Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ulises De la Cruz-Mosso | -- | 1490 | 2023-02-25 17:22:13 | | | |
| 2 | Catherine Yang | Meta information modification | 1490 | 2023-02-27 02:56:45 | | | | |
| 3 | Catherine Yang | Meta information modification | 1490 | 2023-02-27 07:29:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pesqueda-Cendejas, K.; Campos-López, B.; Mora-García, P.E.; Moreno-Ortiz, J.M.; De La Cruz-Mosso, U. Nutrients’ Role in Epigenetic Modifications. Encyclopedia. Available online: https://encyclopedia.pub/entry/41665 (accessed on 07 February 2026).
Pesqueda-Cendejas K, Campos-López B, Mora-García PE, Moreno-Ortiz JM, De La Cruz-Mosso U. Nutrients’ Role in Epigenetic Modifications. Encyclopedia. Available at: https://encyclopedia.pub/entry/41665. Accessed February 07, 2026.
Pesqueda-Cendejas, Karen, Bertha Campos-López, Paulina E. Mora-García, José M. Moreno-Ortiz, Ulises De La Cruz-Mosso. "Nutrients’ Role in Epigenetic Modifications" Encyclopedia, https://encyclopedia.pub/entry/41665 (accessed February 07, 2026).
Pesqueda-Cendejas, K., Campos-López, B., Mora-García, P.E., Moreno-Ortiz, J.M., & De La Cruz-Mosso, U. (2023, February 25). Nutrients’ Role in Epigenetic Modifications. In Encyclopedia. https://encyclopedia.pub/entry/41665
Pesqueda-Cendejas, Karen, et al. "Nutrients’ Role in Epigenetic Modifications." Encyclopedia. Web. 25 February, 2023.
Copy Citation
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by an aberrant immune response and persistent inflammation. Epigenetic changes are dynamic and susceptible to modification in response to environmental stimuli, which provides a feasible intervention to influence epigenetic homeostasis through an “epigenetic diet.” The term epigenetic diet was previously described by Daniel et al., referring to the consumption of certain foods, such as soy, grapes, vegetables, and green tea, which exert mechanisms against aging and cancer.
systemic lupus erythematosus
methyl donor nutrients
DNA methylation
miRNAs
1. Folate
Folate, also known as vitamin B9, is an essential micronutrient vital for cellular functions, such as DNA synthesis and methylation [1]. Its immune functions have been previously described. According to Courtermanche et al. [2], dietary folate deficiency is likely to affect most dividing cells and could cause DNA breaks in human T lymphocytes, affecting their proliferation and increasing apoptosis rate. Similarly, folate deficiency affects cell function and T helper cell differentiation, which suggests that folate plays a relevant role in maintaining Th cell homeostasis [3]. In this sense, folate may directly influence the immune system by influencing immune cell homeostasis and indirectly by influencing the DNA methylation of immune genes.
Under normal dietary conditions, the folate absorbed is metabolized to its bioactive form of 5-methyltetrahydrofolate (5-methylTHF); this metabolite is required to maintain the flux of methyl groups for the re-methylation of homocysteine to methionine. Methionine is the substrate for SAM or S-adenosyl-L-methionine (AdoMet), a methyl group donor for methylation reactions that most commonly occurs at the 5 positions of cytosine to generate 5-methylcytosine [4]. The evidence suggests that folate-deficient diets may induce DNA hypomethylation. A study conducted on women aged 65–80 reported that inadequate folate intake during 7 weeks resulted in DNA hypomethylation in leukocytes [5], which highlights the role of folate in the modulation of DNA methylation (Figure 1).
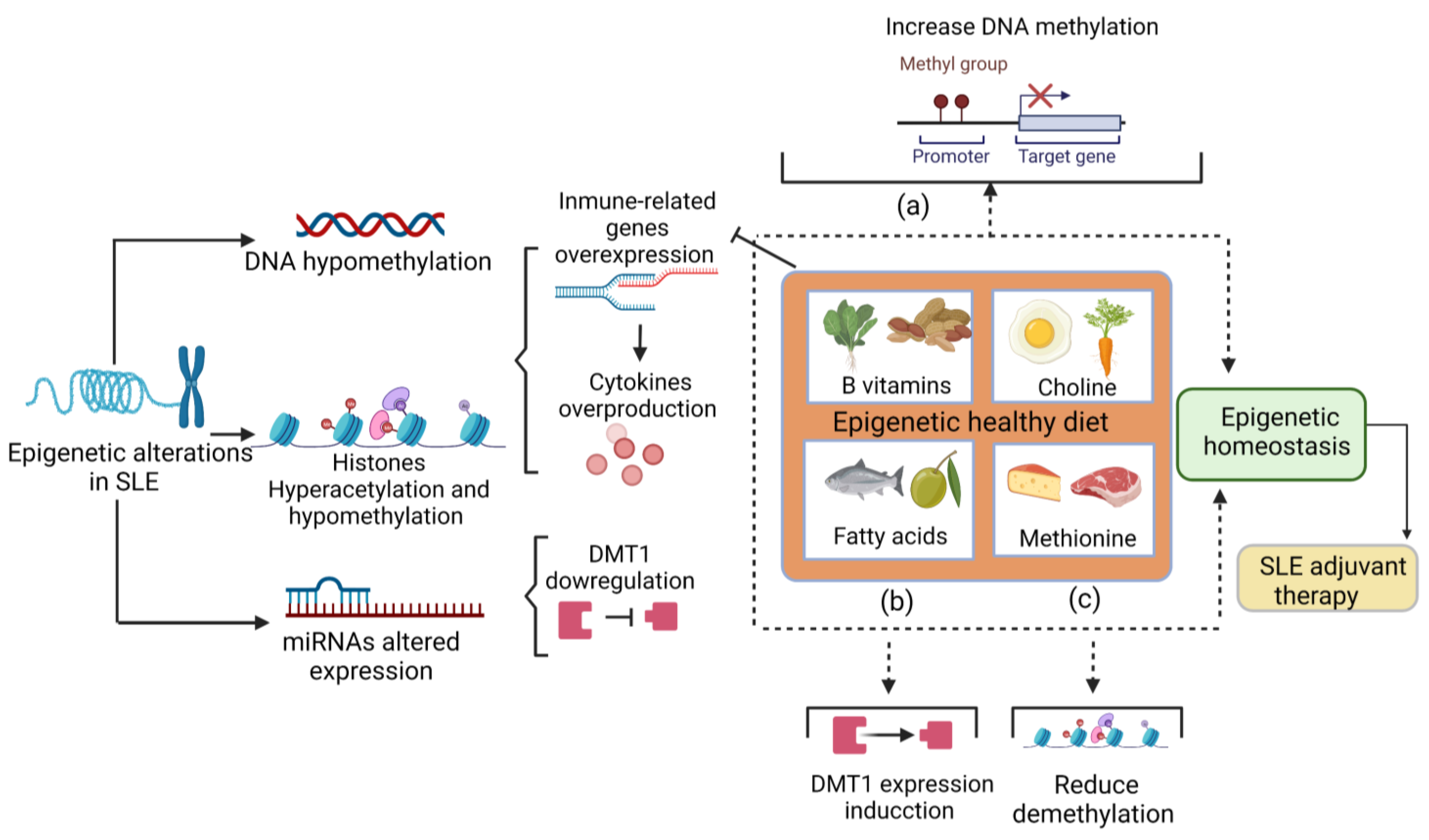
Figure 1. The role of the diet in epigenetic changes. Nutrients have a role in epigenetic modifications. (a) B vitamins and choline donate methyl groups to promote DNA methylation and could reduce the expression of immune-related genes and subsequently reduce cytokine overproduction. (b) Fatty acids stimulate DNMT1 expression, which could compensate for the downregulation of DNMT1 in SLE patients. (c) The methionine intake reduces hydroxymethylation a step before demethylation. Altogether, these mechanisms promote epigenetic homeostasis. Therefore, a healthy epigenetic diet that provides an adequate amount of these nutrients acts as an adjuvant therapy for SLE. SLE: systemic lupus erythematosus. DNMT1: DNA methyltransferase 1.
In humans and rats, hypomethylation may be induced by low dietary folate and reversed by folate repletion. In a randomized controlled trial, supplementation with 400 µg per day of folate led to an increase in DNA methylation of 31% in leucocytes and 25% in colonic mucosa; however, the statistical effect was marginal [5]. In a similar study, DNA hypomethylation by folate repletion was reversed with supplementation of 286–516 µg for 3 weeks [6]. Notably, DNA hypomethylation reversibility depends on folate depletion duration. In a study conducted in rats with folate deficiency for 9, 18, 24, and 36 weeks, the repletion of the adequate folate diet just increased the DNA methylation in those with 9 weeks of folate depletion [7], which suggests that hypomethylation is not reversibly after prolonged folate deficiency.
2. Choline and Betaine
Choline is an essential nutrient involved in synthesizing the neurotransmitter acetylcholine, methyl group donor, betaine, and phospholipids. It has also been associated with preventing cognition alterations, hepatic steatosis, cardiovascular disease, and cancer. Choline also has a relevant role in epigenetic regulation through SAM synthesis (Figure 1) during its oxidation to betaine [8].
Studies of global and gene-specific DNA methylation in rodent models exposed to a maternal choline-deficient diet showed global and specific hypomethylation of cyclin-dependent kinase 3 (CDKN3), calbindin1, and vascular endothelial growth factor (VEGF-C) genes. Notably, this study also observed global hypomethylation but gene-specific hypermethylation of insulin-like growth factor-2 (IGF-2) [9]. This demonstrates that methyl donor nutrients could act differently by depending on global or gene-specific methylation and by depending on the gene evaluated; however, more studies are necessary to prove this theory. Another similar study has demonstrated that Maternal choline supply modifies fetal histone and DNA methylation in rat fetal liver and brain, suggesting that choline exerts epigenetic mechanisms during stages of embryonic development [10].
Betaine is a nonessential nutrient found in several food sources and can be synthesized from choline; it has an essential role in DNA methylation, playing as a methyl donor. Recently, it has been suggested that betaine plays an anti-inflammatory role through the betaine NF-κB signaling pathway, and NLRP3 inflammasome inhibition [11]. Due to its epigenetic and anti-inflammatory effects, adequate choline and betaine intake should be ensured in SLE patients.
3. B Vitamins
B vitamins comprise a group of water-soluble vitamins that perform essential cellular functions in catabolic and anabolic enzymatic reactions [12] but also play a relevant role in epigenetic regulation, acting as methyl donors or as cofactors on the one-carbon metabolism (Figure 1) [13].
Vitamin B12, also known as cobalamin, in one-carbon metabolism acts as a cofactor for the enzyme methionine synthase; thus, it is a regulator of DNA methylation [13]. Cobalamin deprivation induced global hypomethylation in a murine model, even when combined with a folate-rich diet [14]. This indicates that the high consumption of other methyl donors cannot compensate for the cobalamin deficiency. Vitamin B12 could also directly affect the immune system; its deficiency has been associated with TNF-alfa overproduction [15]; thus, adequate cobalamin consumption may have anti-inflammatory effects on SLE.
Pyridoxine or vitamin B6 serves as a coenzyme in the transfer of a one-carbon unit from serine to tetrahydrofolate (THF) to generate glycine and 5,10 methylenetetrahydrofolate (MTHF) [13]. Moreover, its role in methylation, it has been reported that B6 deficiency results in inflammation and has been involved in inflammatory diseases, such as rheumatoid arthritis [16]; however, there is no evidence of its specific role in SLE.
Riboflavin has potent anti-inflammatory and antioxidant effects; its deficiency has been associated with disturbing MTHFR activity and to the risk of cancer by influencing DNA methylation patterns. In SLE, pyridoxine has been inversely associated with atherosclerotic plaque, a common alteration in these patients; there is no evidence of pyridoxine and DNA methylation in SLE; however, an adequate intake of this vitamin may reduce cardiovascular alterations in SLE and avoid disturbances of the MTHFR activity [17][18].
4. Methionine
Methionine is an essential sulfur-containing amino acid that must be consumed in the diet. Eggs, fish, dairy products, and some meats are sources of it. Regarding its role in DNA methylation, it serves as a precursor of SAM; thus, when the concentration of methionine is low, SAM synthesis is reduced, and DNA methylation is theoretically reduced [9]. A study conducted on 168 women of reproductive age showed that high methionine intake reduces hydroxymethylation (a step that precedes demethylation) (Figure 1) [19]. However, methionine’s biological effects are controversial because it is necessary to preserve DNA methylation patterns; however, in cancer, evidence suggests that methionine restriction inhibits cancer cell growth and may enhance the efficacy of chemotherapeutic agents. In addition, methionine has been involved in oxidative and aging events [20][21]. Due to these possible adverse effects of methionine, it needs to be clarified how to be the recommendation for methionine intake in SLE patients; however, an adequate but not excessive amount of methionine may be included in the dietary recommendations for SLE.
5. Fatty Acids
Fatty acids are no part of the methyl donor nutrients; however, emerging findings support that fatty acids can modify the epigenome. Until now, the exact mechanisms through which fatty acids influence epigenetic changes were not known; however, some mechanisms have suggested that short-chain fatty acids, such as butyric, propionic, and valeric, can inhibit histone deacetylase activity. On the other hand, variation in energy intake led to changes in cellular NAD+/NADH which may alter histone deacetylase activities; therefore, indirectly, fatty acid could modulate histone deacetylase activity through energy changes [22].
The evidence in the murine model and humans reported that PUFAs and saturated fatty acid intake might alter the methylation status; however, the specificity of such effects still needs to be clarified [22]. In human colorectal cancer cells, PUFA treatment increases the expression of DNMTs in HT29/219 (Figure 1) but suppresses other cell lines [23]. Thus, more evidence about fatty acids and their epigenetic roles is necessary.
Regarding the immune system, PUFAs, specifically omega-3 fatty acids, have been widely related to immune function due to their anti-inflammatory properties. In macrophages, treatment with omega-3 decreases pro-inflammatory cytokine synthesis and increases IL-10 cytokine production [24]. Additionally, omega-3 fatty acid promotes M2 polarization in murine models and may modulate T cell activation. Even omega-3 fatty acid supplementation has demonstrated beneficial effects in T-mediated diseases, such as autoimmune hepatitis [24] and probably SLE.
6. Other Environmental Factors That Impact DNA Methylation
Chronic alcohol consumption could promote DNA hypomethylation; it has been demonstrated that alcohol can inhibit methionine synthase activity in the liver, resulting in a significant reduction in s-adenosyl methionine levels [25].
In addition, tobacco compounds can promote cobalamin and folate inactivation and interfere with one-carbon metabolism, consequently interfering with the availability of methyl groups [9]. Therefore, promoting healthy habits in SLE patients, such as avoiding or at least limiting alcohol intake and smoking, is crucial in treating the disease.
References
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488.
- Courtemanche, C.; Huang, A.C.; Elson-Schwab, I.; Kerry, N.; Ng, B.Y.; Ames, B.N. Folate deficiency and ionizing radiation cause DNA breaks in primary human lymphocytes: A comparison. FASEB J. 2004, 18, 209–211.
- Wu, C.H.; Huang, T.C.; Lin, B.F. Folate deficiency affects dendritic cell function and subsequent T helper cell differentiation. J. Nutr. Biochem. 2017, 41, 65–72.
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA Methylation: A Review of Molecular Mechanisms and the Evidence for Folate’s Role. Adv. Nutr. 2012, 3, 21–38.
- Lu, Q.; Qiu, X.; Hu, N.; Wen, H.; Su, Y.; Richardson, B.C. Epigenetics, disease, and therapeutic interventions. Ageing Res. Rev. 2006, 5, 449–467.
- Jacob, R.A.; Gretz, D.M.; Taylor, P.C.; James, S.J.; Pogribny, I.P.; Miller, B.J.; Henning, S.M.; Swendseid, M.E. Moderate Folate Depletion Increases Plasma Homocysteine and Decreases Lymphocyte DNA Methylation in Postmenopausal Women. J. Nutr. 1998, 128, 1204–1212.
- Pogribny, I.P.; Ross, S.A.; Wise, C.; Pogribna, M.; Jones, E.A.; Tryndyak, V.P.; James, S.J.; Dragan, Y.P.; Poirier, L.A. Irreversible global DNA hypomethylation as a key step in hepatocarcinogenesis induced by dietary methyl deficiency. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2006, 593, 80–87.
- Wiedeman, A.; Barr, S.; Green, T.; Xu, Z.; Innis, S.; Kitts, D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513.
- Mahmoud, A.; Ali, M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608.
- Davison, J.M.; Mellott, T.J.; Kovacheva, V.P.; Blusztajn, J.K. Gestational Choline Supply Regulates Methylation of Histone H3, Expression of Histone Methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA Methylation of Their Genes in Rat Fetal Liver and Brain. J. Biol. Chem. 2009, 284, 1982–1989.
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070.
- Kennedy, D. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68.
- Cappuccilli, M.; Bergamini, C.; Giacomelli, F.A.; Cianciolo, G.; Donati, G.; Conte, D.; Natali, T.; La Manna, G.; Capelli, I. Vitamin B Supplementation and Nutritional Intake of Methyl Donors in Patients with Chronic Kidney Disease: A Critical Review of the Impact on Epigenetic Machinery. Nutrients 2020, 12, 1234.
- Kulkarni, A.; Dangat, K.; Kale, A.; Sable, P.; Chavan-Gautam, P.; Joshi, S. Effects of Altered Maternal Folic Acid, Vitamin B12 and Docosahexaenoic Acid on Placental Global DNA Methylation Patterns in Wistar Rats. PLoS ONE 2011, 6, e17706.
- Peracchu, M.; Bamonti, C.; Pomati, M.; De Franceschi, M.; Scalabrino, G. Human cobalamin deficiency: Alterations in serum tumor necrosis factor- α and epidermal growth factor. Eur. J. Haematol. 2001, 67, 123–127.
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of B vitamins on the one-carbon transfer pathways. Chem. Biol. Interact. 2006, 163, 113–132.
- Lourdudoss, C.; Elkan, A.C.; Hafström, I.; Jogestrand, T.; Gustafsson, T.; van Vollenhoven, R.; Frostegård, J. Dietary micronutrient intake and atherosclerosis in systemic lupus erythematosus. Lupus 2016, 25, 1602–1609.
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950.
- Pauwels, S.; Duca, R.; Devlieger, R.; Freson, K.; Straetmans, D.; Van Herck, E.; Huybrechts, I.; Koppen, G.; Godderis, L. Maternal Methyl-Group Donor Intake and Global DNA (Hydroxy)Methylation before and during Pregnancy. Nutrients 2016, 8, 474.
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684.
- Kitada, M.; Ogura, Y.; Monno, I.; Xu, J.; Koya, D. Effect of Methionine Restriction on Aging: Its Relationship to Oxidative Stress. Biomedicines 2021, 9, 130.
- Burdge, G.C.; Lillycrop, K.A. Fatty acids and epigenetics. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 156–161.
- Sarabi, M.M.; Naghibalhossaini, F. The impact of polyunsaturated fatty acids on DNA methylation and expression of DNMTs in human colorectal cancer cells. Biomed. Pharmacother. 2018, 101, 94–99.
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028.
- Lu, S.C.; Huang, Z.Z.; Yang, H.; Mato, J.M.; Avila, M.A.; Tsukamoto, H. Changes in methionine adenosyltransferase and S -adenosylmethionine homeostasis in alcoholic rat liver. Am. J. Physiol.-Gastrointest. Liver Physiol. 2000, 279, G178–G185.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
28 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




