Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronaldo Thomatieli-Santos | -- | 1580 | 2023-03-01 02:13:06 | | | |
| 2 | Conner Chen | + 6 word(s) | 1586 | 2023-03-01 06:55:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Volpe-Fix, A.R.; De França, E.; Silvestre, J.C.; Thomatieli-Santos, R.V. Exercise and Their Immunomodulation Effect. Encyclopedia. Available online: https://encyclopedia.pub/entry/41760 (accessed on 13 January 2026).
Volpe-Fix AR, De França E, Silvestre JC, Thomatieli-Santos RV. Exercise and Their Immunomodulation Effect. Encyclopedia. Available at: https://encyclopedia.pub/entry/41760. Accessed January 13, 2026.
Volpe-Fix, Andressa Roehrig, Elias De França, Jean Carlos Silvestre, Ronaldo Vagner Thomatieli-Santos. "Exercise and Their Immunomodulation Effect" Encyclopedia, https://encyclopedia.pub/entry/41760 (accessed January 13, 2026).
Volpe-Fix, A.R., De França, E., Silvestre, J.C., & Thomatieli-Santos, R.V. (2023, March 01). Exercise and Their Immunomodulation Effect. In Encyclopedia. https://encyclopedia.pub/entry/41760
Volpe-Fix, Andressa Roehrig, et al. "Exercise and Their Immunomodulation Effect." Encyclopedia. Web. 01 March, 2023.
Copy Citation
Depending on the intensity and volume, physical exercise can stimulate oxidative stress and muscle inflammation to generate muscle recovery. The practice of physical exercise is considered a potent immunomodulator, during physical exercise, both in trained and sedentary individuals, it is possible to observe a brief increase in the number of circulating leukocytes, which are mobilized from the lymphatic system, vessel walls, and spleen, indicating the ability of exercise to influence different cell compartments.
flavonoids
physical exercise
inflammation
1. Introduction
The use of plant-based or natural supplements has been growing, as has the research on their properties [1]. The benefits of these supplements seem to be linked to the presence of food bioactive compounds (FBC) in the composition of leaves, roots, seeds, fungi, or seaweed [2]. FBCs are substances related to the secondary metabolism of plants, and its function is to protect against environmental aggressions [3]. There are several types of FBCs, with a huge variety of functions, with polyphenols being the most abundant [4]. It is known that FBCs bring benefits to the organism that consumes them [5], and due to the anti-inflammatory, antioxidant, and immunoregulatory [6] capacities demonstrated by some of these compounds, they have been investigated in the context of physical exercise.
The practice of physical exercise is considered a potent immunomodulator. Beyond protecting the body against pathogens, the immune system also plays a crucial role in tissue remodeling after injury [7]. Physical exercise can induce significant injuries in the skeletal muscle tissue, leading to a consequent drop in performance. For the immune system to rescue muscle performance, improvement in pro-anti/inflammatory balance must occur to allow for muscle regeneration. However, muscle regeneration takes time [8], and hypothetically, the magnitude of cell injury (during exercise and the inflammatory phase) can be too much and make the recovery phase difficult [8]. Immunonutrition strategies to counter these deleterious effects on the immune system have been proposed [6].
2. Exercise and Their Immunomodulation Effect
During physical exercise, both in trained and sedentary individuals, it is possible to observe a brief increase in the number of circulating leukocytes, which are mobilized from the lymphatic system, vessel walls, and spleen, indicating the ability of exercise to influence different cell compartments [9].
In healthy people, moderate training seems to be associated with improved immunity from the point of view of antigen recognition, presentation, and elimination mechanisms, in addition to the organization of the immune response, protecting or attenuating the symptoms of infections and reducing the days with symptoms in case of illness [10][11].
On the other hand, strenuous exercise for prolonged periods has the opposite effect. After running a marathon, a picture of immunosuppression is generated, characterized by a marked decrease in the number of T cells, circulating NK cells, and neutrophils, a decrease in their activities and functions, in addition to a decrease in the salivary concentration of IgA [9].
In the above context, the theory of the ‘Open Window’ period after performing strenuous exercise was postulated. The ‘Open Window’ period is related to the moment after exhausting exercises that can last from 3 h to 72 h depending on the parameters analyzed, during which a lower functionality of the immune system is observed, increasing the risk and probability of opportunistic infections, mainly upper respiratory tract infections (URTIs) [12]. Performing several acute exercise sessions with strenuous characteristics without adequate recovery time can result in chronic immunosuppression.
The modulatory effect that exercise poses on the immune system can be explained by an S-curve graphic model. Therefore, people undergoing moderate training are less likely to develop infections, especially URTIs, while amateur athletes are more likely to develop infections than people who train moderately or are sedentary. On the other hand, professional athletes, despite the high training overload, are less likely than amateur athletes to develop these infections [13][14].
The intensity and duration of physical exercise are determining factors for different changes in the immune system. Studies show that moderate aerobic training (30 to 60 min, 3 to 5 days a week at an intensity between 60 and 80% of VO2 max), as opposed to other intensities and volumes, results in an improvement of the immune system in the face of inflammation, in the capacity of phagocytic activity of neutrophils and monocytes [9][12][15] and a lower risk of URTIs [13]. The guiding mechanisms of exercise immunology are still discussed; however, hormonal and metabolic changes induced by exercise seem to play a relevant role [16][17][18][19][20].
Immune System and Muscle Recovery after Exercise
The immune system plays a crucial role in tissue remodeling after injury. The adaptation of skeletal muscle tissue in response to physical exercise depends on the immune system’s function. It has been postulated that muscle adaptation/regeneration depends on the inflammatory response in a coordinated (five waves/phases) and time-dependent process [7].
The practice of physical exercise can induce significant injuries in the skeletal muscle tissue, leading to a consequent drop in performance, i.e., loss of muscle functionality. Resolving the muscle injury is paramount to restoring muscle performance. For this, during and after physical exercise, metabolites are released from damaged muscle tissue (such as CK, LDH, troponin, and complement C4) that act as damage-associated molecular patterns (DAMPs). DAMPs (the first wave) trigger an inflammatory response (i.e., recruiting immune system cells such as neutrophils, monocytes, and CD8+ T cells from the other sites to remove myofiber debris in the injured areas) [21]. In the second wave, neutrophils secrete IL-1 and IL-8 (which activate M1 macrophages to the lesioned region). M1 macrophages infiltrate skeletal muscle tissue to phagocytose cellular debris and secrete significant amounts of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and nitric oxide for a proper inflammatory response [21]. During the second wave, infiltrated CD8+ T cells also secrete TNF-α, IFN-γ, IL-1α, and IL-13. Pro-inflammatory cytokines such as IL1-β and TNF-α stimulate IL-6 and COX-2, which are essential to induce myoblast proliferation and differentiation (i.e., the stimulation of the myogenesis process mediated mainly by prostaglandins (PGEs and PGDs)) [22]. In the third wave, Treg cells (in response to elevated IL-6) secrete IL-33, and IL-10 stimulates the phenotypic shift from inflammatory M1 macrophages to anti-inflammatory M2 macrophages (which secrete IGF-1, IL-4, and IL-13) [21]. Anti-inflammatory cytokines (such as IL-4, IL-10, IL-13, and IL-33) provide a favorable environment for growth factors (such as IGF-1 and TGF-β) to promote the recruitment of satellite cells (to injury areas) and the differentiation, growth, and maturation of new muscle fibers [22]. Complete muscle recovery (and possible overcompensation) occurs with the maturation (fourth wave) of new muscle fibers [7]. The muscle injury/regeneration process is summarized in Figure 1.
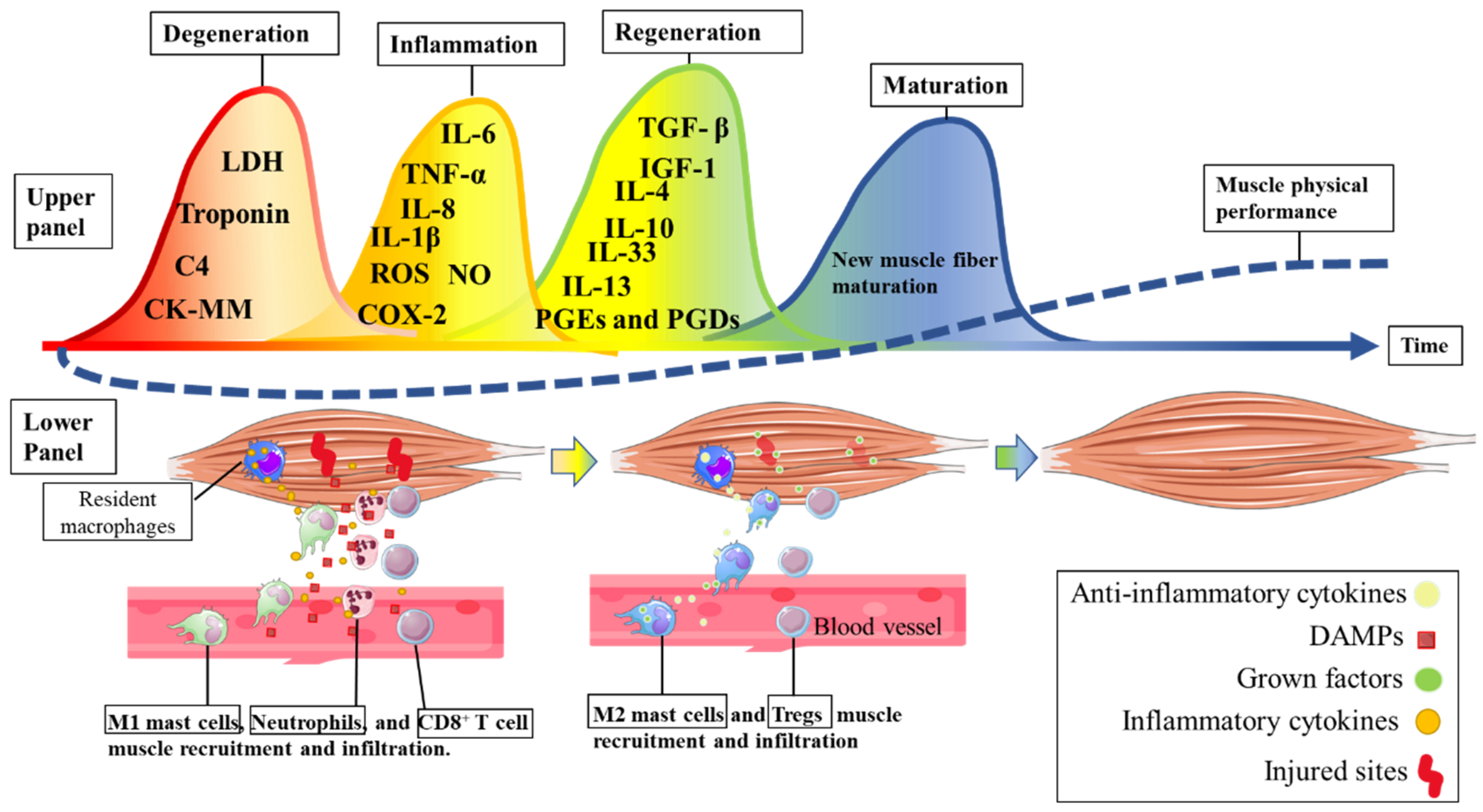
Figure 1. The immunological response to a strenuous bout of physical exercise. In the upper panel, the different processes (five waves) induced by muscle injury during and after physical exercise are described. The lower panel describes immune cell recruitment after muscle cell injury. Acronyms: Complement C4, C4; CK, creatine kinase; LDH, lactate dehydrogenase; COX-2, ciclooxigenase-2; IGF1, insulin grow factor-1; IL, interleukin; PGE and PGD, prostaglandins E and D, respectively; TGF-β, transforming growth factor-beta; TNF, tumor necrosis factor.
The first wave (DAMPS) appears not to be necessary to induce better muscle adaptation [23]. On the other hand, CK secretion above normal levels has been associated with poor physical performance in athletes [19] and clinical patients [24]. Elevated CK in blood plasma occurs mainly after unaccustomed exercises or exercise protocols with weightlifting, eccentric exercises, downhill running, and prolonged exercise (e.g., ultramarathons). Well-trained individuals or those accustomed to repeated exercise to induce muscle damage showed lower muscle CK release into the bloodstream and a lower performance decrement than untrained or unaccustomed individuals [25]. Interestingly, supplementation strategies (e.g., mainly with FBC antioxidant and anti-inflammatory characteristics) decrease muscle damage after physical exercise that induces muscle damage [26]. Additionally, it is well known that FBCs increase athletic performance, such as creatine, taurine, citrulline, and nitrate, and also decrease muscle damage through antioxidant and anti-inflammatory mechanisms [26]. Therefore, preventing muscle damage with FBCs is used in sports nutrition to avoid declining athletic performance.
As described earlier, the second wave (inflammation) responds to the first wave (muscle damage) to promote the removal of damaged cell debris and to induce tissue regeneration. Muscle damage associated with the inflammatory process (second wave) has also been associated with decreased performance in athletes [27] and clinical patients [24]. For instance, in an experimental study, an acute IL-6 injection impaired endurance performance in healthy subjects and increased fatigue sensation [28]. Additionally, evidence has shown that the acute use of paracetamol (an inhibitor of COX-1, COX-2, and IL-6) increases athletic performance [29]. Therefore, managing inflammation-related processes might be the main reason for using sports-related pharmacological anti-inflammatory drugs because the major reason for using pharmacological anti-inflammatory drugs in athletes is to treat pain or injury, to treat illness, and to enhance performance [30]. However, the chronic and indiscriminate use of pharmacological anti-inflammatories (which interfere with the second wave described in Figure 1) can hinder the adaptation to training such as muscle strength and hypertrophy [31].
Physical exercise breaks body homeostasis, and restoring the broken balance depends on the ability of different physiological systems and cellular biochemistry to act in coordination. In this context, for exercise immunology, there is an ambiguous scenario in which metabolic, hormonal, and cytokine changes alter cell traffic directing immune system cells to skeletal muscle, despite the risk of increasing the likelihood of opportunistic infections [3][32][33]. Probably, the greater the muscle damage, the longer the cell mobilization time in the skeletal muscle and the greater the vulnerability in other body sites [8]. Therefore, strategies that can accelerate the muscle regeneration process and maintain greater control of the pro/anti-inflammatory balance after exercise can contribute to the faster restoration of muscle and immunological homeostasis. Immunonutrition strategies to counter these deleterious effects on the immune system have been proposed [6].
References
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; Hayes, L.D.; Milic, M.; Padulo, J. Herbal medicine for sports: A review. J. Int. Soc. Sport. Nutr. 2018, 15, 14.
- Williams, M. Dietary supplements and sports performance: Herbals. J. Int. Soc. Sport. Nutr. 2006, 3, 1.
- Ruiz-Iglesias, P.; Gorgori-González, A.; Massot-Cladera, M.; Castell, M.; Pérez-Cano, F.J. Does flavonoid consumption improve exercise performance? Is it related to changes in the immune system and inflammatory biomarkers? A systematic review of clinical studies since 2005. Nutrients 2021, 13, 1132.
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.; Barros, L.; Ferreira, I.C. Food bioactive compounds and emerging techniques for their extraction: Polyphenols as a case study. Foods 2020, 10, 37.
- Vieira, J.M.; Gutierres, J.M.; Carvalho, F.B.; Stefanello, N.; Oliveira, L.; Cardoso, A.M.; Morsch, V.M.; Pillat, M.M.; Ulrich, H.; Duarte, M.M.F. Caffeine and high intensity exercise: Impact on purinergic and cholinergic signalling in lymphocytes and on cytokine levels. Biomed. Pharmacother. 2018, 108, 1731–1738.
- Bermon, S.; Castell, L.M.; Calder, P.C.; Bishop, N.C.; Blomstrand, E.; Mooren, F.C.; Krüger, K.; Kavazis, A.N.; Quindry, J.C.; Senchina, D.S.; et al. Consensus Statement Immunonutrition and Exercise. Exerc. Immunol. Rev. 2017, 23, 8–50.
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297.
- Kurowski, M.; Seys, S.; Bonini, M.; Del Giacco, S.; Delgado, L.; Diamant, Z.; Kowalski, M.L.; Moreira, A.; Rukhadze, M.; Couto, M. Physical exercise, immune response, and susceptibility to infections—Current knowledge and growing research areas. Allergy 2022, 77, 2653–2664.
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63.
- Grande, A.J.; Keogh, J.; Silva, V.; Scott, A.M. Exercise versus no exercise for the occurrence, severity, and duration of acute respiratory infections. Cochrane Database Syst. Rev. 2020, 4, CD010596.
- Laddu, D.R.; Lavie, C.J.; Phillips, S.A.; Arena, R. Physical activity for immunity protection: Inoculating populations with healthy living medicine in preparation for the next pandemic. Prog. Cardiovasc. Dis. 2021, 64, 102.
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Sha, W. Upper respiratory tract infection is reduced in physically fit and active adults. Br. J. Sport. Med 2011, 45, 987–992.
- Hull, J.H.; Loosemore, M.; Schwellnus, M. Respiratory health in athletes: Facing the COVID-19 challenge. Lancet Respir. Med. 2020, 8, 557–558.
- Malm, C. Susceptibility to infections in elite athletes: The S-curve. Scand. J. Med. Sci. Sport. 2006, 16, 4–6.
- van de Weert-van Leeuwen, P.B.; Arets, H.G.M.; van der Ent, C.K.; Beekman, J.M. Infection, inflammation and exercise in cystic fibrosis. Respir. Res. 2013, 14, 32.
- Ortega, E. The “bioregulatory effect of exercise” on the innate/inflammatory responses. J. Physiol. Biochem. 2016, 72, 361–369.
- Williams, N.C.; Killer, S.C.; Svendsen, I.S.; Jones, A.W. Immune nutrition and exercise: Narrative review and practical recommendations. Eur. J. Sport Sci. 2019, 19, 49–61.
- Nieman, D.C.; Mitmesser, S.H. Potential impact of nutrition on immune system recovery from heavy exertion: A metabolomics perspective. Nutrients 2017, 9, 513.
- Von Ah Morano, A.E.; Dorneles, G.P.; Peres, A.; Lira, F.S. The role of glucose homeostasis on immune function in response to exercise: The impact of low or higher energetic conditions. J. Cell. Physiol. 2020, 235, 3169–3188.
- Nieman, D.C.; Lila, M.A.; Gillitt, N.D. Immunometabolism: A multi-omics approach to interpreting the influence of exercise and diet on the immune system. Annu. Rev. Food Sci. Technol. 2019, 10, 341–363.
- Yang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Transl. 2018, 13, 25–32.
- Alvarez, A.M.; DeOcesano-Pereira, C.; Teixeira, C.; Moreira, V. IL-1β and TNF-α Modulation of Proliferated and Committed Myoblasts: IL-6 and COX-2-Derived Prostaglandins as Key Actors in the Mechanisms Involved. Cells 2020, 9, 2005.
- Damas, F.; Phillips, S.M.; Libardi, C.A.; Vechin, F.C.; Lixandrão, M.E.; Jannig, P.R.; Costa, L.A.R.; Bacurau, A.V.; Snijders, T.; Parise, G.; et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol. 2016, 594, 5209–5222.
- Jäkel, B.; Kedor, C.; Grabowski, P.; Wittke, K.; Thiel, S.; Scherbakov, N.; Doehner, W.; Scheibenbogen, C.; Freitag, H. Hand grip strength and fatigability: Correlation with clinical parameters and diagnostic suitability in ME/CFS. J. Transl. Med. 2021, 19, 159.
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Eccentric Exercise: Physiological Characteristics and Acute Responses. Sport. Med. 2017, 47, 663–675.
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief Review. Sport. Med.-Open 2019, 5, 1.
- Fatouros, I.G.; Jamurtas, A.Z. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186.
- Robson-Ansley, P.J.; de Milander, L.; Collins, M.; Noakes, T.D. Acute interleukin-6 administration impairs athletic performance in healthy, trained male runners. Can. J. Appl. Physiol. 2004, 29, 411–418.
- Grgic, J.; Mikulic, P. Effects of Paracetamol (Acetaminophen) Ingestion on Endurance Performance: A Systematic Review and Meta-Analysis. Sports 2021, 9, 126.
- Pedersen, J.R.; Andreucci, A.; Thorlund, J.B.; Koes, B.; Møller, M.; Storm, L.K.; Bricca, A. Prevalence, frequency, adverse events, and reasons for analgesic use in youth athletes: A systematic review and meta-analysis of 44,381 athletes. J. Sci. Med. Sport 2022, 25, 810–819.
- Lundberg, T.R.; Howatson, G. Analgesic and anti-inflammatory drugs in sports: Implications for exercise performance and training adaptations. Scand. J. Med. Sci. Sport. 2018, 28, 2252–2262.
- Pedersen, B.K.; Toft, A.D. Effects of exercise on lymphocytes and cytokines. Br. J. Sport. Med. 2000, 34, 246.
- Padilha, C.S.; Von Ah Morano, A.E.; Krüger, K.; Rosa-Neto, J.C.; Lira, F.S. The growing field of immunometabolism and exercise: Key findings in the last 5 years. J. Cell. Physiol. 2022, 237, 4001–4020.
More
Information
Subjects:
Physiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
817
Revisions:
2 times
(View History)
Update Date:
01 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




