Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qing-Xiang Amy Sang | -- | 3495 | 2023-02-28 16:09:13 | | | |
| 2 | Rita Xu | Meta information modification | 3495 | 2023-03-01 02:54:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khamis, Z.I.; Sarker, D.B.; Xue, Y.; Al-Akkary, N.; James, V.D.; Zeng, C.; Li, Y.; Sang, Q.A. Human Brain with Induced Pluripotent Stem Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/41749 (accessed on 07 February 2026).
Khamis ZI, Sarker DB, Xue Y, Al-Akkary N, James VD, Zeng C, et al. Human Brain with Induced Pluripotent Stem Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/41749. Accessed February 07, 2026.
Khamis, Zahraa I., Drishty B. Sarker, Yu Xue, Nancy Al-Akkary, Viviana D. James, Changchun Zeng, Yan Li, Qing-Xiang Amy Sang. "Human Brain with Induced Pluripotent Stem Cells" Encyclopedia, https://encyclopedia.pub/entry/41749 (accessed February 07, 2026).
Khamis, Z.I., Sarker, D.B., Xue, Y., Al-Akkary, N., James, V.D., Zeng, C., Li, Y., & Sang, Q.A. (2023, February 28). Human Brain with Induced Pluripotent Stem Cells. In Encyclopedia. https://encyclopedia.pub/entry/41749
Khamis, Zahraa I., et al. "Human Brain with Induced Pluripotent Stem Cells." Encyclopedia. Web. 28 February, 2023.
Copy Citation
Induced pluripotent stem cells (iPSCs) are crucial for disease modeling and cell-based therapy because they serve as an infinite source of specific human cell types. Brain cancer is a group of diverse and rapidly growing malignancies that originate in the central nervous system (CNS) and have a poor prognosis. The complexity of brain structure and function makes brain cancer modeling extremely difficult, limiting pathological studies and therapeutic developments. Advancements in human pluripotent stem cell technology have opened a window of opportunity for brain cancer modeling, providing a wealth of customizable methods to simulate the disease in vitro.
human brain cancer
induced pluripotent stem cell technology
tumor microenvironment
isogenic cells
1. Introduction
Early embryonic development marks the division and transformation of a totipotent zygote into pluripotent cells that can give rise to all cell types of the body [1]. The induction of pluripotency in somatic cells is of enormous scientific interest and is a powerful tool for medicine and basic biological research [2]. Before the advent of culture-induced reprogramming, the production of pluripotent cells was mostly achieved using exposure-based approaches such as somatic cell nuclear transfer (SCNT), cell fusion, or reprogramming by cell extracts [3]. Induced pluripotent stem cell (iPSC) technology later emerged as the most viable option for pluripotent stem cell production due to its simplicity and reproducibility [4]. The pioneering work of Takahashi and Yamanaka in 2006, which reprogrammed mouse somatic cells into pluripotent stem cells by introducing a specified set of transcription factors, was a breakthrough in iPSC derivation [5]. This discovery subsequently fueled other studies to generate iPSCs from different human somatic cells using defined factors [6][7][8]. Human iPSC production from skin or blood cells represents an abundant source of human cell types needed for therapeutic purposes. Hence, iPSC reprogramming is now one of the most prominent trends in basic research fields and in medicine. It is applied in disease modeling, drug discovery [9][10][11], and cell therapy for the treatment of different kinds of diseases, such as retinal diseases [12][13], neurodegenerative diseases and injuries [14][15][16], thrombocytopenia [17], and cancer [18][19].
Models using iPSCs are compelling alternatives to animal models for studying neural disorders and brain tumors because iPSC-derived neurons possess similar genetic information, cell structure, and neuronal connections as those found in the human brain. Pathological models are now available for major neurological disorders that have been used to elucidate pathogenic mechanisms and to evaluate drugs preclinically [10][20][21]. However, brain tumor modeling using iPSCs is still a challenging and underexplored area of research.
Despite appreciable improvement in cancer survivorship, brain cancers continue to show poor prognosis, largely attributable to the absence of suitable brain tumor models that limit the study of complex brain tumor biology and drug development. Glioma and medulloblastoma are two primary brain tumors with the highest incidence in adults and children, respectively [22]. The most aggressive glioma variant, glioblastoma, frequently develops therapeutic resistance to hand patients a scanty median survival of 15 months [10][22][23][24]. Medulloblastoma, which accounts for 20% of all childhood brain malignancies [25][26], shows a better response to radiation and chemotherapy. However, the search for medulloblastoma treatment modalities without long-term adverse effects is ongoing [22]. The current scenario implies that obtaining reliable glioma and medulloblastoma models would be the key to understanding tumor biology and finding effective treatment strategies to enhance brain cancer survival.
The past decade has seen a gradual emergence of iPSC-derived human brain tumor platforms [10][27][28][29] resulting from the recent development of methods for capturing tumorigenic mutations and organoid formation. The advent of customizable and highly efficient genome editing technologies like CRISPR has made brain tumor modeling plausible, since engineered systems offer targeted study of patient-specific diseases with germline or somatic mutations [30]. Apart from tumor cells, modeling blood vessels and other supporting cells of the brain tumor microenvironment is also of paramount interest as they affect how tumor cells respond to stress and develop therapeutic resistance [24]. Therefore, creating a faithful brain tumor avatar with all its essential components is crucial for predicting drug response and improving treatment outcomes for dreadful brain malignancies [23].
2. In Vitro Modeling of the Human Brain with Induced-Pluripotent Stem Cells
Since their discovery, iPSCs have enabled unprecedented advancements in studying human brain physiology and pathobiology. Researchers have successfully used iPSCs to generate various types of brain cells in 2D cultures to model specific brain regions in 3D organoid systems and to restructure the blood–brain barrier (BBB) in vitro. Collectively, these models contributed to the generation of a brain-like organ that may recapitulate the architecture of a developing human brain.
2.1. Deriving Brain Microenvironment Cells Using iPSCs
The human brain consists of two major cell types—the neurons and the glial cells—that work in concert to ensure the proper execution of its functions. While the neurons are the primary cells responsible for signal transmission and processing, the glial cells, composed of astrocytes, oligodendrocytes, and microglia, support various neuronal functions and play active roles in synaptogenesis and brain development [31]. These brain-specific cells can be differentiated in vitro from iPSCs in 2D monolayer cultures (Figure 1). Despite their inherent advantages, the 2D culture models fail to properly mimic the complex structure of the human brain, ranging from cell–cell and cell–matrix interactions to cell polarity and migration [32].
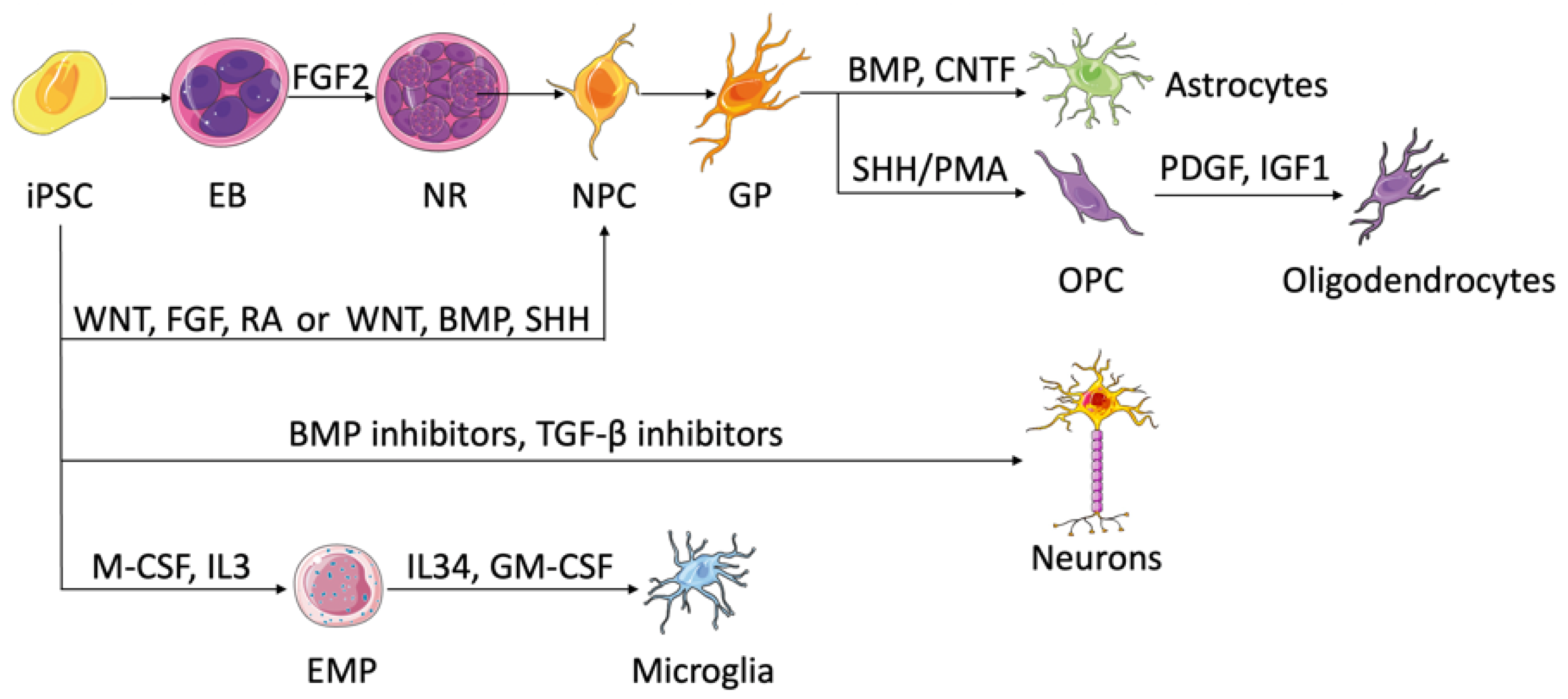
Figure 1. Brain cells and their differentiation from iPSCs. NPCs exist in neural rosettes and develop glial phenotypes spontaneously, which can differentiate into astrocytes by the addition of STAT pathway activators, such as BMP and CNTF. Following SHH or PMA addition, NPCs can turn into oligodendrocytes. PDGF and IGF1 are used in the process of maturation. NPCs can also come from iPSCs directly. Wnt, FGF, and RA establish anterior–posterior identities of NPCs, while Wnt, BMP, and SHH form dorsal–ventral identities. Neuron formation from iPSCs requires dual SMAD signaling inhibition using BMP and TGF-β inhibitors. Myelopoiesis takes place upon the addition of M-CSF and IL3. EMPs generate microglia once treated with IL34 and GM-CSF. EB, embryonic body; NR, neural rosette; NPC, neural progenitor cell; GP, glial phenotype; OPC, oligodendrocyte progenitor cell; EMP, erythromyeloid progenitor.
2.1.1. Neural Progenitor Cells and Neurons
Neural progenitor cells (NPCs) serve as precursor cells that can differentiate into neurons, astrocytes, and oligodendrocytes, so the generation of NPCs is considered substantial for the study of neurodevelopment and neurological diseases. With the advent of iPSCs, various protocols have been developed to differentiate iPSCs into NPCs [33]. Historically, this differentiation involves the spontaneous formation of small spheres called embryoid bodies (EB) if culturing iPSCs in suspension, which after the addition of specific growth factors such as fibroblast growth factor-2 (FGF-2) would generate neural tube-like structures called neural rosettes [34]. The cells of the neural rosettes have characteristics similar to embryonic neuroepithelia and mainly contain NPCs [33]. However, the variety in iPSC lines and experimental setups created variations in differentiated NPCs and urged the search for an alternative [35]. In 2009, Chambers et al. established a protocol for neural induction that involves dual inhibition of SMAD signaling using bone morphogenetic protein (BMP) pathway inhibitors and transforming growth factor beta (TGF-β) pathway inhibitors [36]. This dual-SMAD inhibition protocol circumvented the EB formation and its associated variability in monolayer cultures, as well as induced efficient differentiation in a short period of time. Nowadays, various protocols for neural differentiation of iPSCs are available with slight variations from the aforementioned methods.
It is important to note that NPCs can be generated from iPSCs as region-specific subtypes based on the available morphogens. While wingless/integrated (Wnt), FGF, and retinoic acid (RA) morphogens generate progenitors along the anterior–posterior axis, exposure to Wnt, BMP, and sonic hedgehog (SHH) results in cells with dorsal–ventral identities (reviewed by Tao et al.) [37]. In addition to regional patterning, one can enrich certain neural progenitors by exploiting the intrinsic time course needed for the production of different progenitor subtypes. Mitogens and γ-secretase inhibitors are combined to promote the rise of target progenitors and keep them in the cell cycle, which enriches the population of target progenitor cells by preventing them from differentiating to the next stage. Cultured in a neurotrophic factor-rich medium, regionally patterned neural progenitors can give rise to various neuronal subtypes such as dopaminergic, glutamatergic, GABAergic, and cholinergic. When engrafted into the mouse brain, neuron subtypes appear to localize in different areas, following functional maturation dictated by their locally intrinsic programs that have not been fully understood but take a long time, more than 6 months in certain cases [33][37]. The availability of in vitro human iPSC-derived neurons enables in-depth studies of neurological diseases that are caused by damage in specific neural subtypes.
2.1.2. Astrocytes
Differentiation of iPSCs into astrocytes takes a prolonged time and occurs in three steps: (1) neural induction of iPSCs into NPCs through EB formation, (2) expansion of NPCs until they acquire a glial phenotype, and (3) differentiation of glial progenitors into astrocytes driven by several factors, such as BMP and ciliary neurotrophic factor (CNTF), which activate the signal transducer and activator of transcription (STAT) pathway [37][38]. Neural progenitors patterned to different areas of the brain are found to switch to glial cells and then to astrocytes while retaining their regional identities [39]. While modeling astrocytes, the iPSC–astrocytic derivatives should recapitulate not only the structural but also the physiological features of genuine astrocytes [40].
2.1.3. Oligodendrocytes
Involved in axonal myelination, oligodendrocytes emerge late during normal development and follow an intricate spatiotemporal specification. Similar to astrocytes, oligodendrocytic differentiation from iPSCs begins with NPC generation followed by gliogenesis [41][42]. The transition of the gliogenic precursors into oligodendrocyte progenitor cells coexpressing oligodendrocyte transcription factor 2 (Olig2) and NK2 homeobox 2 (NKX2-2) is maintained by the addition of SHH or its agonist purmorphamine (PMA) [43]. The mature oligodendrocytes are finally established with the help of growth factors such as platelet-derived growth factor (PDGF) or insulin-like growth factor 1 (IGF1) [37][44]. Since the differentiation time of the previous protocols is too long, efforts are focused on accelerating the process. Studies show that overexpression of oligodendrocyte-specific transcription factors, such as SPY-box transcription factor 10 (SOX10), can induce fast and efficient differentiation of iPSCs into oligodendrocytes capable of myelinating axons [44][45]. Recently, Shaker et al. developed a protocol that generated myelinating human oligodendrocytes in only 42 days, using an iPSC line that overexpresses SOX10 [46].
2.1.4. Microglia
Microglia are the resident macrophages of the central nervous system (CNS) and the primary gatekeepers against infections. Aside from their role in innate immunity, microglia are critical players in neurological diseases, highlighting the need for the development of in vitro human-like microglia models that can recapitulate their in vivo counterparts. Recent protocols for iPSC-derived microglia rely on the latest developmental studies that trace microglia lineage to erythromyeloid progenitors (EMPs) in the yolk sac [47]. In most studies, myelopoiesis of iPSCs is induced by the addition of macrophage colony stimulation factor (M-CSF) and interleukin 3 (IL3) in the culture medium, where pure microglia precursors occur in the supernatant persistently. For the maturation of microglia, IL34 and granulocyte–macrophage colony-stimulating factor (GM-CSF) are needed. Additional factors, such as stem cell factor (SCF), vascular endothelial growth factor (VEGF), and BMP4, are optional and can be added for enhancing differentiation efficiency. While the differentiated cells exhibited a plethora of microglia structural and functional features compared to primary microglia culture, additional studies are required to validate the results with in vivo microglia [48].
2.2. Derivation of Brain Organoids from iPSC
To more accurately model the human brain, a 3D culture system is required. These 3D structures, also termed organoids, consist of organ-specific progenitor cells capable of spontaneous self-organization and differentiation into various tissue-specific cells that can mimic some of the organ’s structural and functional features [49]. Researchers reviewed using specific factors to achieve corresponding brain organoids from iPSCs or iPSC-derived EBs (Figure 2). In 2008, iPSCs were shown to self-aggregate into EBs when grown in serum-free floating culture, generating neural tissue with apicobasal polarization and cortical fate [50][51]. Another revolution in brain organoids that directed the differentiation of EBs into various brain regions was the introduction of Matrigel, an extracellular matrix derived from a mouse sarcoma cell line, into the culture [52]. When stabilized in Matrigel, EBs spontaneously developed into optical cup characteristics of retinal tissue [53]. Later in 2013, Lancaster et al. produced cerebral organoids by embedding EB-derived neuroectodermal tissue in Matrigel and then culturing the complex in differentiation media with RA supplement [54]. This protocol generated multiple brain regions and heterogeneous cytoarchitecture. However, the cellular diversity of iPSCs, the inherent variability among different batches, and the lack of reproducibility raised concerns about the relevance of this organoid model for applications in disease modeling and drug testing.
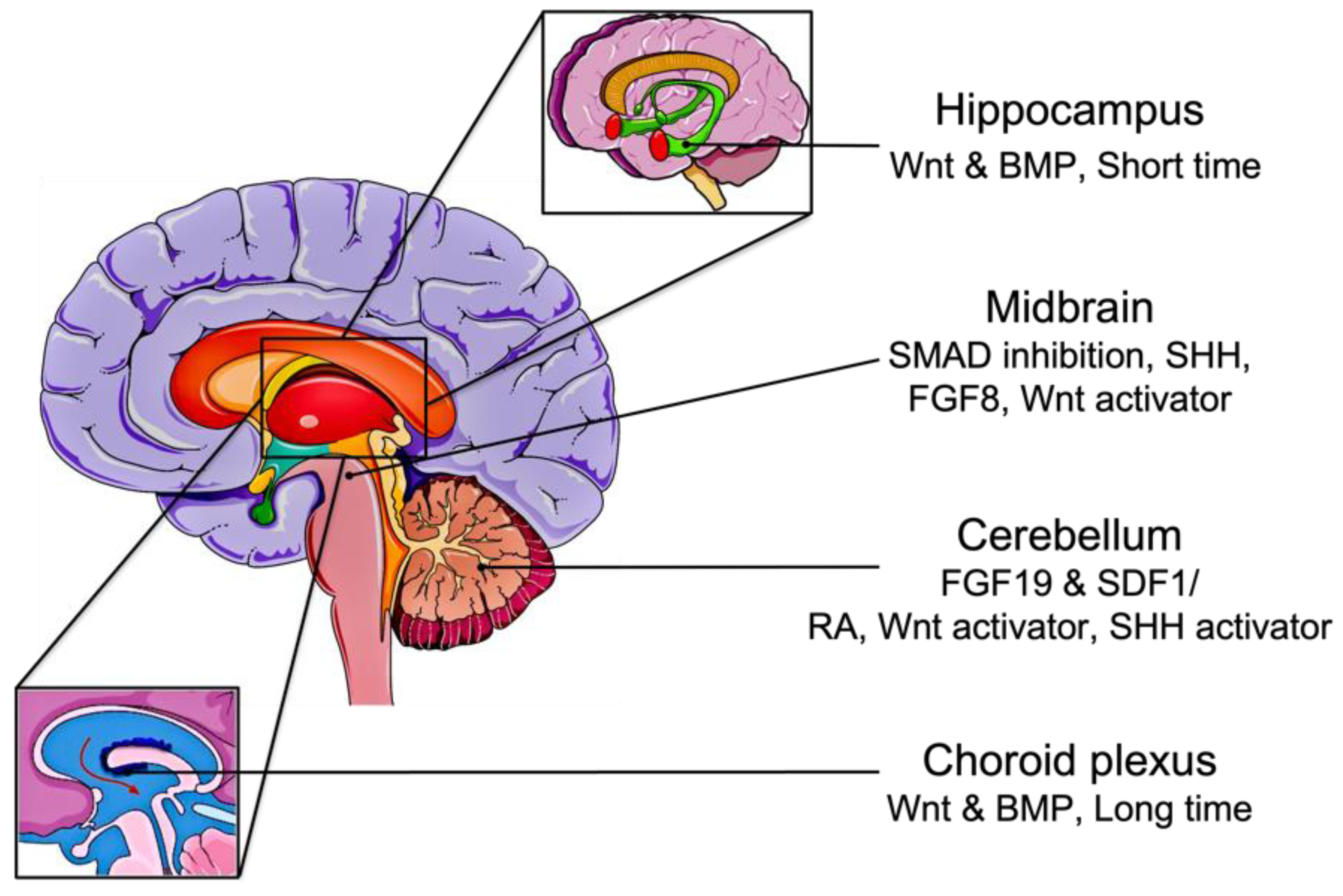
Figure 2. Brain organoid differentiation from iPSCs. The addition of RA, Wnt, and SHH activators generates cerebellar organoids directly from iPSCs. Pluripotent stem cells can be differentiated into cerebellar tissue through FGF19 and SDF1 treatment. Induced PSCs aggregate into embryoid bodies in serum-free suspension. Short-term treatment of Wnt and BMP to EBs develops hippocampus organoids, while long-term treatment generates choroid plexus organoids. If treated with FGF19 and SDF1, EBs can form cerebellum organoids.
Alternatively, other methods have been described to restrict the differentiation of the organoids into specific brain regions. By adding defined signaling molecules, organoids can be patterned into forebrain, midbrain, or hindbrain. For example, the addition of SMAD inhibitors can guide the organoids into dorsal forebrain fate, and if followed by SHH agonists, can promote ventral forebrain identities [55]. Furthermore, treatment of EBs with Wnt and BMP for a short period of time develops hippocampal organoids, while prolonged exposure generates choroid plexus organoids [56]. Midbrain regions are established by the addition of SHH, FGF8, and a Wnt activator to the culture medium after SMAD inhibition [57]. The most elaborate hindbrain structure, the cerebellum, was also formed in vitro using FGF19 and stromal cell-derived factor 1 (SDF1) [58]. Hua et al. demonstrated the formation of cerebellar organoids from iPSCs using the combination of RA, Wnt activator, and SHH activator [59][60].
Even though the addition of external factors has patterned the brain organoids into specific regional identities, these regions lack proper morphogen spatiotemporal gradients, functional connectivity among diverse brain regions, and non-ectodermal cell lineages, such as microglia and endothelial cells (ECs). This can substantially affect brain organoid development, resulting in an unfaithful replica of the human brain [50].
A multitude of studies has emerged to address the aforementioned shortcomings of the patterning protocols. Cederquist et al. engineered a pseudo-signaling center by embedding inducible SHH-expressing iPSCs at one pole of a developing organoid generating forebrain topography reminiscent of in vivo neurodevelopment [61]. Additionally, Rifes et al. implemented a microfluidic approach to create a Wnt-activating gradient that can induce neural tube regionalization [62]. To optimize neural connectivity, assembled organoids are formed by developing individual brain regions first and subsequently fusing them in a coculture. Several studies have reported the fusion of ventral and dorsal forebrain organoids, which resulted in dorsal migration of the interneurons and their integration into cortical microcircuits characteristics of the in vivo process [63][64][65]. More recently, Xiang et al. fused cortical and thalamic organoids to model the reciprocal thalamocortical projections that occur in the human brain [66]. Additionally, the incorporation of microglia into brain organoids that lack an innate source is essential due to their vital roles in neuronal development and immune response regulation. However, Ormel et al. showed that brain organoids developed without dual-SMAD inhibition could spontaneously give rise to mesodermal progenitors and later microglia-like cells. These microglia-like cells exhibited some phenotypes and functions typical of adult microglia [67]. The derived isogenic microglia-like cells were also shown to express phenotypic markers, phagocytose particles, and secrete inflammatory cytokines, as well as the ability to integrate with the dorsal and ventral forebrain organoids [68]. As the organoid grows, vascularization becomes essential to ensure proper delivery of nutrients and oxygen to the core. Recently, Ham et al. reported the generation of vessel-like structures with mature BBB features upon the treatment of cerebral organoids with VEGF [69]. Alternative methods employed to prevent necrosis in the organoid core include transplantation of the organoid in the mouse cortex [70] or growing the organoid as slice cultures [71].
Collectively, despite various pitfalls, these breakthroughs in brain organoid development from human iPSCs have provided unprecedented opportunities to understand and manage brain development and neurological disorders.
2.3. Induced PSC-Derived Blood–Brain Barrier Models
The blood–brain barrier (BBB) is an exceptionally selective barrier with rich composition of unique brain microvascular endothelial cells (BMECs) and other supporting cells (neurons, astrocytes, and pericytes) of the neurovascular unit (NVU). Through its dynamic cellular complexes and tight junctions, the BBB is involved in the maintenance of brain homeostasis and the regulation of any interchange between the bloodstream and the CNS [72]. BBB components express specific molecular transporters controlling the entry of molecules and nutrients into the brain [73]. Through this, the BBB is at the forefront of ensuring optimal CNS function by moderating the influence of systemic fluctuations and harmful substances (e.g., pathogens and toxins) on the brain [74].
Specialized endothelial cells known as BMECs compose the innermost layer of the BBB’s physiological barrier. Alteration of these cells on a structural and functional level is linked to neurological disorders such as traumatic brain injury, Alzheimer’s disease, and Parkinson’s disease [75][76]. Pericytes are multifunctional cells found inside capillaries that also constitute an important part of the NVU. With their stem cell-like properties, they are involved in several physiological processes, including regulation of the BBB, neuroinflammation, vasculogenesis, and angiogenesis. Disruption of pericyte function is also associated with brain pathologies, particularly vascular diseases [77].
In a pioneering approach to generate BMECs from iPSCs, Lippmann et al. employed endothelial and neural codifferentiation of iPSCs in an unconditioned medium followed by expansion of the cells in an endothelial cell medium and selective matrix-aided cell purification. By coculturing these iPSC-derived BMECs (iBMECs) with astrocytes, the group was able to induce the expression of endothelial transporters and receptors, in addition to the recreation of BBB attributes such as well-organized tight junctions and polarized efflux transporter activity [78]. The key steps of endothelial induction and BMEC specification and purification were adopted later in the iBMEC protocols reviewed by Workman and Svendsen [79]. The successful protocols resulted in optimized iPSC seeding density [80], faster differentiation [81], accurate developmental course [82][83], simplified defined media [84], increased iBMEC purity [85], and capacity for cryopreservation [86][87]. However, limitations of the iBMEC protocols still exist and mainly include the generation of a homogeneous epithelial cell population. It is critical for disease pathology and drug screening research to generate endothelial cells conforming as much as possible to their in vivo counterparts at both the transcriptional and the functional levels. To that end, reprogramming the epithelial lineage of BMECs into vascular ECs can be achieved by overexpressing endothelial transcription factors ETV2, ERG, and FL1 [88]. Regarding the acquisition of canonical BBB characteristics (i.e., expression of GLUT1, increased claudin-5, decreased PLVAP, and decreased permeability) by iBMECs, Gastfriend et al. showed in a recent study that activation of Wnt signaling is essential to express the endothelial phenotypes [89].
Pericytes have two distinct origins and can be generated from iPSCs through either mesoderm or neural crest induction [90]. Mesoderm induction can be achieved in several ways, one of which involves the addition of BMP4, activin A, and VEGF [91][92]. Alternatively, multipotent vascular progenitor (MesoT) cells can be generated from iPSCs and differentiated into pericytes by mesoderm induction [93]. Neural crest induction of iPSCs into pericytes relies on canonical Wnt signaling activation in a protocol similar to endothelial cell differentiation [94]. Wnt activation and simultaneous activin/nodal/BMP/TGF-β1 inhibition improve the faithfulness of in vitro BBB models [95]. It is thus evident that neurovascular functions greatly depend on key signaling pathways and interactions between brain-specific pericytes and surrounding cells, such as endothelial cells, astrocytes, and neurons. These pathways include PDGF-BB, TGF-β, and Notch signaling. Specifically, pericyte survival is promoted by PDGF-BB-PDGFRb signaling, while pericyte attachment to endothelial cells is mediated by TGF-β signaling [96]. Notch signaling plays a crucial role in vascular development and arteriovenous specifications [96].
Coculture of iBMECs with pericytes, astrocytes, and neurons is an attractive approach to enhance BBB properties in models, including transendothelial electrical resistance (TEER) and low passive permeability [97][98][99]. This has led to the generation of iPSC-derived NVU supporting cells and combining them with iBMECs for an isogenic model of human BBB [95][100][101][102]. Such models should allow a deeper understanding of physiological processes in both healthy and diseased states. Recently, Marzano et al. showed that brain pericytes and endothelial cell coculture derived from iPSCs showed appropriate inflammatory responses to amyloid-β42 oligomers [103]. Similar observations were made for iPSC-derived astroglial cells [104]. These studies warrant effective functionalization of cells toward reliable BBB modeling.
In vitro models derived from human iPSCs proved to be important tools for research considering interspecies variations in BBB receptor expression and the difficulty of using fresh tissue from human biopsies [105][106]. While 2D models were initially promising, the incorporation of other relevant factors, such as cell–cell interactions and fluid shear stress remained necessary to ensure EC and pericyte function [95][107]. This limitation was addressed by incorporating microfluidic channels synthesized from biomaterials [108] and including several iPSC-derived cell types in the BBB chips [109]. Through the generation of a 3D environment, such as BBB chips and iPSC-derived 3D brain organoids, observation of multilineage differentiation, self-organization into heterogeneous cell populations, and neurodevelopmental/neurodegenerative gene regulation patterns becomes possible [110]. Flow dynamics and BBB–brain tissue interactions can therefore be readily explored, yielding a better understanding of disease pathology and providing a physiologically relevant platform for disease modeling and drug screening.
References
- Mitalipov, S.; Wolf, D. Totipotency, Pluripotency and Nuclear Reprogramming. In Engineering of Stem Cells; Springer: Berlin/Heidelberg, Germany, 2009; pp. 185–199.
- Takahashi, K.; Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 2013, 140, 2457–2461.
- Hochedlinger, K.; Jaenisch, R. Nuclear reprogramming and pluripotency. Nature 2006, 441, 1061–1067.
- Yamanaka, S. Induced pluripotent stem cells: Past, present, and future. Cell Stem Cell 2012, 10, 678–684.
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676.
- Park, I.-H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008, 451, 141–146.
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872.
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920.
- Grskovic, M.; Javaherian, A.; Strulovici, B.; Daley, G.Q. Induced pluripotent stem cells—Opportunities for disease modelling and drug discovery. Nat. Rev. Drug Discov. 2011, 10, 915–929.
- Luo, J.; Li, P. Human pluripotent stem cell-derived brain organoids as in vitro models for studying neural disorders and cancer. Cell Biosci. 2021, 11, 99.
- Urbaniak, A.; Reed, M.R.; Heflin, B.; Gaydos, J.; Piña-Oviedo, S.; Jędrzejczyk, M.; Klejborowska, G.; Stępczyńska, N.; Chambers, T.C.; Tackett, A.J. Anti-glioblastoma activity of monensin and its analogs in an organoid model of cancer. Biomed. Pharmacother. 2022, 153, 113440.
- Deng, W.-L.; Gao, M.-L.; Lei, X.-L.; Lv, J.-N.; Zhao, H.; He, K.-W.; Xia, X.-X.; Li, L.-Y.; Chen, Y.-C.; Li, Y.-P. Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Rep. 2018, 10, 1267–1281.
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kimura, T.; Futagami, T.; Suegami, S.; Takahashi, M. Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Rep. 2016, 7, 619–634.
- Cooper, O.; Seo, H.; Andrabi, S.; Guardia-Laguarta, C.; Graziotto, J.; Sundberg, M.; McLean, J.R.; Carrillo-Reid, L.; Xie, Z.; Osborn, T. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci. Transl. Med. 2012, 4, 141ra190.
- Nakamura, M.; Okano, H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013, 23, 70–80.
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.-Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 2020, 382, 1926–1932.
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous induced pluripotent stem cell–based cell therapies: Promise, progress, and challenges. Curr. Protoc. 2021, 1, e88.
- Ito, T.; Kawai, Y.; Yasui, Y.; Iriguchi, S.; Minagawa, A.; Ishii, T.; Miyoshi, H.; Taketo, M.M.; Kawada, K.; Obama, K. The therapeutic potential of multiclonal tumoricidal T cells derived from tumor infiltrating lymphocyte-derived iPS cells. Commun. Biol. 2021, 4, 694.
- Ueda, T.; Kumagai, A.; Iriguchi, S.; Yasui, Y.; Miyasaka, T.; Nakagoshi, K.; Nakane, K.; Saito, K.; Takahashi, M.; Sasaki, A. Non–clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti–glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 2020, 111, 1478–1490.
- Okano, H.; Morimoto, S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell 2022, 29, 189–208.
- Pasteuning-Vuhman, S.; de Jongh, R.; Timmers, A.; Pasterkamp, R.J. Towards advanced iPSC-based drug development for neurodegenerative disease. Trends Mol. Med. 2021, 27, 263–279.
- Huse, J.T.; Holland, E.C. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer 2010, 10, 319–331.
- Antonica, F.; Aiello, G.; Soldano, A.; Abballe, L.; Miele, E.; Tiberi, L. Modeling Brain Tumors: A Perspective Overview of in vivo and Organoid Models. Front. Mol. Neurosci. 2022, 15, 818696.
- Gopalakrishnan, J. The emergence of stem cell-based brain organoids: Trends and challenges. BioEssays 2019, 41, 1900011.
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19, v1–v88.
- Udaka, Y.T.; Packer, R.J. Pediatric Brain Tumors. Neurol. Clin. 2018, 36, 533–556.
- Krieger, T.G.; Tirier, S.M.; Park, J.; Jechow, K.; Eisemann, T.; Peterziel, H.; Angel, P.; Eils, R.; Conrad, C. Modeling glioblastoma invasion using human brain organoids and single-cell transcriptomics. Neuro-Oncology 2020, 22, 1138–1149.
- Plummer, S.; Wallace, S.; Ball, G.; Lloyd, R.; Schiapparelli, P.; Quiñones-Hinojosa, A.; Hartung, T.; Pamies, D. A Human iPSC-derived 3D platform using primary brain cancer cells to study drug development and personalized medicine. Sci. Rep. 2019, 9, 1407.
- Sancho-Martinez, I.; Nivet, E.; Xia, Y.; Hishida, T.; Aguirre, A.; Ocampo, A.; Ma, L.; Morey, R.; Krause, M.N.; Zembrzycki, A.; et al. Establishment of human iPSC-based models for the study and targeting of glioma initiating cells. Nat. Commun. 2016, 7, 10743.
- Koga, T.; Chen, C.C.; Furnari, F.B. Genome engineering evolves brain tumor modeling. Neurol. Med.-Chir. 2020, 60, 329–336.
- He, F.; Sun, Y.E. Glial cells more than support cells? Int. J. Biochem. Cell Biol. 2007, 39, 661–665.
- Koo, B.; Choi, B.; Park, H.; Yoon, K.J. Past, Present, and Future of Brain Organoid Technology. Mol. Cells 2019, 42, 617–627.
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying Human Neurological Disorders Using Induced Pluripotent Stem Cells: From 2D Monolayer to 3D Organoid and Blood Brain Barrier Models. Compr. Physiol. 2019, 9, 565–611.
- Zhang, S.-C.; Wernig, M.; Duncan, I.D.; Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1129–1133.
- Boulting, G.L.; Kiskinis, E.; Croft, G.F.; Amoroso, M.W.; Oakley, D.H.; Wainger, B.J.; Williams, D.J.; Kahler, D.J.; Yamaki, M.; Davidow, L.; et al. resource A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 279–286.
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280.
- Tao, Y.; Zhang, S.C. Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell 2016, 19, 573–586.
- Rajan, P.; McKay, R.D.G. Multiple Routes to Astrocytic Differentiation in the CNS. J. Neurosci. 1998, 18, 3620–3629.
- Krencik, R.; Weick, J.P.; Liu, Y.; Zhang, Z.J.; Zhang, S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011, 29, 528–534.
- Leventoux, N.; Morimoto, S.; Imaizumi, K.; Sato, Y.; Takahashi, S.; Mashima, K.; Ishikawa, M.; Sonn, I.; Kondo, T.; Watanabe, H.; et al. Human Astrocytes Model Derived from Induced Pluripotent Stem Cells. Cells 2020, 9, 2680.
- Douvaras, P.; Wang, J.; Zimmer, M.; Hanchuk, S.; O’Bara, M.A.; Sadiq, S.; Sim, F.J.; Goldman, J.; Fossati, V. Efficient Generation of Myelinating Oligodendrocytes from Primary Progressive Multiple Sclerosis Patients by Induced Pluripotent Stem Cells. Stem Cell Rep. 2014, 3, 250–259.
- Wang, S.; Bates, J.; Li, X.; Schanz, S.; Chandler-Militello, D.; Levine, C.; Maherali, N.; Studer, L.; Hochedlinger, K.; Windrem, M.; et al. Human iPSC-Derived Oligodendrocyte Progenitor Cells Can Myelinate and Rescue a Mouse Model of Congenital Hypomyelination. Cell Stem Cell 2013, 12, 252–264.
- Lu, Q.R.; Sun, T.; Zhu, Z.; Ma, N.; Garcia, M.; Stiles, C.D.; Rowitch, D.H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 2002, 109, 75–86.
- Chanoumidou, K.; Mozafari, S.; Baron-Van Evercooren, A.; Kuhlmann, T. Stem cell derived oligodendrocytes to study myelin diseases. Glia 2020, 68, 705–720.
- García-León, J.A.; Kumar, M.; Boon, R.; Chau, D.; One, J.; Wolfs, E.; Eggermont, K.; Berckmans, P.; Gunhanlar, N.; de Vrij, F.; et al. SOX10 Single Transcription Factor-Based Fast and Efficient Generation of Oligodendrocytes from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 655–672.
- Shaker, M.R.; Pietrogrande, G.; Martin, S.; Lee, J.H.; Sun, W.; Wolvetang, E.J. Rapid and Efficient Generation of Myelinating Human Oligodendrocytes in Organoids. Front. Cell. Neurosci. 2021, 15, 631548.
- Hasselmann, J.; Blurton-Jones, M. Human iPSC-derived microglia: A growing toolset to study the brain’s innate immune cells. Glia 2020, 68, 721–739.
- Haenseler, W.; Rajendran, L. Concise Review: Modeling Neurodegenerative Diseases with Human Pluripotent Stem Cell-Derived Microglia. Stem Cells 2019, 37, 724–730.
- Wang, Z.; Wang, S.N.; Xu, T.Y.; Miao, Z.W.; Su, D.F.; Miao, C.Y. Organoid technology for brain and therapeutics research. CNS Neurosci. Ther. 2017, 23, 771–778.
- Benito-Kwiecinski, S.; Lancaster, M.A. Brain organoids: Human neurodevelopment in a dish. Cold Spring Harb. Perspect. Biol. 2020, 12, a035709.
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 2008, 3, 519–532.
- Kleinman, H.K.; McGarvey, M.L.; Hassell, J.R.; Star, V.L.; Cannon, F.B.; Laurie, G.W.; Martin, G.R. Basement membrane complexes with biological activity. Biochemistry 1986, 25, 312–318.
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56.
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379.
- Pasca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 2015, 12, 671–678.
- Sakaguchi, H.; Kadoshima, T.; Soen, M.; Narii, N.; Ishida, Y.; Ohgushi, M.; Takahashi, J.; Eiraku, M.; Sasai, Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 2015, 6, 8896.
- Qian, X.; Jacob, F.; Song, M.M.; Nguyen, H.N.; Song, H.; Ming, G.L. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 2018, 13, 565–580.
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015, 10, 537–550.
- Hua, T.; Bejoy, J.; Song, L.; Wang, Z.; Zeng, Z.; Zhou, Y.; Li, Y.; Sang, Q.-X.A. Cerebellar Differentiation from Human Stem Cells Through Retinoid, Wnt, and Sonic Hedgehog Pathways. Tissue Eng. Part A 2021, 27, 881–893.
- Hua, T.; Liu, C.; Kiran, S.; Gray, K.; Jung, S.; Meckes, D.G.; Li, Y.; Sang, Q.-X.A. Phenotypic, metabolic, and biogenesis properties of human stem cell-derived cerebellar spheroids. Sci. Rep. 2022, 12, 12880.
- Cederquist, G.Y.; Asciolla, J.J.; Tchieu, J.; Walsh, R.M.; Cornacchia, D.; Resh, M.D.; Studer, L. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 2019, 37, 436–444.
- Rifes, P.; Isaksson, M.; Rathore, G.S.; Aldrin-Kirk, P.; Møller, O.K.; Barzaghi, G.; Lee, J.; Egerod, K.L.; Rausch, D.M.; Parmar, M.; et al. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat. Biotechnol. 2020, 38, 1265–1273.
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 2017, 14, 743–751.
- Birey, F.; Andersen, J.; Makinson, C.D.; Islam, S.; Wei, W.; Huber, N.; Fan, H.C.; Metzler, K.R.C.; Panagiotakos, G.; Thom, N. Assembly of functionally integrated human forebrain spheroids. Nature 2017, 545, 54–59.
- Xiang, Y.; Tanaka, Y.; Patterson, B.; Kang, Y.J.; Govindaiah, G.; Roselaar, N.; Cakir, B.; Kim, K.Y.; Lombroso, A.P.; Hwang, S.M.; et al. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 2017, 21, 383–398.e387.
- Xiang, Y.; Tanaka, Y.; Cakir, B.; Patterson, B.; Kim, K.Y.; Sun, P.; Kang, Y.J.; Zhong, M.; Liu, X.; Patra, P.; et al. hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell 2019, 24, 487–497.e487.
- Ormel, P.R.; Vieira de Sá, R.; Van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis van Berlekom, A. Microglia innately develop within cerebral organoids. Nat. Commun. 2018, 9, 4167.
- Song, L.; Yuan, X.; Jones, Z.; Vied, C.; Miao, Y.; Marzano, M.; Hua, T.; Sang, Q.-X.A.; Guan, J.; Ma, T. Functionalization of brain region-specific spheroids with isogenic microglia-like cells. Sci. Rep. 2019, 9, 11055.
- Ham, O.; Jin, Y.B.; Kim, J.; Lee, M.O. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2020, 521, 84–90.
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441.
- Qian, X.; Su, Y.; Adam, C.D.; Deutschmann, A.U.; Pather, S.R.; Goldberg, E.M.; Su, K.; Li, S.; Lu, L.; Jacob, F. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell 2020, 26, 766–781. e769.
- Fernández-López, D.; Faustino, J.; Daneman, R.; Zhou, L.; Lee, S.Y.; Derugin, N.; Wendland, M.F.; Vexler, Z.S. Neurobiology of Disease Blood-Brain Barrier Permeability Is Increased After Acute Adult Stroke but Not Neonatal Stroke in the Rat. J. Neurosci. 2012, 32, 9588–9600.
- Benson, P.F.; Joseph, M.C. The Blood-Brain Barrier. Dev. Med. Child Neurol. 1961, 3, 510–514.
- Bhalerao, A.; Sivandzade, F.; Archie, S.R.; Chowdhury, E.A.; Noorani, B.; Cucullo, L. In vitro modeling of the neurovascular unit: Advances in the field. Fluids Barriers CNS 2020, 17, 22.
- Jarazo, J.; Barmpa, K.; Modamio, J.; Saraiva, C.; Sabaté-Soler, S.; Rosety, I.; Griesbeck, A.; Skwirblies, F.; Zaffaroni, G.; Smits, L.M.; et al. Parkinson’s Disease Phenotypes in Patient Neuronal Cultures and Brain Organoids Improved by 2-Hydroxypropyl-β-Cyclodextrin Treatment. Mov. Disord. 2021, 37, 80–94.
- Lacalle-Aurioles, M.; Cassel de Camps, C.; Zorca, C.E.; Beitel, L.K.; Durcan, T.M. Applying hiPSCs and Biomaterials Towards an Understanding and Treatment of Traumatic Brain Injury. Front. Cell. Neurosci. 2020, 14, 594304.
- Brown, L.S.; Foster, C.G.; Courtney, J.-M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and neurovascular function in the healthy and diseased brain. Front. Cell. Neurosci. 2019, 13, 282.
- Lippmann, E.S.; Azarin, S.M.; Kay, J.E.; Nessler, R.A.; Wilson, H.K.; Al-Ahmad, A.; Palecek, S.P.; Shusta, E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012, 30, 783–791.
- Workman, M.J.; Svendsen, C.N. Recent advances in human iPSC-derived models of the blood–brain barrier. Fluids Barriers CNS 2020, 17, 30.
- Wilson, H.K.; Canfield, S.G.; Hjortness, M.K.; Palecek, S.P.; Shusta, E.V. Exploring the effects of cell seeding density on the differentiation of human pluripotent stem cells to brain microvascular endothelial cells. Fluids Barriers CNS 2015, 12, 1–12.
- Hollmann, E.K.; Bailey, A.K.; Potharazu, A.V.; Neely, M.D.; Bowman, A.B.; Lippmann, E.S. Accelerated differentiation of human induced pluripotent stem cells to blood–brain barrier endothelial cells. Fluids Barriers CNS 2017, 14, 13.
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621.
- Qian, T.; Maguire, S.E.; Canfield, S.G.; Bao, X.; Olson, W.R.; Shusta, E.V.; Palecek, S.P. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci. Adv. 2017, 3, e1701679.
- Neal, E.H.; Marinelli, N.A.; Shi, Y.; McClatchey, P.M.; Balotin, K.M.; Gullett, D.R.; Hagerla, K.A.; Bowman, A.B.; Ess, K.C.; Wikswo, J.P. A simplified, fully defined differentiation scheme for producing blood-brain barrier endothelial cells from human iPSCs. Stem Cell Rep. 2019, 12, 1380–1388.
- Praça, C.; Rosa, S.C.; Sevin, E.; Cecchelli, R.; Dehouck, M.-P.; Ferreira, L.S. Derivation of brain capillary-like endothelial cells from human pluripotent stem cell-derived endothelial progenitor cells. Stem Cell Rep. 2019, 13, 599–611.
- Pong, S.; Lizano, P.; Karmacharya, R. Derivation, expansion, cryopreservation and characterization of brain microvascular endothelial cells from human induced pluripotent stem cells. JoVE J. Vis. Exp. 2020, 165, e61629.
- Wilson, H.K.; Faubion, M.G.; Hjortness, M.K.; Palecek, S.P.; Shusta, E.V. Cryopreservation of brain endothelial cells derived from human induced pluripotent stem cells is enhanced by rho-associated coiled coil-containing kinase inhibition. Tissue Eng. Part C Methods 2016, 22, 1085–1094.
- Lu, T.M.; Houghton, S.; Magdeldin, T.; Barcia Durán, J.G.; Minotti, A.P.; Snead, A.; Sproul, A.; Nguyen, D.H.T.; Xiang, J.; Fine, H.A.; et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc. Natl. Acad. Sci. USA 2021, 118, e2016950118.
- Gastfriend, B.D.; Nishihara, H.; Canfield, S.G.; Foreman, K.L.; Engelhardt, B.; Palecek, S.P.; Shusta, E.V. Wnt signaling mediates acquisition of blood–brain barrier properties in naïve endothelium derived from human pluripotent stem cells. eLife 2021, 10, e70992.
- Yamazaki, T.; Mukouyama, Y.-S. Tissue specific origin, development, and pathological perspectives of pericytes. Front. Cardiovasc. Med. 2018, 5, 78.
- Chan, X.Y.; Black, R.; Dickerman, K.; Federico, J.; Levesque, M.; Mumm, J.; Gerecht, S. Three-Dimensional Vascular Network Assembly from Diabetic Patient-Derived Induced Pluripotent Stem Cells. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2677–2685.
- Orlova, V.V.; Van Den Hil, F.E.; Petrus-Reurer, S.; Drabsch, Y.; Ten Dijke, P.; Mummery, C.L. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 2014, 9, 1514–1531.
- Colunga, T.; Hayworth, M.; Kreß, S.; Reynolds, D.M.; Chen, L.; Nazor, K.L.; Baur, J.; Singh, A.M.; Loring, J.F.; Metzger, M. Human pluripotent stem cell-derived multipotent vascular progenitors of the mesothelium lineage have utility in tissue engineering and repair. Cell Rep. 2019, 26, 2566–2579.e2510.
- Faal, T.; Phan, D.T.; Davtyan, H.; Scarfone, V.M.; Varady, E.; Blurton-Jones, M.; Hughes, C.C.; Inlay, M.A. Induction of mesoderm and neural crest-derived pericytes from human pluripotent stem cells to study blood-brain barrier interactions. Stem Cell Rep. 2019, 12, 451–460.
- Stebbins, M.J.; Gastfriend, B.D.; Canfield, S.G.; Lee, M.-S.; Richards, D.; Faubion, M.G.; Li, W.-J.; Daneman, R.; Palecek, S.P.; Shusta, E.V. Human pluripotent stem cell–derived brain pericyte–like cells induce blood-brain barrier properties. Sci. Adv. 2019, 5, eaau7375.
- Jeske, R.; Albo, J.; Marzano, M.; Bejoy, J.; Li, Y. Engineering Brain-Specific Pericytes from Human Pluripotent Stem Cells. Tissue Eng. Part B Rev. 2020, 26, 367–382.
- Appelt-Menzel, A.; Cubukova, A.; Günther, K.; Edenhofer, F.; Piontek, J.; Krause, G.; Stüber, T.; Walles, H.; Neuhaus, W.; Metzger, M. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri-and multipotent stem cells. Stem Cell Rep. 2017, 8, 894–906.
- Delsing, L.; Dönnes, P.; Sánchez, J.; Clausen, M.; Voulgaris, D.; Falk, A.; Herland, A.; Brolén, G.; Zetterberg, H.; Hicks, R. Barrier properties and transcriptome expression in human iPSC-derived models of the blood–brain barrier. Stem Cells 2018, 36, 1816–1827.
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014, 4, 4160.
- Canfield, S.G.; Stebbins, M.J.; Faubion, M.G.; Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. An isogenic neurovascular unit model comprised of human induced pluripotent stem cell-derived brain microvascular endothelial cells, pericytes, astrocytes, and neurons. Fluids Barriers CNS 2019, 16, 25.
- Canfield, S.G.; Stebbins, M.J.; Morales, B.S.; Asai, S.W.; Vatine, G.D.; Svendsen, C.N.; Palecek, S.P.; Shusta, E.V. An isogenic blood–brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J. Neurochem. 2017, 140, 874–888.
- Delsing, L.; Kallur, T.; Zetterberg, H.; Hicks, R.; Synnergren, J. Enhanced xeno-free differentiation of hiPSC-derived astroglia applied in a blood–brain barrier model. Fluids Barriers CNS 2019, 16, 27.
- Marzano, M.; Chen, X.; Russell, T.A.; Medina, A.; Wang, Z.; Hua, T.; Zeng, C.; Wang, X.; Sang, Q.-X.; Tang, H. Studying the Inflammatory Responses to Amyloid Beta Oligomers in Brain-Specific Pericyte and Endothelial Co-Culture from Human Stem Cells. Front. Chem. Eng. 2022, 4, 927188.
- Griffin, K.; Bejoy, J.; Song, L.; Hua, T.; Marzano, M.; Jeske, R.; Sang, Q.-X.A.; Li, Y. Human stem cell-derived aggregates of forebrain astroglia respond to amyloid beta oligomers. Tissue Eng. Part A 2020, 26, 527–542.
- Song, H.W.; Foreman, K.L.; Gastfriend, B.D.; Kuo, J.S.; Palecek, S.P.; Shusta, E.V. Transcriptomic comparison of human and mouse brain microvessels. Sci. Rep. 2020, 10, 12358.
- Warren, M.S.; Zerangue, N.; Woodford, K.; Roberts, L.M.; Tate, E.H.; Feng, B.; Li, C.; Feuerstein, T.J.; Gibbs, J.; Smith, B.; et al. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol. Res. 2009, 59, 404–413.
- Thosar, S.S.; Johnson, B.D.; Johnston, J.D.; Wallace, J.P. Sitting and endothelial dysfunction: The role of shear stress. Med. Sci. Monit. 2012, 18, REV173–REV180.
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular tissue engineering: Progress, challenges, and clinical promise. Cell Stem Cell 2018, 22, 340–354.
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24, 995–1005.e1006.
- Lee, C.T.; Bendriem, R.M.; Wu, W.W.; Shen, R.F. 3D brain Organoids derived from pluripotent stem cells: Promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 2017, 24, 59.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
515
Revisions:
2 times
(View History)
Update Date:
01 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




