Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xing-Xing Fan | -- | 2810 | 2023-02-28 08:58:13 | | | |
| 2 | Rita Xu | -3 word(s) | 2807 | 2023-02-28 10:19:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xie, Y.; Liu, W.; Li, D.; Hou, J.; Coghi, P.S.; Fan, X. Vaccines in Solid Tumor. Encyclopedia. Available online: https://encyclopedia.pub/entry/41730 (accessed on 07 February 2026).
Xie Y, Liu W, Li D, Hou J, Coghi PS, Fan X. Vaccines in Solid Tumor. Encyclopedia. Available at: https://encyclopedia.pub/entry/41730. Accessed February 07, 2026.
Xie, Ya-Jia, Wen-Qian Liu, Dan Li, Jin-Cai Hou, Paolo Saul Coghi, Xing-Xing Fan. "Vaccines in Solid Tumor" Encyclopedia, https://encyclopedia.pub/entry/41730 (accessed February 07, 2026).
Xie, Y., Liu, W., Li, D., Hou, J., Coghi, P.S., & Fan, X. (2023, February 28). Vaccines in Solid Tumor. In Encyclopedia. https://encyclopedia.pub/entry/41730
Xie, Ya-Jia, et al. "Vaccines in Solid Tumor." Encyclopedia. Web. 28 February, 2023.
Copy Citation
Conventional vaccines are widely used to boost human natural ability to defend against foreign invaders, such as bacteria and viruses. Therapeutic cancer vaccines attracted the most attention for anti-cancer therapy. According to the main components, it can be divided into five types: cell, DNA, RNA, peptide, and virus-based vaccines. They mainly perform through two rationales: (1) it trains the host immune system to protect itself and effectively eradicate cancer cells; (2) these vaccines expose the immune system to molecules associated with cancer that enable the immune system to recognize and destroy cancer cells.
cancer vaccines
immunosuppressive TME
solid tumor
1. Background
Vaccines provide a new opportunity for the prevention and treatment of infectious diseases. The pandemic of COVID-19 promoted the rapid development of vaccine technology and made cancer vaccines re-emerge in public focus [1]. Cancer vaccines are active immunotherapies that use nucleic acid sequences, peptides, proteins, and exosomes containing tumor-specific antigens (TSAs) or tumor-associated antigens (TAAs) to induce a specific immune response and eventually suppress tumor growth. With the successful identification of tumor antigens, personalized neoantigens vaccines and immune checkpoint inhibitors that reverse tumor-induced immune depletion, cancer vaccines have been regarded as a potentially promising therapeutic strategy in the immunotherapy of solid tumors [2]. However, the antitumor efficiency of cancer vaccines is weakened and impaired due to the highly immunosuppressive characteristics of the tumor microenvironment (TME) (Figure 1) [3][4]. In recent years, combined cancer vaccines with various immunotherapies or standardized therapies have become an effective strategy to reverse immunosuppressive TME and improve clinical outcomes [5][6]. Moreover, the availability and low cost of high-throughput sequencing technology have led to the identification of many tumor neoantigens. The in-depth research on immune mechanisms and various new vaccine platforms have widely promoted the research of cancer vaccines.
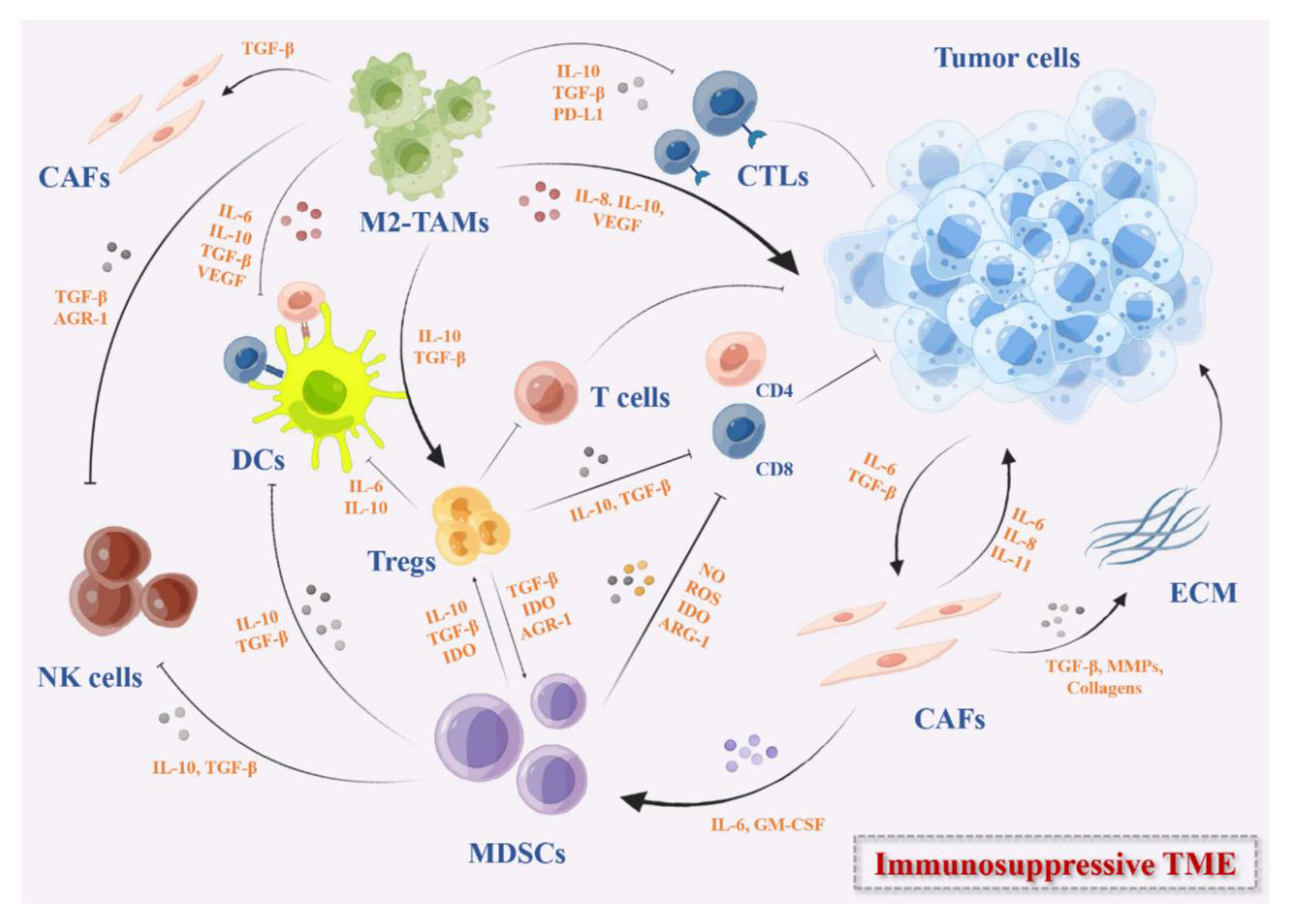
Figure 1. The immunosuppressive TME in solid tumors. These immunosuppressive cells include MDSCs, DCs, M2-TAMs, Tregs, and CAFs. They secrete immunosuppressive cytokines such as IL-10, IDO, TGF-β, growth factors such as VEGF, the checkpoints ligands such as PD-L1, or express checkpoints on the cell surface that can inhibit the activation of DC-mediated T cells and effector T cells directly or indirectly, remodel the ECM, and promote the angiogenesis in TME.
2. Cell-Based Cancer Vaccines
Cell-based cancer vaccines are the main form of original cancer vaccine. For instance, dendritic cell [7] is a specialized antigen-presenting cell and plays a vital role in initiating a specific T cell response in innate antitumor immunity [8]. The dendritic cell-based [7] vaccine has achieved significant results in clinical trials. It is capable of presenting cancer antigens through MHC-I and MHC-II molecules, thereby initiating an antigen-specific immune response [9][10]. The first FDA-approved DC-based vaccine Sipuleucel-T was successfully used for the treatment of metastatic prostate cancer in 2020 [11]. Studies have indicated that Sipuleucel-T prolonged the overall survival of patients with prostate cancer and reduced the risk of death [12]. Although DCs inhibit tumor growth, tumor-infiltrating DCs usually show impaired or defective function in various tumors which exacerbate immunosuppressive effects and promote tumor development [13][14]. In addition, various types of immune cells such as tumor-associated macrophages (TAMs), myelogenous inhibitory cells (MDSCs), and regulatory T cells (Tregs) in TME also inhibit the effector T cell response and release cytokines to affect the function of DCs [15].
To enhance the anti-cancer immune response, many DC vaccines have been prepared and loaded with various TAAs or adjuvants to development of vaccines against TME, which mainly focuses on five categories: autologous dendritic cells, autologous dendritic cells loaded with tumor lysates, autologous DC transfected or pulsed with TAA-encoded RNA, autologous DC loaded with recombinant TAAs or TAA-derived peptides, and other DCs [16]. TAA targets are expressed at high levels in different tumor cells, and the most common TAAs include MUC1, WT1, CEA, mesothelin, and mutated KRAS [17]. It is generally suggested that immature DCs induce tolerance to itself, while mature DCs resist foreign antigens and exercise immune response. Therefore, stimulating mature DCs is the primary key factor for vaccine preparation [18]. The activation of DC vaccine currently mainly adopts “mature cocktail” therapy composed of proinflammatory cytokines TNF-α, IL-1β, IL-6, and Toll-like receptor agonists. The monocyte-derived DCs (MoDCs) exposure to a “maturation cocktail” while loaded with antigens enhances antigens capture, processing, and presentation on MHC I and MHC II molecules, increases the expression of co-stimulatory molecules CD80 and CD86, and induces DCs to initiate immature T cells [19].
The selection of appropriate antigens and antigens loading methods is crucial for DC vaccine to stimulate immune response. Common tumor antigens include tumor lysates, specific TAA-based peptides, protein, mRNA, and even whole tumor [20]. The whole tumor lysates contain a variety of tumor antigens, such as TSAs. However, other unrelated antigens are also present in the tumor lysates, resulting in decreased specificity that hinders antigens processing and presentation of DCs [21]. Peptide- or protein-based DC vaccines can reduce the incidence of autoimmune-related adverse reactions while maintaining tumor selectivity [17]. Peptides can be loaded directly onto MHC-I and MHC-II molecules on the DCs surface whereas protein and tumor cell MHC-I pathways are not specifically targeted and need to be processed and presented by DCs to induce T cells [22]. In contrast to peptide-based DC vaccines, the advantage of protein-based DC vaccines is not limited to selected haplotypes. Multiple epitopes appear on different haplotypes, thereby inducing an immune response against a broad spectrum of antigens [23]. Gene-edited DC is another effective antigen-loading method, transfecting mRNA encoding TSAs or TAAs into DCs, which avoids the need to identify haplotypes in patients and induces T cell immune response [10]. In addition, the combination of cancer vaccine with currently used cancer therapies such as radiotherapy, chemotherapy, immune checkpoint inhibitors (ICIs), and adoptive T cell therapy is an effective method to improve immunogenicity and inhibit the growth of malignant tumors.
3. DNA-Based Vaccine
DNA vaccines are now considered as a potential strategy to fight solid tumors by activating the immune system. Compared with traditional vaccines, DNA vaccines have shown great advantages in many aspects: (1) inducing both humoral immunity and cellular immunity; (2) simple and flexible design; (3) high safety, no pathogen infection risk, less adverse reactions; (4) and low cost and high production speed, and is suitable for large-scale production [24].
DNA vaccines are double-stranded nucleotides that encode a specific tumor antigen-encoding gene or immunostimulatory molecule that is transported to the host cell by a variety of delivery methods. DNA vaccines reach the cytoplasm through the cell membrane of APC and migrate to the nucleus for replication, transcription, and antigen production. The host cells express the target antigen and present the antigen through the MHC signaling pathway, thereby activating CD4+ T cells and CD8+ T cells and inducing immune responses [25]. DNA vaccines with built-in unmethylated CpG motif can bias the immune response to Th1, which is conducive to the induction of CTLs to kill the tumor, with a strong immune stimulation [26].
Although DNA vaccines have been shown to enhance antitumor immune responses, they are generally less immunogenic and less effective in clinical trials, primarily due to different resistance mechanisms during tumor development [27]. Therefore, optimizing the delivery system is essential to induce an effective immune response against tumor-associated antigens. The most common delivery methods of DNA vaccine are intradermal (ID) delivery and intramuscular (IM) delivery. Compared with IM delivery, ID delivery induces enhanced expression of antigens, leading to higher immunogenicity. Due to the high density of complex DCs network in dermis, the antigens are better exposed to DCs to initiate the immune response, thereby ID is the most suitable route for DNA delivery [28]. In recent years, several physical and chemical methods have been developed for DNA vaccine delivery, including gene gun delivery, electroporation, microneedles arrays, liposomes, virosomes, and nanoparticles [28][29]. Thus, optimizing the delivery system is a potential method to enhance the immunogenicity of DNA vaccines.
In addition, adjuvants are used as immunostimulatory to enhance the immunogenicity of antigens, so the development of new DNA vaccine adjuvants also significantly affects the efficacy of DNA vaccines [30]. CpG oligonucleotide (CpG ODN) activates the innate immune system and increases the number of CD8+ T cells by binding to intracellular homologous TLR-9 receptors [31]. Many cytokines that enhance cellular and humoral immune responses have been used as DNA vaccine adjuvants such as chemokines, interleukins, granulocyte/macrophage colony-stimulating factor (GM-CSF), co-stimulatory molecules, and signaling molecules to induce the immune response via Th1 and Th2 cellular pathways [32]. Studies have revealed that codon-optimized GM-CSF linked to DNA vaccine boosts IFN-γ production in specific CD8+ T cells and CD4+ T cells and polarizes Th1 immune response [33]. The plenty of DNA vaccine experiments with adjuvants have been conducted in mice or other animals, but few experiments have been conducted in human bodies, thus pending further, more in-depth research and.
4. RNA-Based Vaccine
The FDA approval of two kinds of COVID-19 mRNA vaccines (mRNA-1273 and BNT162b2) to respond to the COVID-19 pandemic has generated widespread interest in mRNA vaccines [34]. Similar to DNA vaccines, mRNA vaccines also induce both humoral and cellular immunity. Rationally, the mRNA encoding TSAs or TAAs enters the cytoplasm to bind with the ribosome of the host cell and translate. The antigenic proteins are degraded by the proteasome in the cytoplasm into antigenic peptides that are loaded onto MHC I for antigen-specific CD8 T cell activation. Cross-presentation of extracellular proteins on MHC I or loading onto MHC II activates CD4 T cells [35][36].
RNA vaccines have more advantages compared with DNA vaccine: mRNA is translated in splinter cells and non-splinter cells. Unlike DNA vaccines that need to migrate to the nucleus, mRNA only needs to be transferred into cytoplasm, and mRNA protein expression rate and quantity are generally higher than DNA vaccines; the mRNA vaccine is not integrated into the host genome sequence, and there is no risk of infection or insertion mutation [37][38]. However, there are some limitations in mRNA vaccines development. On the one side, the naked mRNA is rapidly degraded by extracellular RNases. On the other side, mRNA has inherent immunogenicity, which activates interferon-related reactions to further promote mRNA degradation, leading to decreased antigen expression [39].
The applications of mRNA vaccines have been limited by inefficient in vivo delivery. The mRNA are macromolecular substances that are unable to reach the cytoplasm through the lipid bilayer membrane of cell membrane, greatly limiting its clinical application. In order to solve the problem that mRNA is difficult to transmit through the cell membrane, different vectors have been developed to deliver mRNA, mainly including viral vectors, non-viral vectors, and dendritic cell-based vectors. Among many carriers, lipid nanoparticles (LNPs) are the most widely used delivery vehicles, which usually consist of four parts: (1) ionizable or cationic lipids for interaction with mRNA molecules; (2) auxiliary phospholipids similar to phospholipid bilayer; (3) cholesterol analog for stabilizing that LNP structure; (4) and polyethylene glycol (PEG) [40]. The ionizable lipid is a determining factor in the potency of the LNP, as it is positively charged at acidic pH and enhances the encapsulation of negatively charged mRNA by electrostatic interaction. In acidic environments, positively charged lipids interact with the ionic endosome membrane to promote membrane fusion and destabilization, resulting in mRNA release from the LNP and endosome [37]. However, ionizable lipids are essentially unchanged at physiological pH, which is a physiological property to promote endosome escape of mRNA.
A number of clinical studies have been conducted on mRNA packaged with LNP. The mRNA-4157 vaccine is a personalized mRNA vaccine encoding multiple antigens and delivering via LNP developed by Moderna in the United States [41]. Two clinical studies on the safety, tolerability, and immunogenicity of mRNA-4157 combined with pembrolizumab in the treatment of solid tumors are ongoing (NCT03313778/ NCT03897881). MRNA-4157 has shown remarkable safety and tolerability and induced potent antigen-specific T cell response.
Transfection of mRNA into DC was the first mRNA-based vaccine to enter clinical trials. At present, there are two delivery methods of DC-based mRNA vaccine, i.e., in vitro loaded DCs and in vivo targeted DCs. Although the procedure of ex vivo loading of DCs is complex and costly, it can achieve accurate antigen stimulation and high-efficiency transfection. DC-based mRNA vaccine is loaded in vitro by obtaining immature DCs from peripheral blood of patients, loading antigen-encoded mRNA after cells maturation, and returning to patients to initiate immune response and exert anti-cancer activity [42][43].
In a Phase I/II study, the immune response following vaccination with dendritic cells via mRNA electroporation with single-step antigen loading and TLR activation was explored in patients with stage III and IV melanoma. Participants were melanoma patients who demonstrated expression of melanoma-associated tumor antigen gp100 and tyrosinase. The results showed that intranodal administration of mRNA-optimized DC exerted great feasibility and safety, but limited TAA-specific immune response was observed (NCT01530698) [44]. In another Phase I/II trial of vaccine therapy with mRNA-transfected DCs in patients with advanced malignant melanoma, 16 of 31 patients showed tumor-specific immune responses, and the survival rate of those with responders was improved compared with non-responders. Most patients also respond to autologous DC antigens (NCT01278940) [45].
5. Peptide-Based Cancer Vaccines
Peptide-based cancer vaccines, usually consisting of a series of amino acids derived from tumor antigen or immune activating peptide from bacteria or other hosts, offer a strong immune stimulating effect [46][47]. The peptide-based vaccine has the advantages of convenient production, high speed, low carcinogenic potential, excellent safety profiles, insusceptible pathogen contamination, high chemical stability, low cost, and easy storage [46][48]. However, peptide-based vaccines are easily degraded by enzymes and have weak immunogenicity, which are difficult to induce robust and long-term immune response.
In order to promote the immunogenicity of peptide-based vaccines, it is important to optimize the sequence length of the peptide. Short peptides, approximately 8 to 12 amino acids in length, are presented without passing through a professional APC and directly bind to MHC I molecules of APCs, resulting in temporary T cell response and immune tolerance [49][50][51]. MHC II molecules can be combined with long peptides with a length of 12–20 amino acids. The peptides are assembled into peptide–MHC II complexes, which are delivered to the cell surface to be recognized by CD4+ T helper cells, triggering a specific T cell reaction and migrating to the tumor microenvironment to play an immune mechanism to inhibit tumor growth [50][52]. Therefore, long peptide vaccines are more likely to induce sustained and effective antitumor activity responses.
The use of adjuvants protects the antigens from degradation and enhances specific immune response to antigens. TLR agonists have proven to be a promising adjuvant for peptide-based vaccines [53][54][55]. TLR is a pattern recognition receptor (PRR) that recognizes pathogen-associated molecular patterns (PAMPs). TLR is able to absorb antigens and provide key cytokines to stimulate and mediate TH1 and TH17 immune responses [53]. Studies have assembled new antigenic peptide and CpG ODN to form PCNPs nanocomposites, which are capable of simultaneously delivering new antigenic peptide and adjuvant to protect CpG ODN from nuclease-mediated degradation in serum, inducing effective an antigen presentation process and activating antigen-specific T cells [53][55]. Moreover, the combination of peptide vaccine with ICIs has achieved a very significant effect on tumor regression [7][56].
6. Virus-Based Cancer Vaccines
Most viruses have natural immunogenicity, and their genetic material can be engineered to contain sequences encoding tumor antigens. Besides inducing local immune responses, local administration of many virus-based cancer vaccines also initiates systemic immune response, resulting in “abscopal effect”. The series of immune responses caused by virus infection eventually achieve effective and persistent antitumor immunity. Virus-based cancer vaccines are mainly divided into three forms: oncolytic virus vaccines, virus vector vaccines, and inactivated, live-attenuated or subunit vaccines against viruses that can induce tumors [57][58].
According to the report, an estimated 13% of cancers are related to viral infections in worldwide [59]. So far, hepatitis B virus (HBV), hepatitis C virus (HCV), human papillomavirus (HPV), merkel cell polyomavirus (MCV), Epstein–Barr virus (EBV), human herpesvirus type 8 (HHV-8), human T cell lymphotropic virus type 1 (HTLV-1), and human immunodeficiency virus (HIV) are common carcinogenic viruses in humans [60]. These DNA and RNA viruses produce carcinogenic effects via several different distinct mechanisms [61]. At present, many types of preventive vaccines have been used for HPV and HBV in clinical trials, but they provide limited benefits for eliminating pre-existing infections [62][63][64][65]. Moreover, therapeutic vaccines are urgently required to reduce the burden of the virus-related precancerous lesions and cancers.
Viruses are commonly used as vaccine vectors for gene delivery, owing to low cost and relative ease of production, purification, and storage [57]. The main types of virus vectors are adenovirus, alphavirus, poxviral (fowlpox, canarypox (ALVAC), vaccinia virus, and modified virus Ankara), and oncolytic virus (measles virus, herpes simplex virus (HSV), and vesicular stomatitis virus). Many studies have inserted TAAs, proinflammatory cytokines (GM-CSF, TNF-α, IL-2, IL-7, IL-12, and IL-23) and chemokines into the viral genome to intensify T cell activation and augment immune cell recruitment, leading to obtain better immune stimulation effects [66][67][68].
Oncolytic viruses, as an emerging immunotherapeutic agent, are able to expressly kill tumor cells and reverse immunosuppression by modulating TME components [69]. Talimogene laherparepvec (T-VEC), as a genetically modified herpes simplex oncolytic virus, was used in a phase II study of patients with unresectable stage IIIB-IV melanoma [70]. This study revealed that T-VEC induced systemic immune activity and revised the immunosuppressive TME, thus expanding the curative effect of other immunotherapeutic drugs in combination therapy [71][72].
References
- Jhaveri, R. The COVID-19 mRNA Vaccines and the Pandemic: Do They Represent the Beginning of the End or the End of the Beginning? Clin. Ther. 2021, 43, 549–556.
- Gupta, M.; Wahi, A.; Sharma, P.; Nagpal, R.; Raina, N.; Kaurav, M.; Bhattacharya, J.; Rodrigues Oliveira, S.M.; Dolma, K.G.; Paul, A.K.; et al. Recent Advances in Cancer Vaccines: Challenges, Achievements, and Futuristic Prospects. Vaccines 2022, 10, 2011.
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220.
- Tie, Y.; Tang, F.; Wei, Y.Q.; Wei, X.W. Immunosuppressive cells in cancer: Mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022, 15, 61.
- Kim, C.G.; Sang, Y.B.; Lee, J.H.; Chon, H.J. Combining Cancer Vaccines with Immunotherapy: Establishing a New Immunological Approach. Int. J. Mol. Sci. 2021, 22, 8035.
- Joshi, S.; Durden, D.L. Combinatorial Approach to Improve Cancer Immunotherapy: Rational Drug Design Strategy to Simultaneously Hit Multiple Targets to Kill Tumor Cells and to Activate the Immune System. J. Oncol. 2019, 2019, 5245034.
- Gibney, G.T.; Kudchadkar, R.R.; DeConti, R.C.; Thebeau, M.S.; Czupryn, M.P.; Tetteh, L.; Eysmans, C.; Richards, A.; Schell, M.J.; Fisher, K.J.; et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin. Cancer Res. 2015, 21, 712–720.
- Randolph, G.J. Dendritic cells: The first step. J. Exp. Med. 2021, 218, e20202077.
- Mildner, A.; Jung, S. Development and function of dendritic cell subsets. Immunity 2014, 40, 642–656.
- Baldin, A.V.; Savvateeva, L.V.; Bazhin, A.V.; Zamyatnin, A.A., Jr. Dendritic Cells in Anticancer Vaccination: Rationale for Ex Vivo Loading or In Vivo Targeting. Cancers 2020, 12, 590.
- Handy, C.E.; Antonarakis, E.S. Sipuleucel-T for the treatment of prostate cancer: Novel insights and future directions. Future Oncol. 2018, 14, 907–917.
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422.
- Ma, Y.; Shurin, G.V.; Peiyuan, Z.; Shurin, M.R. Dendritic cells in the cancer microenvironment. J. Cancer 2013, 4, 36–44.
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059.
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089.
- Laureano, R.S.; Sprooten, J.; Vanmeerbeerk, I.; Borras, D.M.; Govaerts, J.; Naulaerts, S.; Berneman, Z.N.; Beuselinck, B.; Bol, K.F.; Borst, J.; et al. Trial watch: Dendritic cell (DC)-based immunotherapy for cancer. Oncoimmunology 2022, 11, 2096363.
- Yang, J.; Shangguan, J.; Eresen, A.; Li, Y.; Wang, J.; Zhang, Z. Dendritic cells in pancreatic cancer immunotherapy: Vaccines and combination immunotherapies. Pathol. Res. Pract. 2019, 215, 152691.
- Reis e Sousa, C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006, 6, 476–483.
- Kaur, A.; Baldwin, J.; Brar, D.; Salunke, D.B.; Petrovsky, N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr. Opin. Chem. Biol. 2022, 70, 102172.
- Sprooten, J.; Ceusters, J.; Coosemans, A.; Agostinis, P.; De Vleeschouwer, S.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; Garg, A.D. Trial watch: Dendritic cell vaccination for cancer immunotherapy. Oncoimmunology 2019, 8, e1638212.
- Zhou, J.; Li, L.; Jia, M.; Liao, Q.; Peng, G.; Luo, G.; Zhou, Y. Dendritic cell vaccines improve the glioma microenvironment: Influence, challenges, and future directions. Cancer Med. 2022, 1–15.
- Constantino, J.; Gomes, C.; Falcao, A.; Neves, B.M.; Cruz, M.T. Dendritic cell-based immunotherapy: A basic review and recent advances. Immunol. Res. 2017, 65, 798–810.
- Sabado, R.L.; Bhardwaj, N. Directing dendritic cell immunotherapy towards successful cancer treatment. Immunotherapy 2010, 2, 37–56.
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3153–3164.
- Vellios, N.; van der Zee, K. Dataset on cigarette smokers in six South African townships. Data Brief 2020, 32, 106260.
- Cui, Z. DNA vaccine. Adv. Genet. 2005, 54, 257–289.
- Lopes, A.; Vandermeulen, G.; Preat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146.
- Jorritsma, S.H.T.; Gowans, E.J.; Grubor-Bauk, B.; Wijesundara, D.K. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 2016, 34, 5488–5494.
- Eusebio, D.; Neves, A.R.; Costa, D.; Biswas, S.; Alves, G.; Cui, Z.; Sousa, A. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov. Today 2021, 26, 2575–2592.
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Pappa, A.; Chlichlia, K. DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy. Pharmacol. Ther. 2016, 165, 32–49.
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511.
- Chen, Y.P.; Lin, C.C.; Xie, Y.X.; Chen, C.Y.; Qiu, J.T. Enhancing immunogenicity of HPV16 E(7) DNA vaccine by conjugating codon-optimized GM-CSF to HPV16 E(7) DNA. Taiwan J. Obstet. Gynecol. 2021, 60, 700–705.
- Shrestha, A.C.; Wijesundara, D.K.; Masavuli, M.G.; Mekonnen, Z.A.; Gowans, E.J.; Grubor-Bauk, B. Cytolytic Perforin as an Adjuvant to Enhance the Immunogenicity of DNA Vaccines. Vaccines 2019, 7, 38.
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.S. From COVID-19 to Cancer mRNA Vaccines: Moving from Bench to Clinic in the Vaccine Landscape. Front. Immunol. 2021, 12, 679344.
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582.
- Lorentzen, C.L.; Haanen, J.B.; Met, O.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458.
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41.
- Wu, Z.; Li, T. Nanoparticle-Mediated Cytoplasmic Delivery of Messenger RNA Vaccines: Challenges and Future Perspectives. Pharm. Res. 2021, 38, 473–478.
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharmaceut. Med. 2022, 36, 11–20.
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854.
- mRNA-4157 Cancer Vaccine. Available online: https://www.precisionvaccinations.com/vaccines/mrna-4157-cancer-vaccine (accessed on 5 November 2021).
- Bidram, M.; Zhao, Y.; Shebardina, N.G.; Baldin, A.V.; Bazhin, A.V.; Ganjalikhany, M.R.; Zamyatnin, A.A., Jr.; Ganjalikhani-Hakemi, M. mRNA-Based Cancer Vaccines: A Therapeutic Strategy for the Treatment of Melanoma Patients. Vaccines 2021, 9, 1060.
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728.
- Bol, K.F.; Figdor, C.G.; Aarntzen, E.H.; Welzen, M.E.; van Rossum, M.M.; Blokx, W.A.; van de Rakt, M.W.; Scharenborg, N.M.; de Boer, A.J.; Pots, J.M.; et al. Intranodal vaccination with mRNA-optimized dendritic cells in metastatic melanoma patients. Oncoimmunology 2015, 4, e1019197.
- Kyte, J.A.; Aamdal, S.; Dueland, S.; Saeboe-Larsen, S.; Inderberg, E.M.; Madsbu, U.E.; Skovlund, E.; Gaudernack, G.; Kvalheim, G. Immune response and long-term clinical outcome in advanced melanoma patients vaccinated with tumor-mRNA-transfected dendritic cells. Oncoimmunology 2016, 5, e1232237.
- Liu, W.; Tang, H.; Li, L.; Wang, X.; Yu, Z.; Li, J. Peptide-based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif. 2021, 54, e13025.
- Abd-Aziz, N.; Poh, C.L. Development of Peptide-Based Vaccines for Cancer. J. Oncol. 2022, 2022, 9749363.
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854.
- Bijker, M.S.; van den Eeden, S.J.; Franken, K.L.; Melief, C.J.; Offringa, R.; van der Burg, S.H. CD8 + CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J. Immunol. 2007, 179, 5033–5040.
- Yang, J.; Zhang, Q.; Li, K.; Yin, H.; Zheng, J.N. Composite peptide-based vaccines for cancer immunotherapy (Review). Int. J. Mol. Med. 2015, 35, 17–23.
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; de Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299.
- Zhang, J.; Fan, J.; Skwarczynski, M.; Stephenson, R.J.; Toth, I.; Hussein, W.M. Peptide-Based Nanovaccines in the Treatment of Cervical Cancer: A Review of Recent Advances. Int. J. Nanomed. 2022, 17, 869–900.
- Liang, Z.; Cui, X.; Yang, L.; Hu, Q.; Li, D.; Zhang, X.; Han, L.; Shi, S.; Shen, Y.; Zhao, W.; et al. Co-assembled nanocomplexes of peptide neoantigen Adpgk and Toll-like receptor 9 agonist CpG ODN for efficient colorectal cancer immunotherapy. Int. J. Pharm. 2021, 608, 121091.
- Ammi, R.; de Waele, J.; Willemen, Y.; van Brussel, I.; Schrijvers, D.M.; Lion, E.; Smits, E.L. Poly(I:C) as cancer vaccine adjuvant: Knocking on the door of medical breakthroughs. Pharmacol. Ther. 2015, 146, 120–131.
- Kano, Y.; Iguchi, T.; Matsui, H.; Adachi, K.; Sakoda, Y.; Miyakawa, T.; Doi, S.; Hazama, S.; Nagano, H.; Ueyama, Y.; et al. Combined adjuvants of poly(I:C) plus LAG-3-Ig improve antitumor effects of tumor-specific T cells, preventing their exhaustion. Cancer Sci. 2016, 107, 398–406.
- Tanaka, Y.; Wada, H.; Goto, R.; Osada, T.; Yamamura, K.; Fukaya, S.; Shimizu, A.; Okubo, M.; Minamiguchi, K.; Ikizawa, K.; et al. TAS0314, a novel multi-epitope long peptide vaccine, showed synergistic antitumor immunity with PD-1/PD-L1 blockade in HLA-A*2402 mice. Sci. Rep. 2020, 10, 17284.
- Larocca, C.; Schlom, J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011, 17, 359–371.
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6.
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190.
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616.
- Tashiro, H.; Brenner, M.K. Immunotherapy against cancer-related viruses. Cell Res. 2017, 27, 59–73.
- Ciesielska, U.; Nowinska, K.; Podhorska-Okolow, M.; Dziegiel, P. The role of human papillomavirus in the malignant transformation of cervix epithelial cells and the importance of vaccination against this virus. Adv. Clin. Exp. Med. 2012, 21, 235–244.
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102.
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224, S343–S351.
- Glebe, D.; Goldmann, N.; Lauber, C.; Seitz, S. HBV evolution and genetic variability: Impact on prevention, treatment and development of antivirals. Antivir. Res. 2021, 186, 104973.
- Ye, J.F.; Qi, W.X.; Liu, M.Y.; Li, Y. The combination of NK and CD8+T cells with CCL20/IL15-armed oncolytic adenoviruses enhances the growth suppression of TERT-positive tumor cells. Cell Immunol. 2017, 318, 35–41.
- Chen, L.; Chen, H.; Ye, J.; Ge, Y.; Wang, H.; Dai, E.; Ren, J.; Liu, W.; Ma, C.; Ju, S.; et al. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics 2021, 11, 6668–6681.
- Nakao, S.; Arai, Y.; Tasaki, M.; Yamashita, M.; Murakami, R.; Kawase, T.; Amino, N.; Nakatake, M.; Kurosaki, H.; Mori, M.; et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl. Med. 2020, 12, eaax7992.
- Zhang, Y.; Li, Y.; Chen, K.; Qian, L.; Wang, P. Oncolytic virotherapy reverses the immunosuppressive tumor microenvironment and its potential in combination with immunotherapy. Cancer Cell Int. 2021, 21, 262.
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788.
- Johnson, D.B.; Puzanov, I.; Kelley, M.C. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 2015, 7, 611–619.
- Bommareddy, P.K.; Patel, A.; Hossain, S.; Kaufman, H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017, 18, 1–15.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
721
Revisions:
2 times
(View History)
Update Date:
28 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




