Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Domitilla Mandatori | -- | 1301 | 2023-02-27 11:14:25 | | | |
| 2 | Lindsay Dong | Meta information modification | 1301 | 2023-03-01 01:48:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pelusi, L.; Mandatori, D.; Mastropasqua, L.; Agnifili, L.; Allegretti, M.; Nubile, M.; Pandolfi, A. Intraocular Ienses. Encyclopedia. Available online: https://encyclopedia.pub/entry/41698 (accessed on 07 February 2026).
Pelusi L, Mandatori D, Mastropasqua L, Agnifili L, Allegretti M, Nubile M, et al. Intraocular Ienses. Encyclopedia. Available at: https://encyclopedia.pub/entry/41698. Accessed February 07, 2026.
Pelusi, Letizia, Domitilla Mandatori, Leonardo Mastropasqua, Luca Agnifili, Marcello Allegretti, Mario Nubile, Assunta Pandolfi. "Intraocular Ienses" Encyclopedia, https://encyclopedia.pub/entry/41698 (accessed February 07, 2026).
Pelusi, L., Mandatori, D., Mastropasqua, L., Agnifili, L., Allegretti, M., Nubile, M., & Pandolfi, A. (2023, February 27). Intraocular Ienses. In Encyclopedia. https://encyclopedia.pub/entry/41698
Pelusi, Letizia, et al. "Intraocular Ienses." Encyclopedia. Web. 27 February, 2023.
Copy Citation
Intraocular lenses (IOLs) are tiny artificial devices placed inside the eye, which have the main function of restoring the refractive power of the natural crystalline lens that is removed during cataract surgery.
intraocular lenses
eye
ocular drug delivery
1. Mechanisms
Most intraocular lenses (IOLs) are made of synthetic polymers, which can be divided into two major groups: acrylic and silicone [1]. As a result, unlike the contact lens (CLs) described above, the IOLs are permanent, meaning they are considered devices with intermediate characteristics between ocular inserts (OIs) and CLs [2]. Given that the IOL is implanted during cataract surgery and remains in the eye after surgery, recently this ocular device has received growing attention for its possible use as an optimal delivery system for intraocular drug release (Figure 1). In particular, the use of IOLs was hypothesized for the treatment of the most common complications occurring following cataract surgery, which include inflammation, infection, and posterior capsule opacification.
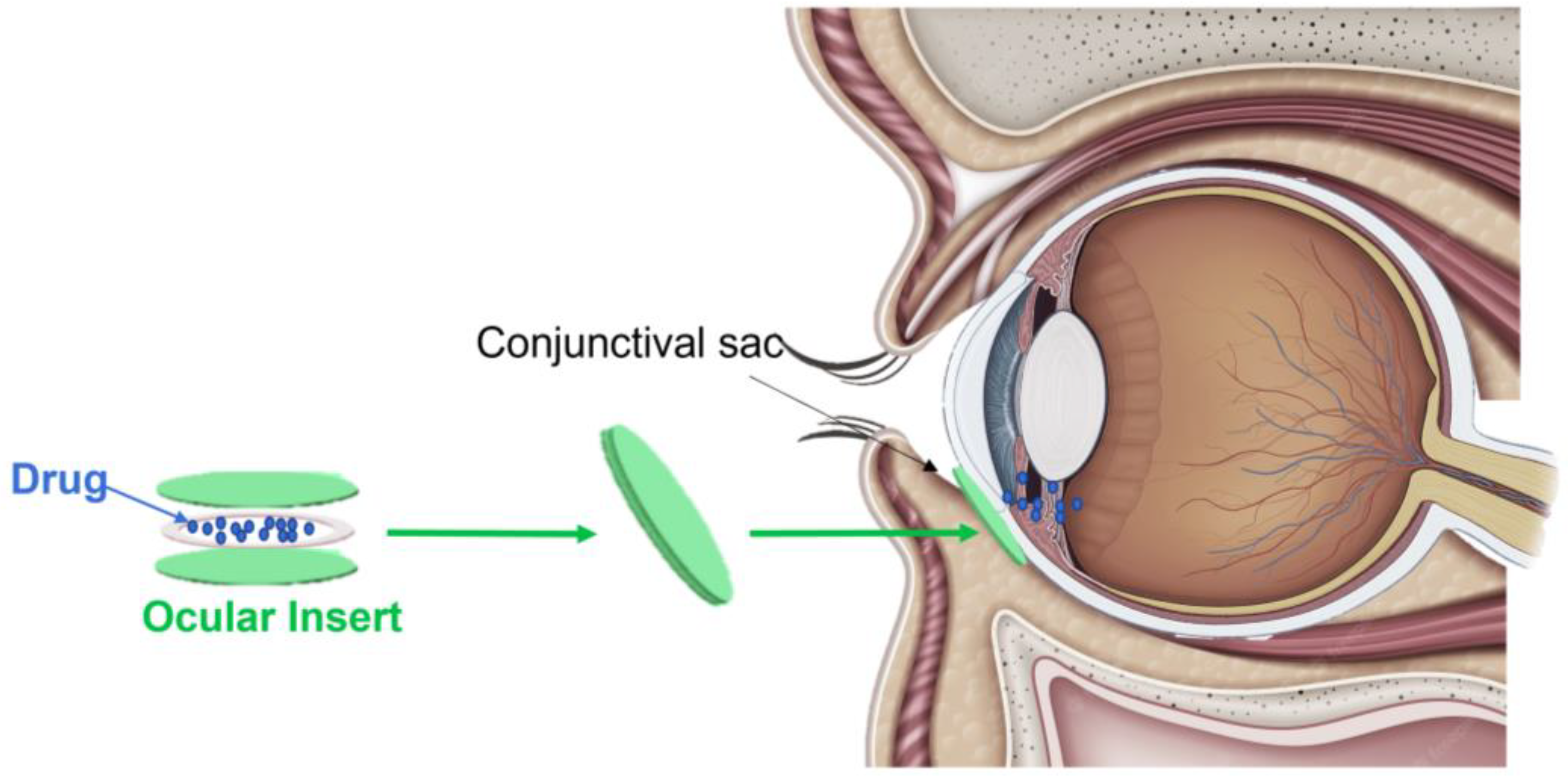
Figure 1. Representative image of a drug-loaded intraocular lens (IOL). A drug-loaded IOL is a permanent, tiny synthetic ocular device composed of acrylic and silicone. Once implanted during cataract surgery into the crystalline lens, it allows sustained intraocular drug release through the diffusion process.
Regarding the drug loading process, two possible approaches are described in the literature. The first one is represented by the drug coating on the IOLs’ surfaces through a soaking method [3]. The second one is based on the use of a separate polymeric drug reservoir attached to the IOLs. During the development of drug-loaded IOLs, in addition to the loading method that is adopted, it is necessary to consider further key aspects to avoid the occurrence of important inconveniences. Given that compared to the other ocular systems, IOLs can efficiently release higher amounts of drugs in the intraocular site, they must be loaded with an effective but non-toxic level of the active compound. Therefore, it is necessary to determine the optimal amount of the drug to load and to understand its pharmacokinetics. In addition, the drug loading should not affect the optical properties or the dioptric power of the IOLs and its position when implanted in the crystalline lens to avoid the phenomenon of blurring vision [4].
Considering all of these aspects, it possible to develop ideal IOLs able to release desired drugs in the intraocular compartments through the diffusion process.
Finally, regarding the main advantages of drug-loaded IOLs, compared to the other drug delivery systems usually used to treat cataract postoperative complications, the choice of these ocular devices could lead to better patient compliance and management. Indeed, drug-loaded IOLs could represent permanent drug delivery systems implanted in a single surgical procedure following cataract surgery [5].
2. Intraocular Lens Drug Release
Based on the characteristic and the advantages described above, recently several studies have paid attention to the development of IOLs loaded with specific drugs (Table 1).
Table 1. Summary of the studies for drug-loaded CLs.
| Clinical Application | OIs Materials | OIs Fabrication Methods | Drug-Loaded | Drug-Loading Technique | Reference |
|---|---|---|---|---|---|
| Ocular Infections | 2-Hydroxylethyl methacrylate (HEMA) and Methyl Methacrylate (MMA) | Cross-linking | Moxifloxacin (MXF) | Soaking | [6] |
| Acrylic | Commercial | Methotrexate (MTX) | Supercritical impregnation technology | [7] | |
| Posterior Capsule Opacification | Hyaluronic Acid (HA) and Chitosan (CHI) | Layer by Layer (LbL) | Paclitaxel (Pac) | Chemical Bonding | [8] |
| Acrylic G-free® material [ethylene glycol phenyl ether acrylate (EGPE), 2-hydroxyethyl methacrylate (HEMA) and poly (propylene glycol) dimethacrylate (PPGDMA)] | Commercial | Moxifloxacin (MXF) and Diclofenac (DFN) | Molecular Imprinting | [9] | |
| Poly-Methyl-Methacrylate (PMMA) | Commercial | 5-fluorouracil (5-Fu) | Chitosan Nanoparticles | [10] | |
| Ocular Inflammatory conditions | Hydrogel: 2-hydroxyethyl methacrylate (HEMA), Methyl Methacrylate (MMA), Methacrylic acid (MAA) | Free-radical polymerization | Indomethacin (IND) | Directly addition to polymeric solution | [11] |
The drugs mainly chosen for the loading studies are antibiotics and anti-inflammatory drugs, which are usually used to control infection and inflammation conditions occurring after cataract surgery.
In this context, the feasibility of using acrylic IOLs for the sustained release of the antibiotic moxifloxacin (MXF), which is commonly used for endophthalmitis prophylaxis after cataract surgery, was investigated. The acrylic IOLs were obtained via the cross-linking of the synthetic co-polymers 2-hydroxylethyl methacrylate (HEMA) and methyl methacrylate (MMA), while the drug loading was performed by soaking the IOLs in a MXF solution. Interestingly, the drug release results obtained in vitro and in vivo showed that the loaded IOLs allowed the constant release of active MXF for up to 2 weeks [6].
Acrylic IOLs were also employed to study the delivery of methotrexate (MTX) [7], an FDA-approved folic acid antagonist [12], to lessen the posterior capsule opacification. Interestingly, the modern technique known as supercritical impregnation [13] was used to load MTX onto the IOLs, and through the use of ex vivo implants in human donor capsular bags, the scholars found that the loaded IOLs sustained the release of MTX for more than 80 days, which induced a decrease in fibrosis by preventing the epithelial–mesenchymal transformation of lens epithelial cells [7].
Xiang (2020) instead demonstrated the capability of IOLs based on polymer hydrogel to load and delivery indomethacin (IND), a non-steroidal anti-inflammatory compound used to prevent ocular inflammation and posterior capsule opacification [11]. The hydrogel lenses were developed through the free-radical polymerization of 2-hydroxyethyl methacrylate (HEMA), methyl methacrylate (MMA), and methacrylic acid (MAA). Instead, IND prodrugs were prepared via the esterification of IND and HEMA and then directly added to the polymeric solution before the free-radical polymerization.
Similarly, hydrogel-based IOLs, composed of the polymers HEMA and 2-butoxyethyl methacrylate (BEM), were used [14] to co-deliver steroidal (dexamethasone sodium phosphate, DSP) and non-steroidal (bromfenac sodium, BFS) active compounds for the treatment of pseudophakic cystoid macular edema [15]. Following the drugs’ binding using two positive charge monomers such as N-2-aminopropyl-methacrylamid (APMA) and acrylamide (AAm), the results obtained in vivo showed that the drug-loaded IOLs allowed the release of BFS and DSP, which both reached therapeutic concentrations in the aqueous humor for about 2 and 8 weeks, respectively [14].
With the aim of improving the drug loading process, several approaches are used to modify the IOLs’ surfaces. Among these, layer-by-layer (LbL) deposition, molecular imprinting, and the coating and the loading of NPs are the most used methods [16].
LbL deposition based on the natural polymers hyaluronic acid (HA) and chitosan (CHI) was used to chemically load the antiproliferative drug paclitaxel (Pac) to prevent posterior capsule opacification following cataract surgery. Importantly, studies in vitro performed to evaluate the drug release highlighted that the HA/CHI multilayer IOLs showed a sustained release profile of Pac, thereby providing support for this novel approach to prevent or treat posterior capsule opacification [8].
Commercial acrylic G-free®-based IOLs were tested to load moxifloxacin (MXF) and the anti-inflammatory diclofenac (DFN) for posterior ocular opacification management. In detail, by using the molecular imprinting approach, which creates molecularly imprinted polymers with tailor-made binding sites complementary to the molecules in terms of their shape, size, and functional groups [17], the surfaces of the IOLs were modified with the functional monomers acrylic acid (AA), methacrylic acid (MAA), and 4-vinylpiridine (4-VP) [9].
The coating technique was instead employed to modify the IOL surfaces with hydrophilic polydopamine (PDA) via dopamine self-polymerization, a technique that exploits the oxidation of dopamine at alkaline pH using dissolved oxygen [18]. The IOLs were then loaded with the antiproliferative drug doxorubicin (DOX). Interestingly, in vitro and in vivo studies demonstrated that such modified IOLs were safe, biocompatible, and effective in inducing cell apoptosis, assuming their use was to prevent postoperative complications such as posterior capsule opacification [19].
Regarding the use of NPs in combination with IOLs, only one study employing this approach was published in 2013. In particular, the scholars modified the surfaces of the synthetic commercial poly-methyl-methacrylate (PMMA) IOLs with chitosan nanoparticles to release 5-fluorouracil (5-Fu), an active compound for the prevention of posterior capsule opacification. The in vitro drug release tests showed the burst release of the 5-Fu from the modified IOLs in the first 2 h, which was sustained for at least 4 days. In addition, the in vivo results performed with New Zealand rabbits demonstrated that at 4 weeks implantation of such nanoparticle-modified IOLs, the animals showed lighter posterior capsule opacification than the control group [10].
References
- Werner, L. Biocompatibility of intraocular lens materials. Curr. Opin. Ophthalmol. 2008, 19, 41–49.
- Lambert, S.R.; Cotsonis, G.; DuBois, L.; Nizam Ms, A.; Kruger, S.J.; Hartmann, E.E.; Weakley, D.R., Jr.; Drews-Botsch, C.G. Infant Aphakia Treatment Study, Long-term Effect of Intraocular Lens vs. Contact Lens Correction on Visual Acuity After Cataract Surgery During Infancy: A Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 365–372.
- Liu, H.; Wu, L.; Fu, S.; Hou, Y.; Liu, P.; Cui, H.; Liu, J.; Xing, L.; Zhang, X. Polylactide-glycoli acid and rapamycin coating intraocular lens prevent posterior capsular opacification in rabbit eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 247, 801–807.
- Liu, Y.-C.; Wong, T.T.; Mehta, J.S. Intraocular lens as a drug delivery reservoir. Curr. Opin. Ophthalmol. 2013, 24, 53–59.
- Topete, A.; Saramago, B.; Serro, A.P. Intraocular lenses as drug delivery devices. Int. J. Pharm. 2021, 602, 120613.
- Filipe, H.P.; Bozukova, D.; Pimenta, A.; Vieira, A.P.; Oliveira, A.; Galante, R.; Topete, A.; Másson, M.; Alves, P.; Coimbra, P.; et al. Moxifloxacin-loaded acrylic intraocular lenses: In vitro and in vivo performance. J. Cataract. Refract. Surg. 2019, 45, 1808–1817.
- Ongkasin, K.; Masmoudi, Y.; Wertheimer, C.M.; Hillenmayer, A.; Eibl-Lindner, K.H.; Badens, E. Supercritical fluid technology for the development of innovative ophthalmic medical devices: Drug loaded intraocular lenses to mitigate posterior capsule opacification. Eur. J. Pharm. Biopharm. 2020, 149, 248–256.
- Huang, H.; Zhu, S.; Liu, D.; Wen, S.; Lin, Q. Antiproliferative drug-loaded multi-functionalized intraocular lens for reducing posterior capsular opacification. J. Biomater. Sci. Polym. Ed. 2021, 32, 735–748.
- Topete, A.; Tang, J.; Ding, X.; Filipe, H.P.; Saraiva, J.A.; Serro, A.P.; Lin, Q.; Saramago, B. Dual drug delivery from hydrophobic and hydrophilic intraocular lenses: In-vitro and in-vivo studies. J. Control. Release 2020, 326, 245–255.
- Huang, X.; Wang, Y.; Cai, J.-P.; Ma, X.-Y.; Li, Y.; Cheng, J.-W.; Wei, R.-L. Sustained Release of 5-Fluorouracil from Chitosan Nanoparticles Surface Modified Intra Ocular Lens to Prevent Posterior Capsule Opacification: An In Vitro and In Vivo Study. J. Ocul. Pharmacol. Ther. 2013, 29, 208–215.
- Xiang, Y.; Zou, M.; Zhang, Y.; Jin, R.; Nie, Y. Drug-loaded and Blue-ray Filtered Hydrogel as a Potential Intraocular Lens for Cataract Treatment. Pharm. Nanotechnol. 2020, 8, 302–312.
- Abdi, F.; Mohammadi, S.S.; Falavarjani, K.G. Intravitreal Methotrexate. J. Ophthalmic Vis. Res. 2021, 657–669.
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259.
- Toffoletto, N.; Salema-Oom, M.; Igea, S.A.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A. Drug-Loaded Hydrogels for Intraocular Lenses with Prophylactic Action against Pseudophakic Cystoid Macular Edema. Pharmaceutics 2021, 13, 976.
- Wielders, L.H.; Lambermont, V.A.; Schouten, J.S.; Biggelaar, F.J.V.D.; Worthy, G.; Simons, R.W.; Winkens, B.; Nuijts, R.M. Prevention of Cystoid Macular Edema After Cataract Surgery in Nondiabetic and Diabetic Patients: A Systematic Review and Meta-Analysis. Am. J. Ophthalmol. 2015, 160, 968–981.e33.
- Topete, A.; Barahona, I.; Santos, L.F.; Pinto, C.A.; Saraiva, J.A.; Serro, A.P.; Saramago, B. The effects of addition of functional monomers and molecular imprinting on dual drug release from intraocular lens material. Int. J. Pharm. 2021, 600, 120513.
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211.
- Fichman, G.; Schneider, J. Dopamine Self-Polymerization as a Simple and Powerful Tool to Modulate the Viscoelastic Mechanical Properties of Peptide-Based Gels. Molecules 2021, 26, 1363.
- Liu, S.; Zhao, X.; Tang, J.; Han, Y.; Lin, Q. Drug-Eluting Hydrophilic Coating Modification of Intraocular Lens via Facile Dopamine Self-Polymerization for Posterior Capsular Opacification Prevention. ACS Biomater. Sci. Eng. 2021, 7, 1065–1073.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
961
Revisions:
2 times
(View History)
Update Date:
02 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




