Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Viviana Frantellizzi | -- | 2107 | 2023-02-27 09:49:25 | | | |
| 2 | Dean Liu | -2 word(s) | 2105 | 2023-02-28 02:15:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gorica, J.; De Feo, M.S.; Corica, F.; Sidrak, M.M.A.; Conte, M.; Filippi, L.; Schillaci, O.; De Vincentis, G.; Frantellizzi, V. Novel Theranostic Approaches Targeting CCR4-Receptor. Encyclopedia. Available online: https://encyclopedia.pub/entry/41693 (accessed on 07 February 2026).
Gorica J, De Feo MS, Corica F, Sidrak MMA, Conte M, Filippi L, et al. Novel Theranostic Approaches Targeting CCR4-Receptor. Encyclopedia. Available at: https://encyclopedia.pub/entry/41693. Accessed February 07, 2026.
Gorica, Joana, Maria Silvia De Feo, Ferdinando Corica, Marko Magdi Abdou Sidrak, Miriam Conte, Luca Filippi, Orazio Schillaci, Giuseppe De Vincentis, Viviana Frantellizzi. "Novel Theranostic Approaches Targeting CCR4-Receptor" Encyclopedia, https://encyclopedia.pub/entry/41693 (accessed February 07, 2026).
Gorica, J., De Feo, M.S., Corica, F., Sidrak, M.M.A., Conte, M., Filippi, L., Schillaci, O., De Vincentis, G., & Frantellizzi, V. (2023, February 27). Novel Theranostic Approaches Targeting CCR4-Receptor. In Encyclopedia. https://encyclopedia.pub/entry/41693
Gorica, Joana, et al. "Novel Theranostic Approaches Targeting CCR4-Receptor." Encyclopedia. Web. 27 February, 2023.
Copy Citation
With the high mortality rate of malignant tumors, there is a need to find novel theranostic approaches to provide an early diagnosis and targeted therapy. The chemokine receptor 4 (CCR4) is highly expressed in various tumors and plays an important role in tumor pathogenesis.
CCR4
theranostics
CXCR4
chemokine receptor
1. CCR4
CCR4 also known as C-C chemokine receptor 4, produces a protein that is a member of the G-protein-coupled receptor family. It is a CC chemokine receptor for MIP-1, RANTES, TARC, and MCP-1. Chemokines are a family of tiny, structurally similar polypeptide molecules that control how different kinds of leukocytes move around inside their cells. The chemokines also have important impacts on cells of the central nervous system and endothelial cells engaged in angiogenesis or angiostasis, as well as on immune system development, homeostasis, and function [1]. CCR4 is part of the G protein-coupled receptor (GPCR) and is a member of the 7-transmembrane domain family of receptors. CCR4 has been used as a target for many drugs of immunotherapy. It is present on the surface of the cell, and it consists of 352 amino acid residues, containing an N-terminal, C-terminal, and 7-transmembrane domains, and three extracellular and intracellular loops. CCR4 passes the signal within the cell by combining with the signaling chemokine; this way it controls cellular growth and the immune system. CCR4 overexpression is associated with many types of tumors. The CCR4 interaction with CCL12 activates intracellular signaling that activates various pathways such as NF-Kb, RAS-MAPK, JAK-STAT, P13K-AKT-mTOR, and PLC-Ca2+, which can regulate angiogenesis, tumor cell proliferation, and apoptosis inhibition. The activation of mitogen-activated protein kinase (MAPK) activates the c-myc transcription factor, which regulates CCR4 overexpression, therefore leading to positive and negative feedback, promoting tumor growth and cell proliferation. Cell proliferation is also generated through the activation of P13K-AKT and RAF-MEK-ERK pathways. Apoptosis is inhibited through the activation of NF-kB, Wnt pathways, and the upregulation of Bcl-2 proteins. MAPK, ERK1/2, and P13K activate the secretion of MMP which promotes the ability of tumors to metastasize. Due to the crucial role of CCR4 in tumors, various target inhibitors have been studied [2][3]. See Figure 1.
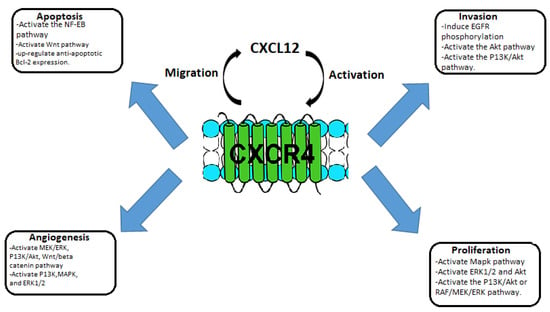
Figure 1. Schematic representation of the role of CXCL12/CCR4 in tumorigenesis and metastasis.
2. CCR4-Targeted Molecular Imaging
Molecular imaging has become crucial in the medical field, especially in the diagnosis of malignant diseases. The goal of molecular imaging is to monitor in real-time biochemical and biological processes taking place in the organism. Through targeted molecular imaging probes, researchers can obtain qualitative, quantitative, and dynamic in vivo imaging, to reveal the pathogenesis and the status of the disease [2]. It also includes multiple image-capture techniques in combination with data retrieved from other medical and nonmedical sources. Single photon emission tomography (SPECT) and positron emission tomography (PET) are the main imaging technologies used. Radionuclide-labeled probes are used to identify and monitor tumors and monitor therapeutic response. The use of highly targeted therapies reduces the need for additional therapies, such as chemotherapy and radiotherapy, and the side effects that they can cause.
2.1. SPECT/CT CCR4-Target Imaging
Single photon emission computed tomography/computed tomography (SPECT/CT) combines two different diagnostic scans into one for a more accurate view of the body region being examined and to track the metabolic processes of the body. The CT scan provides a better anatomical resolution. Single photon emission computed tomography has cost-effective advantages and high sensitivity in the ability to track physiopathological processes in vivo. There are a variety of radiopharmaceuticals and isotopes that can be used in SPECT/CT, some of which have been shown to have utility in tracking CCR4 sensible tumors. Hanaoka et al. developed a radiopharmaceutical for the imaging of CCR4 sensible tumors. They developed a precursor for radiolabeled peptides, containing 14-residue peptidic CXCR4 inhibitor, Ac-TZ14011 creating the radiopharmaceutical 111In-DTPA-Ac-TZ14011. Imaging showed a major intra-tumor biodistribution of the tracer compared to other healthy tissue and blood [4]. Kuil et al., developed a hybrid peptide dendrimer, containing Ac-TZ14011, Cy5.5-like fluorophore, and a DTPA chelate which reduced a specific muscle uptake in molecular imaging [5]. Lesniak et al. evaluated another imaging agent that targets CCR4, POL3026 peptidomimetic template. Through single photon emission computed tomography (SPECT/CT) imaging, biodistribution, and in silico and in vitro studies show [111In]POL-D and [111In]POL-PD significant uptake in U87-stb-CXCR4 tumors compared to the control U87 tumors at 90 min and 24 h post-injection [6]. Other SPECT/CT isotopes targeting CCR4 with promising results are 99mTc-HYNIC, a co-ligand with bifunctional chelator, hydrazino nicotinic acid [7] and 67GA-DOTA-TZ2 [8].
2.2. PET CCR4-Target Imaging
Positron emission tomography is broadly employed as a molecular imaging technique, using radiopharmaceuticals as tracers to track the metabolism of whole tissues and focal lesions to diagnose diseases. PET scans are usually combined with computed tomography (PET/CT) and magnetic resonance imaging (PET/MRI). Through the fusion with anatomical structure imaging of CT or MRI, researchers can obtain information regarding function and metabolism while having high spatial resolution. There is a high variety of PET radiotracers. Lutetium-177 is a low-energy B-emitting radiotracer with a half-life of 6 or 7 days. It has the ability to bind with DOTA/NOTA chelates making it ideal for radiotracer therapy. 177Lu-DOTA-TATE and 177Lu-PSMA-617 can be targeted to a peptide receptor and are ideal as they have a high affinity for tissues expressing such peptides [9]. 68Ga-pentixafor is a promising positron emission tomography (PET) agent targeting CCR4. Pentixafor has a similar structure to CCR4, providing binding stability to plasma proteins. Herrman et al. used 177Lu and 90Y bound with pentixafor to cure Multiple Myeloma (MM) in three patients with advanced disease. The results were promising with one full recovery and a partial one [10]. Other radionuclides have a high affinity for CCR4 sensible tumors and have bifunctional chelating groups; 18F-T140, 18F-AIF-NOTA-T140, 64Cu-T140-2D, 64Cu-DOTA-NFB, 64Cu-NOTA-NFB, 68Ga-DOTA-CPCR4-2 and 68Ga-NOTA-NFB [2].
3. Radiation-Enhanced Expression of Chemokine Receptor CCR4
Radiation therapy, also called radiotherapy, is a treatment that uses high doses of radiation to kill cancer cells and reduce the size of tumors. Radiotherapy is commonly used for the treatment of many types of tumors, by itself or in addition to chemotherapy. Its success depends on accurate and reproducible dose delivery to the target volume and minimization of concomitant doses to healthy tissues. There is, therefore, a requirement for robust dosimetry for all parts of the body, and for all treatment modalities, for patients undergoing radiotherapy. Radiotherapy uses ionizing radiation to eliminate cancer cells through apoptosis, necrosis, and immunogenic cell death. Radiotherapy causes alterations in gene expression in tumor cells, and it can involve the immune system [11]. Irradiated tumor tissues and cancer cells recruit chemokines such as CCL22 and CXCL12 and produce a range of cytokines such as TNF-α, TGF-β, IL-1, IL-6, and GM-CSF which could recruit tumor-infiltrating lymphocytes (TILs) into radiation site. Radiation produces pro-inflammatory factors to recruit TILs and change the inflammatory microenvironment. Tissue radiation generates ROS and kinase activation associated with DNA damage, resulting in the expression of NK cell-activating ligands (NKALs) that activate and secrete the chemokines XCL1, FLT3LG, and CCL5 to recruit dendritic cells (DCs), which, induces tumor suppression in the tumor microenvironment [11]. C-C class chemokine 22 (CCL22) is macrophage-synthetized; its receptor is C-C chemokine receptor 4 (CCR4), and it is expressed on the surface of Th2 cells and regulatory T cells (Treg). High levels of CCL22 have been found in various types of human tumors such as breast, lung, gastrointestinal, and nasopharyngeal tumors, B–CLL, and Hodgkin lymphoma. Radiation treatment increases intracellular and extracellular expression of CCL22, these factors indicate that radiotherapy has immune-stimulating properties. Li H et al. studied the prognosis of patients expressing both CCL22 and CCR4 through Kaplan—Meier survival analysis. The study revealed that such patients had a better prognosis, which was not expected due to Treg cell recruitment by CCL22 and CCR4 [11].
4. Mogalizumab, Humanized Anti-CCR4 Monoclonal Antibody, and Its Applications
4.1. Adult T Cell Leukemia and Sézary Syndrome
Adult T cell Leukemia (ATL) has a very poor prognosis. CCR4 is present in the neoplastic cells of most patients with this type of tumor. Mogamulizumab (see Figure 2) is a novel monoclonal antibody used in immunotherapy targeting the humanized CCR4. This antibody enhances antibody-dependent cellular toxicity through its defucosylated Fc region. Mogamulizumab mediates antibody-dependent cellular toxicity, but it does not mediate complement-dependent cytotoxicity or other antitumor activities. The main effector cells of Mogalizumab are considered to be the natural killer (NK) cells [12]. Mogamulizumab has been proposed as a first-line therapy in ATL patients not suitable for allogeneic stem cell transplantation and patients with refractory ATL. Mogamulizumab has been approved by the Food and Drug Administration and the European Medicines Agency for the treatment of patients with Sézary Syndrome (SS) in 2018 [12]. SS and ATL, both T cell tumors, share similar genetic alterations. Tanaka et al. studied 64 ATL patients treated with Mogalizumab. Forty-five patients received combined therapy with Mogalizumab and vincristine, cyclophosphamide, doxorubicin, prednisone; doxorubicin, ranimustine, prednisone; vindesine, etoposide, carboplatin, prednisone or with cyclophosphamide, doxorubicin, vincristine, prednisolone, whereas 19 received Mogamulizumab monotherapy. Nine patients received an allogeneic hematopoietic stem cell transplant after Mogamulizumab treatment. The patients were evaluated for gene alterations and clinical responses correlated to specific gene alterations. CCR4 was among the 11 genes altered in ATL patients and also one of the six genes altered in SS. CCR4 alterations were present in 25–30% of ATL patients, in addition, this alteration was also correlated to a higher clinical response rate. Most likely, the alterations in the C-terminus guide to a weakened CCR4 internalization upon ligand binding, leading to a higher surface expression of the ligand and leading to increased availability of target molecules for Mogalizumab [12].
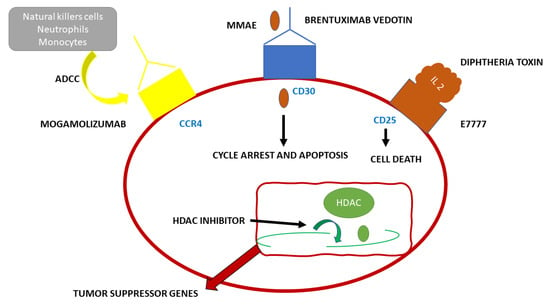
Figure 2. Schematic representation of the functioning of the monoclonal antibody Mogamulizumab.
4.2. Cutaneous Lymphomas
A class of non-Hodgkin lymphomas is primary cutaneous lymphomas, such as mycosis fungoides (MF) and Sézary syndrome (SS). Mycosis fungoides is one of the most common types of Cutaneous T Cell Lymphomas (CTCL). MF originates in the peripheral epidermotropic T cells, in specific in the memory T cells (CD45RO+), expressing the T cell receptor (TCR) and CD4+ immunophenotype [13]. Vaidya et al. in their 2022 publication showed that MF has an incidence of six cases per million per year in the United States and Europe. That would be accountable for 4% of all cases of non-Hodgkin lymphomas. It is more common in male adults over 50 years of age. The pathology is more common amongst dark skinned races rather than Caucasians or Asians [14]. Sezary syndrome is accountable for 3% of all T cell cutaneous lymphomas and it expresses three specific findings: lymphadenopathy, erythroderma with pruritus, and most specifically, atypical circulating lymphocytes called Sezary or Lutzner cells. Sezary Syndrome is part of the leukemic phase of T cell cutaneous lymphomas that rarely compromises the bone marrow; its compromission is found only in advanced forms of the disease. In the World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) classification, MF and SS are listed as separate diseases [15]. Monochemotherapy with gemcitabine or pegylated liposomal doxorubicin is effective, but not in all patients, and the duration of response is limited. Allogeneic hematopoietic stem cell transplant is resolutive, but it has a high treatment-associated mortality rate and relapses are not uncommon. Therefore, new targeted therapies have been developed targeting surface molecules expressed on the surface of tumor cells, such as CCR4, HDACs, CD30, and CD25, CD52, specific for MF and SS. Mogamulizumab, whose antitumor activity is mediated by antibody-dependent cellular cytotoxicity, is a humanized monoclonal antibody against CCR4. Mogamulizumab has been approved for CCR4+ ATL and for peripheral T cell lymphoma (PTCL) and cutaneous T cell lymphoma (CTCL). Sugaya et al. enrolled seven patients with MF, and their overall response (ORR) was 28.6% with no complete response (CR) [16]. Duvic et al. conducted a phase ½ study in 41 pre-treated patients with CTCL. Thirty-eight patients were treated with Mogalizumab, the ORR was 36.8%: 47.1% in SS (n = 17), and 28.6% in MF (n = 21). Ogura et al. conducted a multicenter phase II study of Mogalizumab. They enrolled 38 patients, 37 received Mogalizumab therapy and exhibited clinically meaningful outcomes in patients with CTCL and PTCL [17]. The MAVORIC (Mogamulizumab anti-CCR4 Antibody Versus ComparatOR In CTCL) study is a phase 3 multicentric clinical trial for relapsed or refractory MF-SS. The study revealed that Mogamulizumab has a better effect on survival than the HDAC inhibitor vorinostat. Response rates based on tissues were: 68% in blood, 17% in lymph nodes 0.42% in the skin, and 0% in viscera. The higher response rate was seen in the blood, due to the presence of natural killer cells, neutrophils, or monocytes, which are essential for ADCC. Common side effects were fever, pruritus, nausea, skin eruptions, infusion reactions, and lymphopenia. In 2018, Mogamulizumab has been approved for the treatment of patients with CTCL who have received at least one prior systemic therapy by the FDA and the European Medicines Agency [16].
References
- Lim, H.D.; Lane, J.R.; Canals, M.; Stone, M.J. Systematic Assessment of Chemokine Signaling at Chemokine Receptors CCR4, CCR7 and CCR10. Int. J. Mol. Sci. 2021, 22, 4232.
- Liu, N.; Wan, Q.; Cheng, Z.; Chen, Y. Radionuclide-Labeled Peptides for Imaging and Treatment of CXCR4- Overexpressing Malignant Tumors. Curr. Top. Med. Chem. 2019, 19, 17–32.
- Ogura, M.; Ishida, T.; Hatake, K.; Taniwaki, M.; Ando, K.; Tobinai, K.; Fujimoto, K.; Yamamoto, K.; Miyamoto, T.; Uike, N.; et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1157–1163.
- Hanaoka, H.; Mukai, T.; Tamamura, H.; Mori, T.; Ishino, S.; Ogawa, K.; Iida, Y.; Doi, R.; Fujii, N.; Saji, H. Development of a 111In-labeled peptide derivative targeting a chemokine receptor, CXCR4, for imaging tumors. Nucl. Med. Biol. 2006, 33, 489–494.
- Kuil, J.; Buckle, T.; Oldenburg, J.; Yuan, H.; Borowsky, A.D.; Josephson, L.; van Leeuwen, F.W. Hybrid peptide dendrimers for imaging of chemokine receptor 4 (CXCR4) expression. Mol. Pharm. 2011, 8, 2444–2453.
- Lesniak, W.G.; Sikorska, E.; Shallal, H.; Behnam Azad, B.; Lisok, A.; Pullambhatla, M.; Pomper, M.G.; Nimmagadda, S. Structural characterization and in vivo evaluation of β-Hairpin peptidomimetics as specific CXCR4 imaging agents. Mol. Pharm. 2015, 12, 941–953.
- Mikaeili, A.; Erfani, M.; Sabzevari, O. Synthesis and evaluation of a (99m)Tc-labeled chemokine receptor antagonist peptide for imaging of chemokine receptor expressing tumors. Nucl. Med. Biol. 2017, 54, 10–17.
- Sano, K.; Masuda, R.; Hisada, H.; Oishi, S.; Shimokawa, K.; Ono, M.; Fujii, N.; Saji, H.; Mukai, T. A radiogallium-DOTA-based bivalent peptidic ligand targeting a chemokine receptor, CXCR4, for tumor imaging. Bioorg. Med. Chem. Lett. 2014, 24, 1386–1388.
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Hänscheid, H.; Schirbel, A.; Buck, A.K.; Kropf, S.; Schwaiger, M.; et al. pentixather: Comprehensive Preclinical Characterization of a First CXCR4-directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362.
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J. Nucl. Med. 2016, 57, 248–251.
- Li, H.; Chen, X.; Zeng, W.; Zhou, W.; Zhou, Q.; Wang, Z.; Jiang, W.; Xie, B.; Sun, L.Q. Radiation-Enhanced Expression of CCL22 in Nasopharyngeal Carcinoma is Associated With CCR4(+) CD8 T Cell Recruitment. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 126–139.
- Tanaka, N.; Mori, S.; Kiyotani, K.; Ota, Y.; Gotoh, O.; Kusumoto, S.; Nakano, N.; Suehiro, Y.; Ito, A.; Choi, I.; et al. Genomic determinants impacting the clinical outcome of mogamulizumab treatment for adult T-cell leukemia/lymphoma. Haematologica 2022, 107, 2418–2431.
- Hossain, C.; Jennings, T.; Duffy, R.; Knoblauch, K.; Gochoco, A.; Chervoneva, I.; Shi, W.; Alpdogan, S.O.; Porcu, P.; Pro, B.; et al. The histological prevalence and clinical implications of folliculotropism and syringotropism in mycosis fungoides. Chin. Clin. Oncol. 2019, 8, 6.
- Amorim, G.M.; Niemeyer-Corbellini, J.P.; Quintella, D.C.; Cuzzi, T.; Ramos, E.S.M. Clinical and epidemiological profile of patients with early stage mycosis fungoides. Bras Derm. 2018, 93, 546–552.
- Lopez, A.T.; Bates, S.; Geskin, L. Current Status of HDAC Inhibitors in Cutaneous T-cell Lymphoma. Am. J. Clin. Dermatol. 2018, 19, 805–819.
- Sugaya, M. Clinical Guidelines and New Molecular Targets for Cutaneous Lymphomas. Int. J. Mol. Sci. 2021, 22, 11079.
- Duvic, M.; Pinter-Brown, L.C.; Foss, F.M.; Sokol, L.; Jorgensen, J.L.; Challagundla, P.; Dwyer, K.M.; Zhang, X.; Kurman, M.R.; Ballerini, R.; et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood 2015, 125, 1883–1889.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
693
Revisions:
2 times
(View History)
Update Date:
28 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




