Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angela Picerno | -- | 1094 | 2023-02-27 17:16:52 | | | |
| 2 | Camila Xu | Meta information modification | 1094 | 2023-02-28 03:15:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sallustio, F.; Picerno, A.; Montenegro, F.; Cimmarusti, M.T.; Di Leo, V.; Gesualdo, L. The Human Virome. Encyclopedia. Available online: https://encyclopedia.pub/entry/41709 (accessed on 07 February 2026).
Sallustio F, Picerno A, Montenegro F, Cimmarusti MT, Di Leo V, Gesualdo L. The Human Virome. Encyclopedia. Available at: https://encyclopedia.pub/entry/41709. Accessed February 07, 2026.
Sallustio, Fabio, Angela Picerno, Francesca Montenegro, Maria Teresa Cimmarusti, Vincenzo Di Leo, Loreto Gesualdo. "The Human Virome" Encyclopedia, https://encyclopedia.pub/entry/41709 (accessed February 07, 2026).
Sallustio, F., Picerno, A., Montenegro, F., Cimmarusti, M.T., Di Leo, V., & Gesualdo, L. (2023, February 27). The Human Virome. In Encyclopedia. https://encyclopedia.pub/entry/41709
Sallustio, Fabio, et al. "The Human Virome." Encyclopedia. Web. 27 February, 2023.
Copy Citation
Members of the virome may collaborate with the human host to retain mutualistic functions in preserving human health. Although a person’s virome can alter over time, viral presence in a person can be extremely stable. Since viruses are present in the environment all the time, it is easy to imagine where the members of the changeable virome originate.
virus
glomerulonephritis
virome
hepatitis
1. The Human Virome and Its Body Habitats
Members of the virome may collaborate with the human host to retain mutualistic functions in preserving human health. Evolutionary theories contend that a particular microbe’s ubiquitous existence may signify a successful partnership with the host. The higher risk of cervical cancer in women infected with high-risk Human Papillomavirus (HPV) strains is an example of how some persistent DNA viral infections are clearly related with disease. This may be comparable to the bacterial infections that persist in the microbiome and have the potential to cause disease but are not always manifest [1].
Although a person’s virome can alter over time, viral presence in a person can be extremely stable. Since viruses are present in the environment all the time, it is easy to imagine where the members of the changeable virome originate. However, recognized host defenses and underappreciated processes are probably the main components of the systems that support viral–host cohabitation. Viruses produce proteins that control the cell cycle [2], host gene expression, and host immunological responses [3][4][5][6]. Micro RNAs that control cellular functions are also encoded by viruses [7]. Consequently, viruses that are latent and persistently infected are constantly engaging with the host in a variety of ways. The intricate impacts of interactions between the virus and host during infection are being studied by many virologists. Awaring that viruses frequently target particular cellular pathways to control the cell and encourage viral replication, but each virus may employ a different strategy to accomplish the same control [7][8]. More research on the functional relationships between viruses and host cells will be carried out in the future on the human virome.
The potential interactions between the viral and bacterial communities kept by complex organisms are an essential aspect of virome investigations.
2. The Role of the Viruses in Health and Disease
2.1. Bacteriophages
Bacteriophages that attack bacteria are present in the human virome, and these phages might indirectly affect the host by changing the fitness and composition of infected bacteria. Due to the existence of various host bacteria, different anatomical regions may have rather varied phage compositions. Phages are widely spread throughout the human body. The effects of phage predation on the bacterial populations in humans are not well understood. Phage therapy, in which phages are purposefully administered to human patients to cure bacterial infections, provides a window on phage predation and host health. This strategy is becoming increasingly popular as drug-resistant bacteria start to appear [9]. Phage cocktails have recently been employed in numerous investigations to treat bacterial infection in a small number of patients, and their apparent efficacy [10] has inspired bigger clinical trials. Phages can transfer DNA between cells, giving bacterial genomes new functionality and potentially altering their fitness and pathogenicity [11]. Recent investigations on animals and in vitro have suggested that phages might directly interact with the host immune system. Without the involvement of bacteria, immunological responses can be triggered by phages via Toll-like receptor (TLR) signaling. Through the nucleotide-sensing receptor TLR9, Lactobacillus, Escherichia, and Bacteroides phages can promote the synthesis of Interleukin 12 (IL-12), IL-6, IL-10, and Interferon γ (IFN) [12][13]. The interactions between phages, bacteria, and the host immune system probably play significant roles in maintaining host immunological homeostasis (Figure 1). Phages can modify bacterial fitness and composition in a way that indirectly affects the host. Some phages and human cell viruses have the ability to integrate into the cells of their respective hosts, sometimes giving the host cells additional capabilities [14].
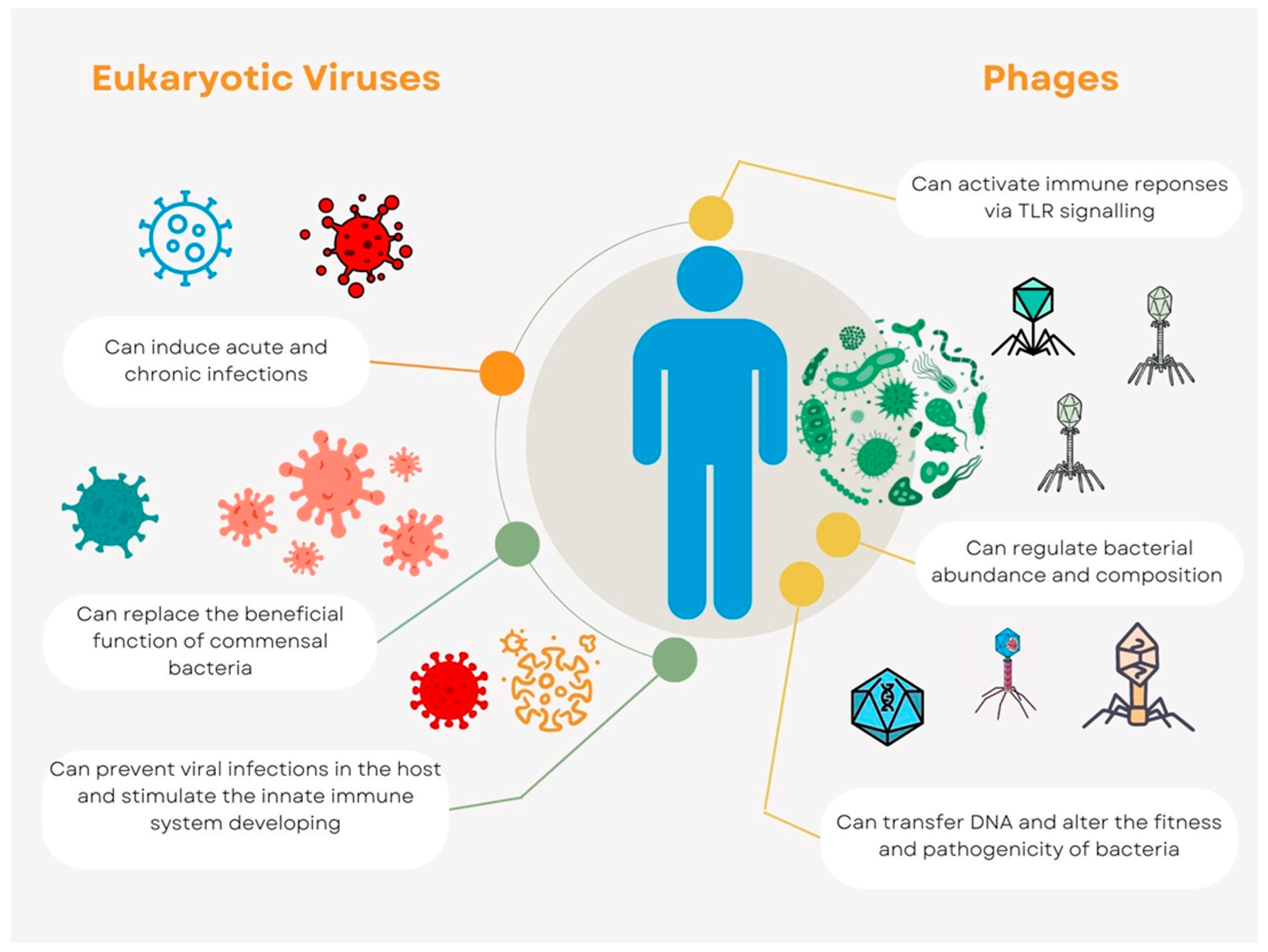
Figure 1. Virome interconnections with host. The host health is impacted by eukaryotic viruses in both negative and positive ways (green and orange lines, respectively). Phages interact with the host through the bacterial population that is associated with it, and these interactions, either directly or indirectly, could have unknown implications (yellow lines) on human health.
2.2. Eukariotic Viruses
Numerous investigations have found a high level of inter-individual variation in the human virome [15]. The virome, on the other hand, is typically quite consistent over time in a healthy adult, paralleling stability in the cellular microbiome. Virome instability is frequently linked to disease states. Virome populations have a variety of effects on their human hosts. Eukaryotic viruses that attack human cells generate immunological reactions, start infections, and occasionally even illness.
When viruses are detected at locations with a local microbiota, they frequently include both viruses that replicate in the human cells there and viruses that infect the microbiota. It is only now beginning to be determined how much circulation exists between the sites. Often, respiratory viral infections can induce exacerbation of some glomerulonephritis such as IgA nephropathy (IgAN), causing onset of gross hematuria and hypertension. The reasons are not clear but may be partly due to mucosal immune system dysfunction [16][17][18][19]. It is unknown if small circular DNA viruses (Anelloviridae and Redondoviridae) that are common in respiratory samples and can also be discovered in feces emerge in the gut as a result of gastrointestinal tract replication or as a result of salivation [14]. Anelloviridae have been detected in greater frequency in immunocompromised patients, including as lung transplant recipients, Human Immunodeficiency Virus (HIV)-positive people, and people on immunosuppressive medications because of inflammatory bowel disease, indicating that they are generally under host immune control [20][21][22]. Another family of extensively occurring tiny circular DNA viruses that are typically identified in the respiratory system is the recently discovered Redondoviridae. They are viruses of the human oral cavity and respiratory tract associated with periodontitis and critical illness [23][24].
3. Virus Infection and Nephropathy
Viral infections are involved in several glomerular diseases, but the pathogenetic links between viral infection and kidney disease are often difficult to establish. The known mechanisms differ for each different viral nephropathy [25]. Generally, acute glomerulonephritis, a viral infection of the glomerulus, causes the local release of cytokines which leads to glomerular cell proliferation [26]. This acute nephropathy resolves spontaneously if the viral infection is rapidly cleared. Chronic glomerulonephritis results from the continuous formation of immune complexes, due to persistent viral infection. Additionally, viral proteins can exacerbate inflammatory conditions and worsen glomerulopathy. Evidence suggests that several viruses such as HIV-1 and hepatitis viruses can cause glomerular damage after both acute and chronic infection. As for cases associated with other viral infections, such as parvovirus B19 and cytomegalovirus (CMV), the mechanisms remain incompletely understood.
References
- Cover, T.L.; Blaser, M.J. Helicobacter Pylori in Health and Disease. Gastroenterology 2009, 136, 1863–1873.
- Nascimento, R.; Costa, H.; Parkhouse, R.M.E. Virus Manipulation of Cell Cycle. Protoplasma 2012, 249, 519–528.
- Versteeg, G.A.; García-Sastre, A. Viral Tricks to Grid-Lock the Type I Interferon System. Curr. Opin. Microbiol. 2010, 13, 508–516.
- Spriggs, M.K. One Step Ahead of the Game: Viral Immunomodulatory Molecules. Annu. Rev. Immunol. 2003, 14, 101–130.
- Khan, N.; Gowthaman, U.; Pahari, S.; Agrewala, J.N. Manipulation of Costimulatory Molecules by Intracellular Pathogens: Veni, Vidi, Vici!! PLoS Pathog. 2012, 8, e1002676.
- Horst, D.; Verweij, M.C.; Davison, A.J.; Ressing, M.E.; Wiertz, E.J.H.J. Viral Evasion of T Cell Immunity: Ancient Mechanisms Offering New Applications. Curr. Opin. Immunol. 2011, 23, 96–103.
- Carl, J.W.; Trgovcich, J.; Hannenhalli, S. Widespread Evidence of Viral MiRNAs Targeting Host Pathways. BMC Bioinform. 2013, 14 (Suppl. 2), S3.
- Zhao, Z.; Xia, J.; Tastan, O.; Singh, I.; Kshirsagar, M.; Carbonell, J.; Klein-Seetharaman, J. Virus Interactions with Human Signal Transduction Pathways. Int. J. Comput. Biol. Drug Des. 2011, 4, 83.
- Moghadam, M.T.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Jazi, F.M. How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections. Infect. Drug Resist. 2020, 13, 45–61.
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232.
- Taylor, V.L.; Fitzpatrick, A.D.; Islam, Z.; Maxwell, K.L. The Diverse Impacts of Phage Morons on Bacterial Fitness and Virulence. Adv. Virus Res. 2019, 103, 1–31.
- Sweere, J.M.; van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X.; et al. Bacteriophage Trigger Antiviral Immunity and Prevent Clearance of Bacterial Infection. Science 2019, 363, eaat9691.
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25, 285–299.e8.
- Liang, G.; Bushman, F.D. The Human Virome: Assembly, Composition and Host Interactions. Nat. Rev. Microbiol. 2021, 19, 514–527.
- Virgin, H.W. The Virome in Mammalian Physiology and Disease. Cell 2014, 157, 142–150.
- Prasad, N.; Patel, M.R. Infection-Induced Kidney Diseases. Front. Med. 2018, 5, 327.
- Hu, X.; Feng, J.; Zhou, Q.; Luo, L.; Meng, T.; Zhong, Y.; Tang, W.; Deng, S.; Li, X. Respiratory Syncytial Virus Exacerbates Kidney Damages in IgA Nephropathy Mice via the C5a-C5AR1 Axis Orchestrating Th17 Cell Responses. Front. Cell. Infect. Microbiol. 2019, 9, 151.
- MacDonald, N.E.; Wolfish, N.; McLaine, P.; Phipps, P.; Rossier, E. Role of Respiratory Viruses in Exacerbations of Primary Nephrotic Syndrome. J. Pediatr. 1986, 108, 378–382.
- Day, N.K.; Geiger, H.; McLean, R.; Resnick, J.; Michael, A.; Good, R.A. The Association of Respiratory Infection, Recurrent Hematuria, and Focal Glomerulonephritis with Activation of the Complement System in the Cold. J. Clin. Investig. 1973, 52, 1698–1706.
- Young, J.C.; Chehoud, C.; Bittinger, K.; Bailey, A.; Diamond, J.M.; Cantu, E.; Haas, A.R.; Abbas, A.; Frye, L.; Christie, J.D.; et al. Viral Metagenomics Reveal Blooms of Anelloviruses in the Respiratory Tract of Lung Transplant Recipients. Am. J. Transplant. 2015, 15, 200–209.
- Monaco, C.L.; Gootenberg, D.B.; Zhao, G.; Handley, S.A.; Ghebremichael, M.S.; Lim, E.S.; Lankowski, A.; Baldridge, M.T.; Wilen, C.B.; Flagg, M.; et al. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell Host Microbe 2016, 19, 311.
- Li, L.; Deng, X.; Linsuwanon, P.; Bangsberg, D.; Bosco Bwana, M.; Hunt, P.; Martin, J.N.; Deeks, S.G.; Delwart, E.; Hospital, F.G.; et al. AIDS Alters the Commensal Plasma Virome. J. Virol. 2013, 87, 10912–10915.
- Abbas, A.A.; Taylor, L.J.; Dothard, M.I.; Leiby, J.S.; Fitzgerald, A.S.; Khatib, L.A.; Collman, R.G.; Bushman, F.D. Redondoviridae, a Family of Small, Circular DNA Viruses of the Human Oro-Respiratory Tract Associated with Periodontitis and Critical Illness. Cell Host Microbe 2019, 25, 719–729.e4.
- Spezia, P.G.; Macera, L.; Mazzetti, P.; Curcio, M.; Biagini, C.; Sciandra, I.; Turriziani, O.; Lai, M.; Antonelli, G.; Pistello, M.; et al. Redondovirus DNA in Human Respiratory Samples. J. Clin. Virol. 2020, 131, 104586.
- Lai, A.S.H.; Lai, K.N. Viral Nephropathy. Nat. Clin. Pract. Nephrol. 2006, 2, 254.
- Glassock, R.J. Immune Complex-Induced Glomerular Injury in Viral Diseases: An Overview. Kidney Int. Suppl. 1991, 35, S5–S7.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
28 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




