Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, K.; Li, D.; He, X.; Xie, C.; He, H. Organic Matter Sources in Lake Sediments. Encyclopedia. Available online: https://encyclopedia.pub/entry/41743 (accessed on 09 March 2026).
Zhang K, Li D, He X, Xie C, He H. Organic Matter Sources in Lake Sediments. Encyclopedia. Available at: https://encyclopedia.pub/entry/41743. Accessed March 09, 2026.
Zhang, Kai, Dongli Li, Xuejun He, Changyuan Xie, Haibo He. "Organic Matter Sources in Lake Sediments" Encyclopedia, https://encyclopedia.pub/entry/41743 (accessed March 09, 2026).
Zhang, K., Li, D., He, X., Xie, C., & He, H. (2023, February 28). Organic Matter Sources in Lake Sediments. In Encyclopedia. https://encyclopedia.pub/entry/41743
Zhang, Kai, et al. "Organic Matter Sources in Lake Sediments." Encyclopedia. Web. 28 February, 2023.
Copy Citation
Sedimentary organic matter is an important component of the metabolism of a lake’s ecosystem, and it is generally derived from both the watershed and the primary productivity of a lake. Understanding the sources of organic matter in lakes and lake trophic status is important when evaluating the quality of lake ecosystems.
lake ecosystems
surface sediments
organic matter sources
carbon isotopes
distribution characteristics
1. Introduction
With global climate change, the greenhouse effect has led to an increase in CO2 in the atmosphere and caused a large increase in primary production in inland lakes [1]. Although many studies believe that freshwater systems are CO2 sources [2], this is not always the case (including but not limited to): (1) While the dynamic equilibrium of a water body CO2 depends on the physical process, the diffusion rate is only one in ten thousand of that in the atmosphere [3]; (2) A unique physiochemical characteristic of karst water is that its CO2 concentration falls below 1% of dissolved inorganic carbon (DIC) when the pH level exceeds 8 [4]; (3) There are large amounts of recalcitrant dissolved organic carbon in karst aquatic ecosystems (decreased release of carbon compounds) [5]; (4) Carbon cannot meet the growing demands of highly eutrophic lakes [6]; and (5) CO2 can be seasonally and locally depleted when photosynthesis occurs [4]. Therefore, the problem of carbon sinks in terrestrial aquatic ecosystems deserves further attention.
Researchers have found that atmospheric CO2 can be converted into organic carbon via a series of physical, chemical, and biological processes and can be buried in sediments [7][8][9][10]. Compared to allochthonous sources [11], the organic matter (OM) component produced in situ by aquatic plants is termed autochthonous [9]; this OM is synthesized from dissolved CO2 (CO2aq), either photosynthetically or chemosynthetically, by submerged macrophytes, algae, and other microbial organisms. If the resulting autochthonous OM is deposited and buried in lake sediments, it can remove carbon from the short-term carbon cycle and form long-term stable carbon storage, which is similar to the marine biological carbon pump (BCP) effect [12]. Recent studies have revealed a BCP effect in which aquatic photosynthesis takes up DIC derived from carbonate weathering, and part of the generated autochthonous organic carbon is buried [5][9][10][13][14][15][16]. Researchers have presented the carbon sink mechanism of the carbonate-weathering-derived carbon sink coupled with aquatic photosynthesis (coupled carbonate weathering (CCW)), which indicates that carbonate weathering can form long-term effective carbon sinks via organic carbon burial [14][17].
Lakes are a significant component of terrestrial ecosystems, and their sediments provide a rich archive of information on the environmental history of lake watersheds [7][8]. The organic matter (OM) component of lake sediments is widely used to reconstruct paleoclimates and paleoenvironments, and to evaluate the anthropogenic impacts on local and regional ecosystems [9]. The transport of OM to lakes is a significant link in the global carbon cycle [10]. In addition to the significant part of the OM of lake sediments in terrestrial aquatic ecosystems, its significance is also reflected in its participation in nutrient cycles and the regulation of lake ecosystems [18]. As shown in Figure 1, contemporary anthropogenic activities such as land-cover changes and agricultural land-use have resulted in increased carbon (C) and nitrogen (N) discharge from soils into freshwater ecosystems [19][20][21][22], and thus strongly influenced the patterns of organic carbon (OC) input and trophic status [15][16]. Therefore, nutrient metabolism and the associated biogeochemical cycles are tightly coupled with aquatic photosynthetic production. It is well-known that in the transformation and flux of C and N, the main carrier is sedimentary organic matter [7]. In lake aquatic ecosystems, there is a coupled relationship between the water column and biogeochemical cycling in surface sediments [23]. Nutrients recycled within the sediments are liberated and subsequently diffused, and can then be supplied back to the overlying water and affect primary production via a somewhat complicated network of microbially-mediated biochemical reactions (Figure 1) [24].
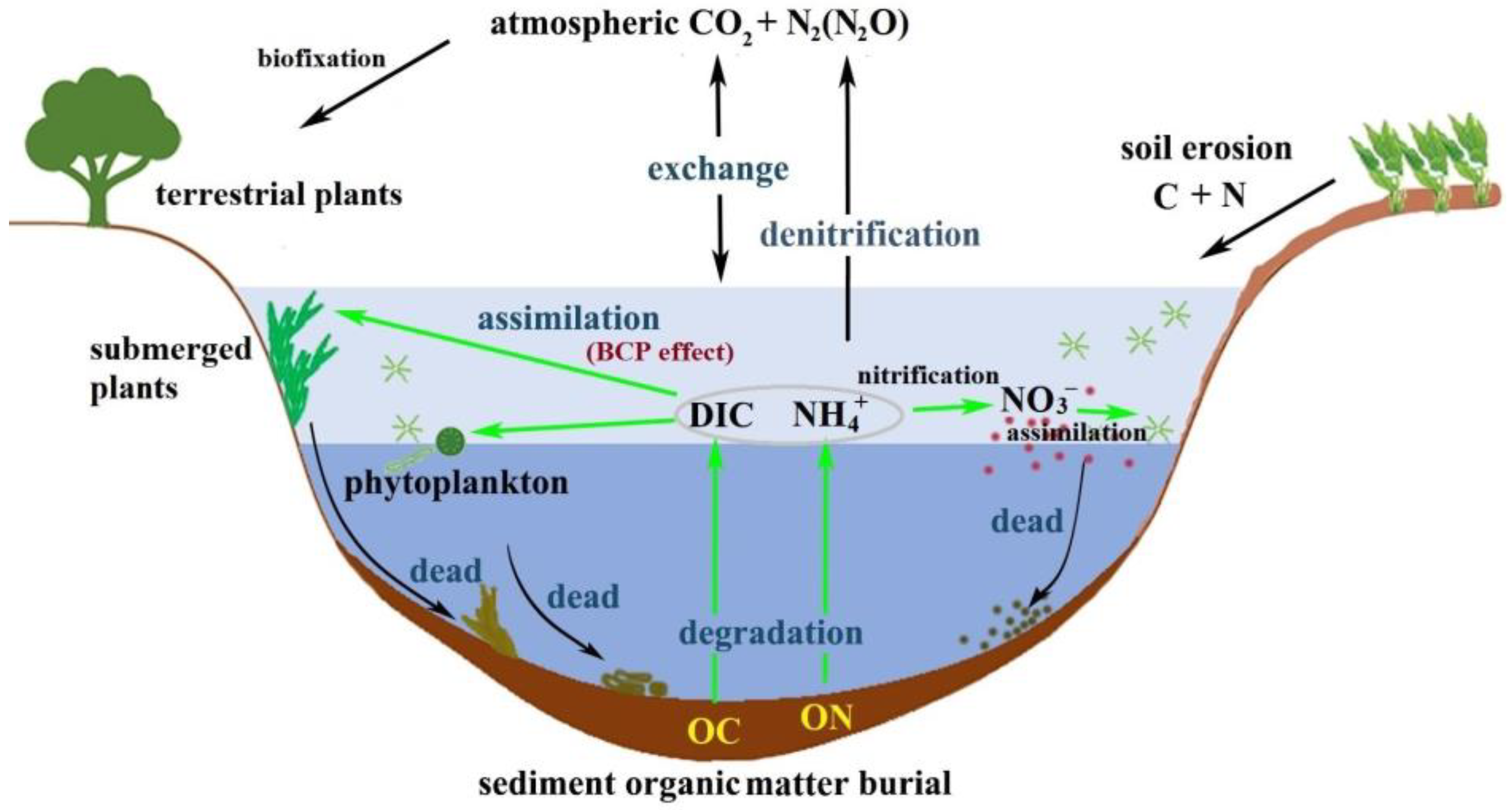
Figure 1. Schematic diagram showing the biogeochemical cycles influencing the organic matter of lake sediments.
2. Significance of the Spatial Distribution of Organic Matter Sources in Lake Sediments
Surface lake sediments have long been considered as the main reservoir of OM, buried in terrestrial aquatic ecosystems. Lakes, especially carbonate lakes, are an important carbon sink in terrestrial ecosystems [8]. The large-scale buried OM in sediments is largely accomplished by the coupling of high sediment deposition/accumulation rates [8] and high storage efficiency, which is 50 times greater than the ocean on average [25]. For lakes, especially large lakes, with complex sedimentary environments, it is important to recognize the spatial distribution of sediments and their composition when assessing a lake’s biogeochemical cycles; this is because the concentration and source may be spatially heterogeneous [26]. In addition, large quantities of nutrients may be released from fine-grained organic sediments at specific locations within the water body [27]. Therefore, it is necessary to map the distribution of sediments and their nutrient composition to create a spatially continuous surface in order to determine specific locations that will be targeted in the restoration of eutrophic lakes.
Various regional studies have confirmed the spatial heterogeneity of the organic components of lake sediments (e.g., in Australia (Coombabah Lake [23]), Asia (Nam Co Lake [28]), Europe (Sarbsko Lake [29]), and America (Pyramid Lake [30]). Numerous factors may influence the quantity of OM including the influx of terrestrial OM to the lake, aquatic productivity, various sediment properties, redox conditions, and the rate of microbial activity [23]. Generally, contributions from allochthonous and autochthonous OM, namely, from aquatic and terrestrial sources, have a direct–indirect impact on the spatio-temporal distribution of OM [26][29]. Thus, differentiating autochthonous and allochthonous sources of OM is a significant first process when evaluating the OM content of lake sediments.
3. Application of Tracer Methods in Determining the Sources of OM in Lake Surface Sediments
The diversity of OM sources depends not only on the biogeochemical characteristics of the lake watershed, but also on the social development status of urbanization and industrialization. Due to the large number of sinks and sources of OM in lake sediments, as above-mentioned, determining the constraints on its distribution is a challenging task [31]. However, as the major active component, OM is ultimately derived from allochthonous sources (terrestrial plant debris) or autochthonous sources (in situ primary production) in aquatic systems. Multiple bulk sediment parameters and molecular biomarkers have long been used to clarify the source of OM including C/N (atomic) ratios, stable organic carbon isotope ratios of OC (δ13Corg) [32], molecular biomarkers [33], and the combined use of stable carbon isotopes (δ13C) and radiocarbon isotopes (Δ14C) in sediments [34].
Any tracer method has its specific attributes including flaws. Essentially, the utilization of various parameters as source indices relies upon different OM sources having markedly different signatures. Conventional geochemical methods (e.g., δ13Corg and C/N) have often been used to clarify the sinks and sources of OM within lacustrine environments [35]. The C/N molar ratios of bacteria, terrestrial plants, soil, phytoplankton, and aquatic macrophytes fall within a specific range [36]. In general, phytoplankton contain more proteins but are low in fiber, and the molar ratios are usually less than 10 [35]; the protein contents of emergent macrophytes and terrestrial plants are generally low, but the fiber contents are rich, which leads to a relatively high C/N molar ratios (>20) [35]. The study found that the C/N molar ratios of phytoplankton vary from 5 to 8, with an average of ~6 in submerged plants of 12 or more [36]. The δ13Corg values for C4 plants fall between −17% and −9%, while that of terrestrial C3 plants is relatively negative, between −32% and −20% [35]. Macrophytes have more positive δ13Corg values (−30% to −5%) due to their C4-like photosynthesis pathway [37], while phytoplankton have relatively low δ13Corg values (−42% to −20%) due to C3-like photosynthesis, which leads to a large degree of δ13C fractionation [38]. Microbial sources (e.g., bacteria) in lake sediments can be indicated by their low δ13Corg values (−26%) [39]. However, the δ13Corg values are influenced by various environmental factors that may lead to ambiguous and overlapping signatures, and C/N is directly affected by the mineralization of OM. Nevertheless, Wilson et al. (2005) [40] considered that the combined use of C/N molar ratios and δ13Corg was an effective means of identifying OM sources. In general, the combined use of multiple indices can be expected to increase the degree of accuracy when determining the source of OM.
Researchers compiled research on the sources and composition of OM in the surface sediments of Fuxian Lake, the largest lake, and a karst rift lake at the source area of the Pearl River. Although Fuxian Lake is oligotrophic, several studies have shown that it is at risk of becoming polluted [41]. Several previous studies have been conducted on the source, migration, and transformation processes and mechanisms of OM in Fuxian Lake [20][42] as well as on the stability of a carbonate weathering carbon sink [6][9][13]. In order to clarify the impact of anthropogenic activities and regional environmental parameters on the ecosystem of Fuxian Lake, researchers analyzed the spatial distribution characteristics of TN, TOC, δ13Corg, and C/N in the surface sediments of Fuxian Lake, and researchers used the results to distinguish the main sources of OM at different water depths to provide a basis for an environmental assessment of the lake.
4. Evaluation of the Nutrient Content of Surface Sediments
Numerous approaches have been used to evaluate the status and health of lake ecosystems. Considering the rapid increase in the accumulation rate of organic matter in the surface sediments of Fuxian Lake, an organic index and the organic nitrogen content of the sediments were used to evaluate the degree of organic contamination, which has been proven to be effective [42][43][44]. The organic index (=Org − C(%) × Org − N(%)) is commonly applied to assess the level of the organic contamination of lake surface sediments, and the organic nitrogen content (TN(%) × 0.95) is an important index for determining the degree to which nitrogen pollution affects the surface sediments of a lake [42][43][44].
The organic index of Fuxian Lake ranged from 0.19 to 1.65, with an average of 0.66. This can be classed as grade IV, which indicates that there is a potential risk of organic pollution. Greater values occurred in the southern marginal area, especially the northern area, which is close to the mouth of one of the major inflowing streams. The organic nitrogen index values were 0.14%~0.43%, with a mean of 0.25%. These results show that there is a serious risk of nitrogen pollution, up to grade IV, throughout the lake, and that the distribution of organic nitrogen content is consistent with that of the organic index (Figure 2). High values occurred in areas of the lake where the catchment is dominated by tourism, agriculture, and forestry, both of which can supply large quantities of allochthonous pollutants, and the deposition of various nutrients and biological residues may be an additional reason for the observed distribution of organic nitrogen pollution. Although modern monitoring shows that Fuxian Lake is an oligotrophic lake and that the contribution of organic matter (mainly, organic carbon) within the lake is mainly autochthonous, there is a potential risk of surface sediments becoming significantly contaminated by organic pollution, especially organic nitrogen pollution. Although this risk has yet to be fully realized, possibly because of the depth of the lake, careful monitoring is required and control measures may need to be adopted in the future.
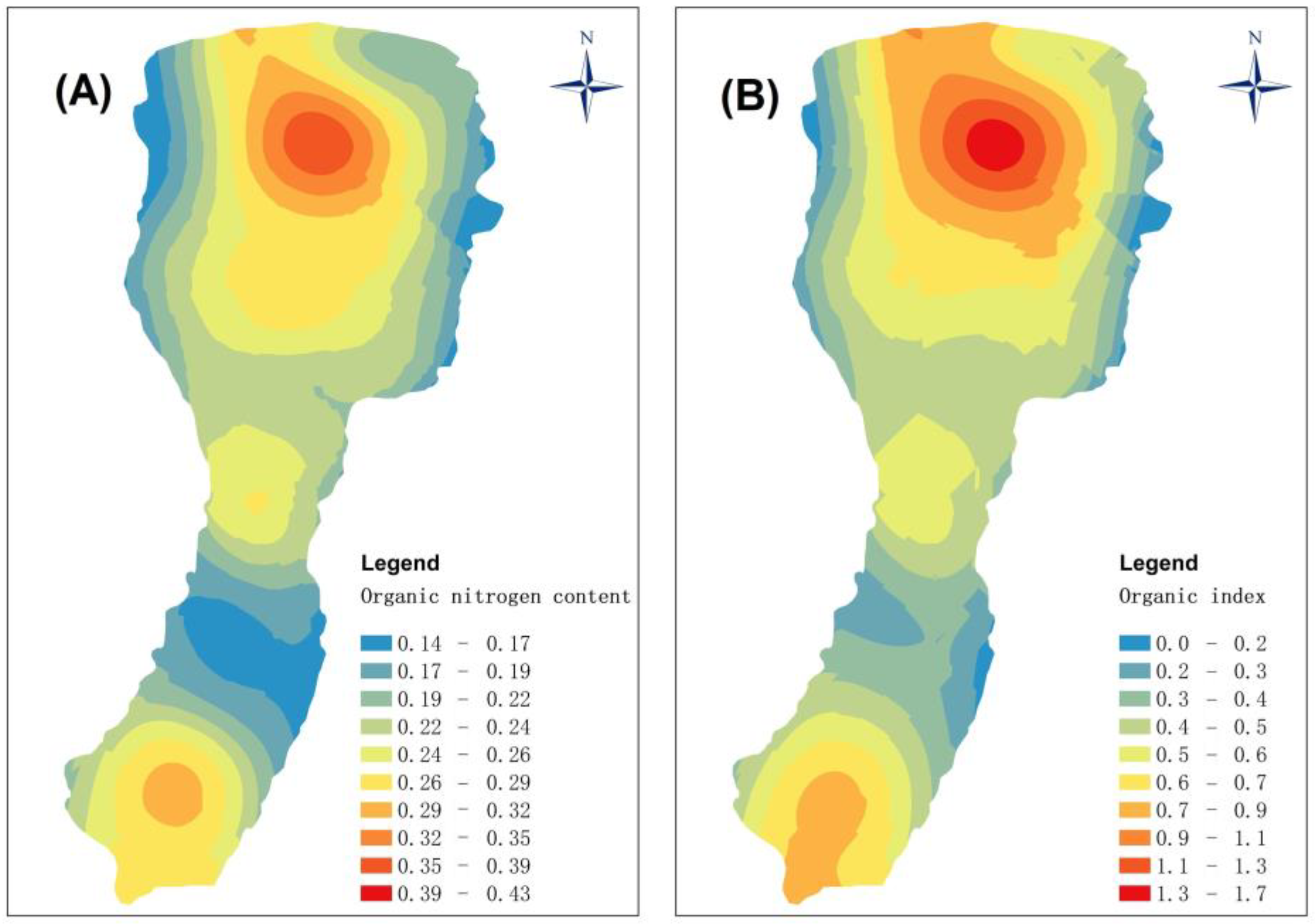
Figure 2. Spatial distribution of the organic nitrogen content (A) and organic index (B) for the surface sediment samples from Fuxian Lake.
References
- Hamdan, M.; Byström, P.; Hotchkiss, E.R.; Al-Haidarey, M.J.; Ask, J.; Karlsson, J. Carbon Dioxide Stimulates Lake Primary Production. Sci. Rep. 2018, 8, 10878.
- Balmer, M.B.; Downing, J.A. Carbon Dioxide Concentrations in Eutrophic Lakes: Undersaturation Implies Atmospheric Uptake. Inland Waters 2011, 1, 125–132.
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 126.
- Zeng, S.; Liu, H.; Liu, Z.; Kaufmann, G.; Zeng, Q.; Chen, B. Seasonal and Diurnal Variations in DIC, NO3− and TOC Concentrations in Spring-Pond Ecosystems under Different Land-Uses at the Shawan Karst Test Site, SW China: Carbon Limitation of Aquatic Photosynthesis. J. Hydrol. 2019, 574, 811–821.
- He, H.; Wang, Y.; Liu, Z.; Bao, Q.; Wei, Y.; Chen, C.; Sun, H. Lake Metabolic Processes and Their Effects on the Carbonate Weathering CO2 Sink: Insights from Diel Variations in the Hydrochemistry of a Typical Karst Lake in SW China. Water Res. 2022, 222, 118907.
- He, H.; LI, X. Study on the Utilization Efficiency of HCO3− by Aquatic Plants and the Buried Flux of Autochthonous Organic Carbon in Fuxian Lake. Quat. Sci. 2021, 41, 1140–1146.
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and Reservoirs as Regulators of Carbon Cycling and Climate. Limnol. Oceanogr. 2009, 54, 2298–2314.
- Mendonça, R.; Müller, R.A.; Clow, D.; Verpoorter, C.; Raymond, P.; Tranvik, L.J.; Sobek, S. Organic Carbon Burial in Global Lakes and Reservoirs. Nat. Commun. 2017, 8, 1694.
- He, H.; Liu, Z.; Chen, C.; Wei, Y.; Bao, Q.; Sun, H.; Yan, H. The Sensitivity of the Carbon Sink by Coupled Carbonate Weathering to Climate and Land-Use Changes: Sediment Records of the Biological Carbon Pump Effect in Fuxian Lake, Yunnan, China, during the Past Century. Sci. Total Environ. 2020, 720, 137539.
- Nõges, P.; Cremona, F.; Laas, A.; Martma, T.; Rõõm, E.-I.; Toming, K.; Viik, M.; Vilbaste, S.; Nõges, T. Role of a Productive Lake in Carbon Sequestration within a Calcareous Catchment. Sci. Total Environ. 2016, 550, 225–230.
- Dixon, R.K.; Turner, D. The Global Carbon Cycle and Climate Change: Responses and Feedbacks from below-Ground Systems. Environ. Pollut. 1991, 73, 245–262.
- Maavara, T.; Lauerwald, R.; Regnier, P.; Van Cappellen, P. Global Perturbation of Organic Carbon Cycling by River Damming. Nat. Commun. 2017, 8, 15347.
- He, H.; Liu, Z.; Chen, C.; Wei, Y.; Bao, Q.; Sun, H.; Hu, Y.; Yan, H. Influence of the Biological Carbon Pump Effect on the Sources and Deposition of Organic Matter in Fuxian Lake, a Deep Oligotrophic Lake in Southwest China. Acta Geochim. 2019, 38, 613–626.
- Liu, Z.; Macpherson, G.; Groves, C.; Martin, J.B.; Yuan, D.; Zeng, S. Large and Active CO2 Uptake by Coupled Carbonate Weathering. Earth-Sci. Rev. 2018, 182, 42–49.
- He, H.; Liu, G.; Zou, Y.; Li, X.; Ji, M.; Li, D. Coupled Action of Rock Weathering and Aquatic Photosynthesis: Influence of the Biological Carbon Pump Effect on the Sources and Deposition of Organic Matter in Ngoring Lake, Qinghai–Tibet Plateau, China. CATENA 2021, 203, 105370.
- He, H.; Liu, Z.; Li, D.; Zheng, H.; Zhao, J.; Chen, C.; Bao, Q.; Wei, Y.; Sun, H.; Yan, H. Recent Environmental Changes in the Yunnan–Guizhou Plateau Inferred from Organic Geochemical Records from the Sediments of Fuxian Lake. Elem. Sci. Anthr. 2021, 9, 068.
- Liu, Z.; Dreybrodt, W.; Wang, H. A New Direction in Effective Accounting for the Atmospheric CO2 Budget: Considering the Combined Action of Carbonate Dissolution, the Global Water Cycle and Photosynthetic Uptake of DIC by Aquatic Organisms. Earth-Sci. Rev. 2010, 99, 162–172.
- Clow, D.W.; Stackpoole, S.M.; Verdin, K.L.; Butman, D.E.; Zhu, Z.; Krabbenhoft, D.P.; Striegl, R.G. Organic Carbon Burial in Lakes and Reservoirs of the Conterminous United States. Environ. Sci. Technol. 2015, 49, 7614–7622.
- Qin, B.; Zhou, J.; Elser, J.J.; Gardner, W.S.; Deng, J.; Brookes, J.D. Water Depth Underpins the Relative Roles and Fates of Nitrogen and Phosphorus in Lakes. Environ. Sci. Technol. 2020, 54, 3191–3198.
- Chen, X.; Feng, M.; Ke, F.; Pan, J.; Fan, F.; Wang, Y.; Li, W. Source and biogeochemical distribution of organic matter in surface sediment in the deep oligotrophic lake fuxian, china. Aquat. Geochem. 2018, 24, 55–77.
- Low-DÉcarie, E.; Fussmann, G.F.; Bell, G. The Effect of Elevated CO2 on Growth and Competition in Experimental Phytoplankton Communities. Glob. Chang. Biol. 2011, 17, 2525–2535.
- Sandrini, G.; Cunsolo, S.; Schuurmans, J.M.; Matthijs, H.C.; Huisman, J. Changes in Gene Expression, Cell Physiology and Toxicity of the Harmful Cyanobacterium Microcystis Aeruginosa at Elevated CO2. Front. Microbiol. 2015, 6, 401.
- Dunn, R.J.; Welsh, D.T.; Teasdale, P.R.; Lee, S.Y.; Lemckert, C.J.; Méziane, T. Investigating the Distribution and Sources of Organic Matter in Surface Sediment of Coombabah Lake (Australia) Using Elemental, Isotopic and Fatty Acid Biomarkers. Cont. Shelf Res. 2008, 28, 2535–2549.
- Downing, J.A.; Watson, S.B.; McCauley, E. Predicting Cyanobacteria Dominance in Lakes. Can. J. Fish. Aquat. Sci. 2001, 58, 1905–1908.
- Einsele, G. Atmospheric Carbon Burial in Modern Lake Basins and Its Significance for the Global Carbon Budget. Glob. Planet. Chang. 2001, 30, 167–195.
- Yu, Z.; Wang, X.; Zhao, C.; Lan, H. Carbon Burial in Bosten Lake over the Past Century: Impacts of Climate Change and Human Activity. Chem. Geol. 2015, 419, 132–141.
- Rydin, E.; Malmaeus, J.; Karlsson, O.; Jonsson, P. Phosphorus Release from Coastal Baltic Sea Sediments as Estimated from Sediment Profiles. Estuar. Coast. Shelf Sci. 2011, 92, 111–117.
- Wang, Y.; Zhu, L.; Wang, J.; Ju, J.; Lin, X. The spatial distribution and sedimentary processes of organic matter in surface sediments of Nam Co, Central Tibetan Plateau. Chin. Sci. Bull. 2012, 57, 4753–4764.
- Woszczyk, M.; Bechtel, A.; Gratzer, R.; Kotarba, M.J.; Kokociński, M.; Fiebig, J.; Cieśliński, R. Composition and Origin of Organic Matter in Surface Sediments of Lake Sarbsko: A Highly Eutrophic and Shallow Coastal Lake (Northern Poland). Org. Geochem. 2011, 42, 1025–1038.
- Tenzer, G.E.; Myers, P.A.; Knoop, P. Sources and Distribution of Organic and Carbonate Carbon in Surface Sediments of Pyramid Lake, Nevada. J. Sediment. Res. 1997, 67, 884–890.
- Zhao, M.; Sun, H.; Liu, Z.; Bao, Q.; Chen, B.; Yang, M.; Yan, H.; Li, D.; He, H.; Wei, Y.; et al. Organic Carbon Source Tracing and the BCP Effect in the Yangtze River and the Yellow River: Insights from Hydrochemistry, Carbon Isotope, and Lipid Biomarker Analyses. Sci. Total Environ. 2022, 812, 152429.
- Tue, N.T.; Hamaoka, H.; Sogabe, A.; Quy, T.D.; Nhuan, M.T.; Omori, K. The Application of δ13C and C/N Ratios as Indicators of Organic Carbon Sources and Paleoenvironmental Change of the Mangrove Ecosystem from Ba Lat Estuary, Red River, Vietnam. Environ. Earth Sci. 2011, 64, 1475–1486.
- Huang, C.; Yao, L.; Zhang, Y.; Huang, T.; Zhang, M.; Zhu, A.-X.; Yang, H. Spatial and Temporal Variation in Autochthonous and Allochthonous Contributors to Increased Organic Carbon and Nitrogen Burial in a Plateau Lake. Sci. Total Environ. 2017, 603–604, 390–400.
- Chen, B.; Zhao, M.; Yan, H.; Yang, R.; Li, H.-C.; Hammond, D.E. Tracing Source and Transformation of Carbon in an Epikarst Spring-Pond System by Dual Carbon Isotopes (13C-14C): Evidence of Dissolved CO2 Uptake as a Carbon Sink. J. Hydrol. 2021, 593, 125766.
- Meyers, P.A. Applications of Organic Geochemistry to Paleolimnological Reconstructions: A Summary of Examples from the Laurentian Great Lakes. Org. Geochem. 2003, 34, 261–289.
- Tyson, R. Sedimentary Organic Matter: Organic Facies and Palynofacies; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012.
- Li, X.; Liu, W.; Xu, L. Evaluation of Lacustrine Organic δ13C as a Lake-Level Indicator: A Case Study of Lake Qinghai and the Satellite Lakes on the Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 532, 109274.
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537.
- Zigah, P.; Minor, E.; Werne, J.; McCallister, S.L. An Isotopic (Δ14C, δ13C, and δ15N) Investigation of Particulate Organic Matter and Zooplankton Biomass in Lake Superior and across a Size-Gradient of Aquatic Systems. Biogeosci. Discuss. 2012, 9, 4399–4439.
- Wilson, G.P.; Lamb, A.L.; Leng, M.J.; Gonzalez, S.; Huddart, D. δ13C and C/N as Potential Coastal Palaeoenvironmental Indicators in the Mersey Estuary, UK. Quat. Sci. Rev. 2005, 24, 2015–2029.
- Tang, F.; Huang, T.; Fan, R.; Luo, D.; Yang, H.; Huang, C. Temporal Variation in Sediment C, N, and P Stoichiometry in a Plateau Lake during Sediment Burial. J. Soils Sediments 2020, 20, 1706–1718.
- Zhang, Z.; Lv, Y.; Zhang, W.; Zhang, Y.; Sun, C.; Marhaba, T. Phosphorus, organic matter and nitrogen distribution characteristics of the surface sediments in Nansi Lake, China. Environ. Earth Sci. 2015, 73, 5669–5675.
- Sui, G. Statement and Evaluation of Organic Matter, Total Nitrogen and Total Phosphate in Surface Layer Sediments in Taihu Lake. J. Lake Sci. 1996, 8, 319–324.
- Lu, S.-Y.; Xu, M.-S.; Jin, X.-C.; Huang, G.-Z.; Hu, W. Pollution Characteristics and Evaluation of Nitrogen, Phosphorus and Organic Matter in Surface Sediments of Lake Changshouhu in Chongqing, China. Environ. Sci. 2012, 33, 393–398.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.9K
Revisions:
2 times
(View History)
Update Date:
01 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





