Nazir, M.S.; Wahjoedi, B.A.; Yussof, A.W.; Abdullah, M.A. Eco-Friendly Extraction and Characterization of Cellulose from Oil Palm Empty Fruit Bunches. BioResources 2013, 8, 2161–2172.
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88.
- Rånby, B.G. Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss. Faraday Soc. 1951, 11, 158–164.
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2014, 132, 41719.
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393.
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872.
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189.
- Brown, A.J. XLIII.—On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439.
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85.
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500.
- Peyre, J.; Pääkkönen, T.; Reza, M.; Kontturi, E. Simultaneous preparation of cellulose nanocrystals and micron-sized porous colloidal particles of cellulose by TEMPO-mediated oxidation. Green Chem. 2015, 17, 808–811.
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557.
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2015, 23, 57–91.
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270.
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The Biopolymer Bacterial Nanocellulose as Drug Delivery System: Investigation of Drug Loading and Release using the Model Protein Albumin. J. Pharm. Sci. 2013, 102, 579–592.
- Raghuwanshi, V.S.; Cohen, Y.; Garnier, G.; Garvey, C.J.; Russell, R.A.; Darwish, T.; Garnier, G. Cellulose Dissolution in Ionic Liquid: Ion Binding Revealed by Neutron Scattering. Macromolecules 2018, 51, 7649–7655.
- Piltonen, P.; Hildebrandt, N.C.; Westerlind, B.; Valkama, J.-P.; Tervahartiala, T.; Illikainen, M. Green and efficient method for preparing all-cellulose composites with NaOH/urea solvent. Compos. Sci. Technol. 2016, 135, 153–158.
- Song, Y.; Sun, Y.; Zhang, X.; Zhou, J.; Zhang, L. Homogeneous Quaternization of Cellulose in NaOH/Urea Aqueous Solutions as Gene Carriers. Biomacromolecules 2008, 9, 2259–2264.
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61.
- Song, D.; Breedveld, V.; Deng, Y. Rheological study of self-crosslinking and co-crosslinking of ammonium zirconium carbonate and starch in aqueous solutions. J. Appl. Polym. Sci. 2011, 122, 1019–1029.
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53.
- Moberg, T.; Sahlin, K.; Yao, K.; Geng, S.; Westman, G.; Zhou, Q.; Oksman, K.; Rigdahl, M. Rheological properties of nanocellulose suspensions: Effects of fibril/particle dimensions and surface characteristics. Cellulose 2017, 24, 2499–2510.
- Mohd, N.; Draman, S.F.S.; Salleh, M.S.N.; Yusof, N.B. Dissolution of cellulose in ionic liquid: A review. In Proceedings of the 6th International Advances in Applied Physics and Materials Science Congress & Exhibition: (APMAS 2016), İstanbul, Türkiye, 1–3 June 2016.
- Grignon, J.; Scallan, A.M. Effect of pH and neutral salts upon the swelling of cellulose gels. J. Appl. Polym. Sci. 1980, 25, 2829–2843.
- Lopez-Sanchez, P.; Schuster, E.; Wang, D.; Gidley, M.J.; Strom, A. Diffusion of macromolecules in self-assembled cellulose/hemicellulose hydrogels. Soft Matter 2015, 11, 4002–4010.
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328.
- Liu, J.; Chinga-Carrasco, G.; Cheng, F.; Xu, W.; Willför, S.; Syverud, K.; Xu, C. Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 2016, 23, 3129–3143.
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364.
- Das, S.K.; Parandhaman, T.; Dey, M.D. Biomolecule-assisted synthesis of biomimetic nanocomposite hydrogel for hemostatic and wound healing applications. Green Chem. 2021, 23, 629–669.
- Seera, S.D.K.; Kundu, D.; Banerjee, T. Physical and chemical crosslinked microcrystalline cellulose-polyvinyl alcohol hydrogel: Freeze–thaw mediated synthesis, characterization and in vitro delivery of 5-fluorouracil. Cellulose 2020, 27, 6521–6535.
- Peng, N.; Hu, D.; Zeng, J.; Li, Y.; Liang, L.; Chang, C. Superabsorbent cellulose–clay nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain. Chem. Eng. 2016, 4, 7217–7224.
- Bodin, A.; Ahrenstedt, L.; Fink, H.; Brumer, H.; Risberg, B.; Gatenholm, P. Modification of Nanocellulose with a Xyloglucan–RGD Conjugate Enhances Adhesion and Proliferation of Endothelial Cells: Implications for Tissue Engineering. Biomacromolecules 2007, 8, 3697–3704.
- Bonilla, M.R.; Lopez-Sanchez, P.; Gidley, M.J.; Stokes, J.R. Micromechanical model of biphasic biomaterials with internal adhesion: Application to nanocellulose hydrogel composites. Acta Biomater. 2016, 29, 149–160.
- Abeer, M.M.; Mohd Amin, M.C.I.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061.
- Maddaloni, M.; Vassalini, I.; Alessandri, I. Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels. Sustain. Chem. 2020, 1, 325–344.
- Jin, T.; Liu, T.; Jiang, S.; Kurdyla, D.; Klein, B.A.; Michaelis, V.K.; Lam, E.; Li, J.; Moores, A. Chitosan nanocrystals synthesis via aging and application towards alginate hydrogels for sustainable drug release. Green Chem. 2021, 23, 6527–6537.
- Chelu, M.; Moreno, J.C.; Atkinson, I.; Cusu, J.P.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.-M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424.
- Mohd Amin, M.C.I.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo- and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473.
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016, 149, 94–101.
- Gulsonbi, M.; Parthasarathy, S.; Raj, K.B.; Jaisankar, V. Green synthesis, characterization and drug delivery applications of a novel silver/carboxymethylcellulose–poly (acrylamide) hydrogel nanocomposite. Ecotoxicol. Environ. Saf. 2016, 134, 421–426.
- Basu, A.; Strømme, M.; Ferraz, N. Towards Tunable Protein-Carrier Wound Dressings Based on Nanocellulose Hydrogels Crosslinked with Calcium Ions. Nanomaterials 2018, 8, 550.
- Lin, F.; Zheng, J.; Guo, W.; Zhu, Z.; Wang, Z.; Dong, B.; Lin, C.; Huang, B.; Lu, B. Smart cellulose-derived magnetic hydrogel with rapid swelling and deswelling properties for remotely controlled drug release. Cellulose 2019, 26, 6861–6877.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
02 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





