Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Huanyu Jiang | -- | 2437 | 2023-02-24 17:14:51 | | | |
| 2 | Camila Xu | Meta information modification | 2437 | 2023-02-27 01:46:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jiang, H.; Ji, P.; Shang, X.; Zhou, Y. Physiological Effects of Nitric Oxide on Cartilage. Encyclopedia. Available online: https://encyclopedia.pub/entry/41647 (accessed on 28 February 2026).
Jiang H, Ji P, Shang X, Zhou Y. Physiological Effects of Nitric Oxide on Cartilage. Encyclopedia. Available at: https://encyclopedia.pub/entry/41647. Accessed February 28, 2026.
Jiang, Huanyu, Piyao Ji, Xiaobin Shang, Yan Zhou. "Physiological Effects of Nitric Oxide on Cartilage" Encyclopedia, https://encyclopedia.pub/entry/41647 (accessed February 28, 2026).
Jiang, H., Ji, P., Shang, X., & Zhou, Y. (2023, February 24). Physiological Effects of Nitric Oxide on Cartilage. In Encyclopedia. https://encyclopedia.pub/entry/41647
Jiang, Huanyu, et al. "Physiological Effects of Nitric Oxide on Cartilage." Encyclopedia. Web. 24 February, 2023.
Copy Citation
Nitric oxide (NO) is a small gaseous molecule that is widely distributed in the human body, and its synthesis is dependent on NO synthase (NOS). NO plays an important role in various physiological processes such as the regulation of blood volume and nerve conduction.
nitric oxide
nitric oxide synthases
osteoarthritis
osteoclasts
osteoblasts
Chondrocytes
1. NO and NO Synthase (NOS)
Nitric oxide (NO) is an unstable, uncharged free radical, and the N atom in NO with free electrons gets rapidly oxidized. Hence, its biological lifetime is only several seconds; subsequently, NO is active only in the immediate proximity of NO-producing cells. Meanwhile, NO has a small size and favorable lipid solubility, thereby exhibiting high membrane permeability [1]. Therefore, NO can rapidly diffuse out of cells and cross through more than several microns into target cells, acting as a paracrine-signaling molecule.
NOS produces NO as a byproduct while oxidizing L-arginine to L-citrulline, using oxygen and nicotinamide adenine dinucleotide phosphate (NADPH) as substrates (Figure 1). In addition, this reaction requires flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and (6R-)5,6,7,8-tetrahydro-L-biopterin (BH4) as cofactors. Among these cofactors, FAD and FMN are responsible for the transfer of electrons from NADPH to heme, and BH4 can bind to the oxygenase domain of NOS as an essential cofactor for substrates. Notably, this reaction is stereospecific; NOS can metabolize L-arginine, but not D-arginine [2]. There are three subtypes of NOS present in the body: neuronal NOS (nNOS; NOS1), inducible NOS (iNOS; NOS2), and endothelial NOS (eNOS; NOS3). They have the same NO production process; however, their structures and functions are tailored to the different body parts. nNOS and eNOS are constitutively expressed (thus also called constitutive NOS, cNOS) and can rapidly produce NO based on an increase in cytoplasmatic calcium. In some specific cases, such as oxidation of BH4 or depletion of L-arginine, eNOS can transform from a NO-producing enzyme into an enzyme that generates superoxide anion (O2−), which is called NOS uncoupling. iNOS is only induced when macrophages are stimulated by cytokines including tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) and other factors such as bacterial toxoids. iNOS produces far more NO as compared to that produced by nNOS or eNOS [3]; this increased production might have various pathological effects.
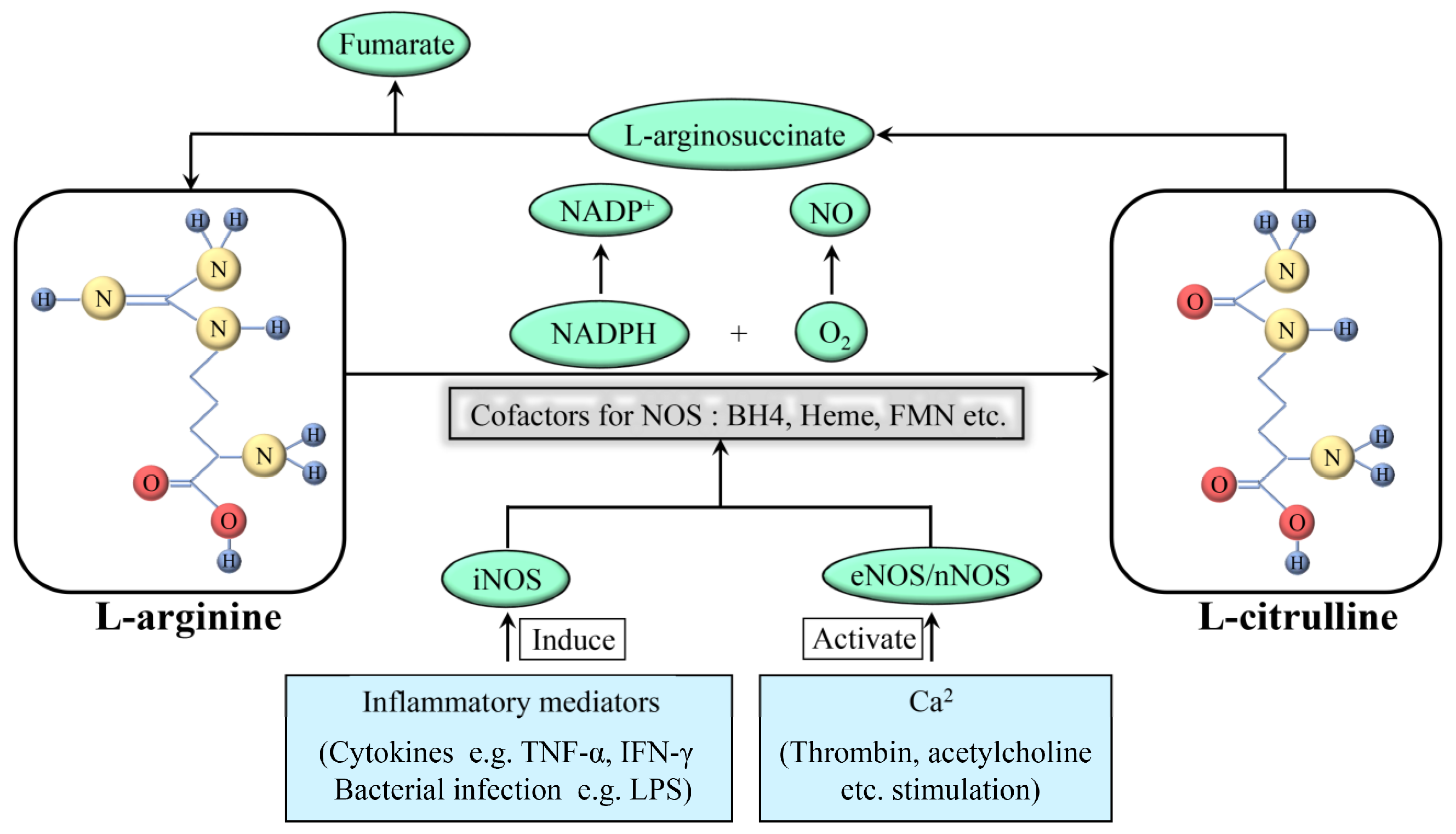
Figure 1. Formation and synthesis of NO. NAPDH: nicotinamide adenine dinucleotide phosphate; NAPD+: oxidation form of NAPDH; NO: nitric oxide; O2: oxygen; iNOS: inducible nitric oxide synthase; eNOS: endothelial nitric oxide synthase; nNOS: neuronal nitric oxide synthase; TNF: tumor necrosis factor; IFN: interferon; LPS: lipopolysaccharide.
NO is a reactive molecule that can act via numerous pathways, depending on the relative concentrations of NO and the surrounding environment in which NO is produced. The effects of NO can be direct or indirect, depending on the NO concentration—NO can show direct effects (<1 µM NO) as well as indirect effects (>1 µM NO). The indirect effects are induced by the reactive nitrogen species, produced by the interaction of NO with superoxide or oxygen [4].
In inflammatory diseases, NO acts as a double-edged sword. It can produce anti-inflammatory effects under physiological conditions. The agents, such as cytokines, promote iNOS activity, resulting in the synthesis of NO in large amounts by monocytes, macrophages, and granulocytes, among many other cells. NO then scavenges free radicals or kills microbes, thereby preventing cell injury.
NO can also act as a pro-inflammatory mediator. Its overproduction can be cytotoxic [5]. NO can react with superoxide and generate peroxynitrite. The subsequent secondary chain reaction leads to the production of NO2 and hydroxide, which can be even more toxic. This results in damaging the normal tissues, thereby increasing inflammation.
2. Physiological Effects of NO on Cartilage
As a chronic evolutionary disease, OA causes structural changes in the normal articular cavity. In order to comprehensively understand the mechanism of OA, the regulation of osseous tissue under physiological conditions should be explored.
The musculoskeletal system is an essential part of the human body’s functions and is affected by the biomechanical environment. The osseous tissue’s shape and density alter with the changes in the biomechanical environment. Qualitatively, the bone mass and density increase in areas with higher loads and decrease in areas with lower loads; this process is called bone remodeling. Bone remodeling consists of two steps: the stimulation of resorption in response to bone formation and the generation of new bone after bone degradation. Studies have shown the complexity of the process, involving multi-tiered communication networks of osteoclasts, osteoblasts, and other cell types in bone [6][7][8]. In addition, the coordination among endocrine, autocrine, and paracrine signals harmonizes human bone remodeling based on the adjustment of these cells in different stages [9]. Therefore, the recruitment, differentiation, and function of cells in bone remodeling are governed by a series of systemic regulators of bone metabolism (parathyroid hormone, vitamin D, and estrogen), local mediators (receptor of activated nuclear factor kappa-B ligand [RANKL], its antagonist osteoprotegerin, and Wnts/sclerostin), free radicals (superoxide, hydrogen peroxide, and NO), and bone matrix components [10]. The subsequent sections focus on the effects of the NO/NOS system on bone processes (Table 1 and Figure 2).
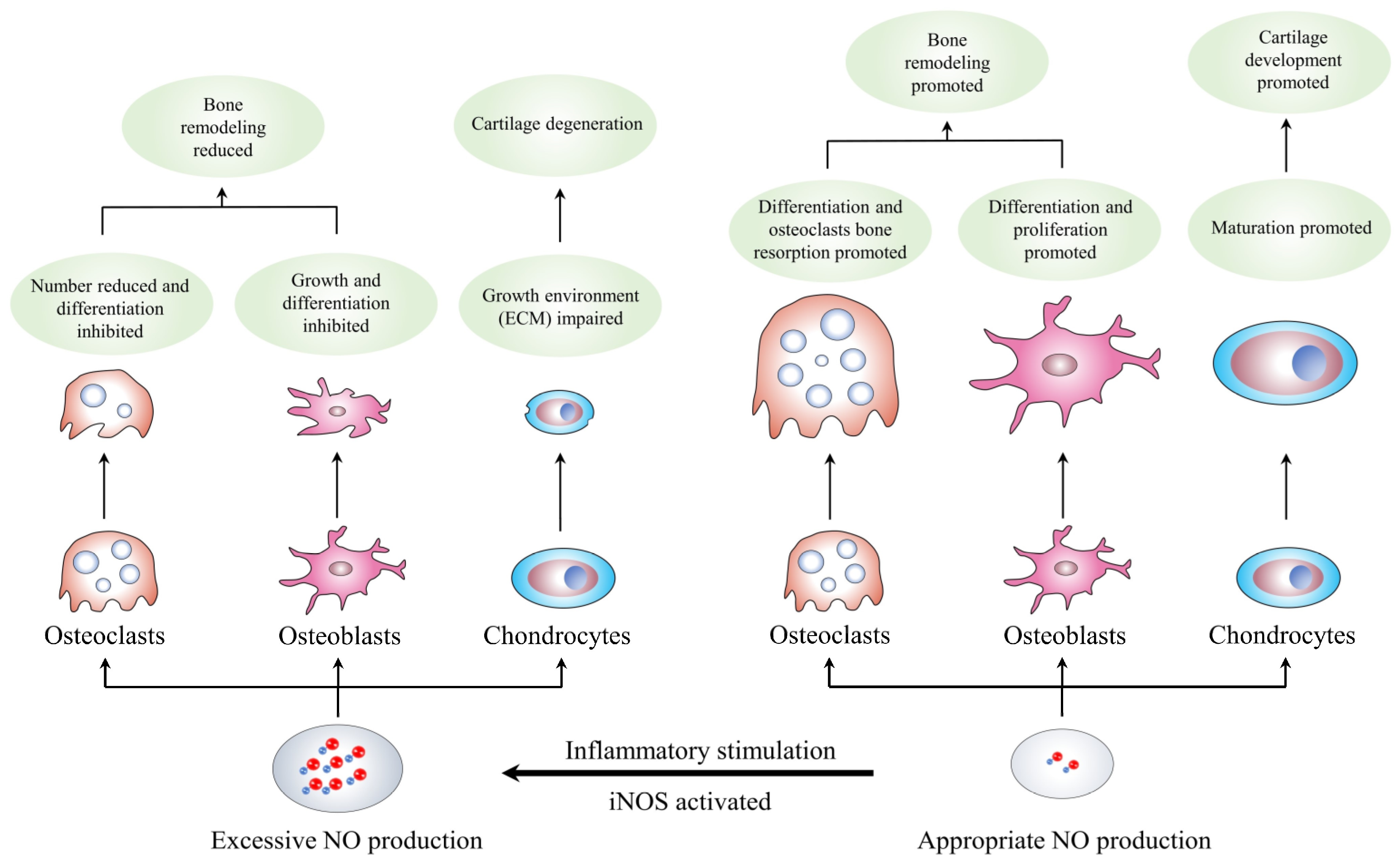
Figure 2. Formation and synthesis of NO. NO: nitric oxide; iNOS: inducible nitric oxide synthase; ECM: extracellular matrix.
Table 1. Possible effect of NO on different type of cells and its related mechanisms.
| Targeted-Cell | Type of Study | NO Concentration | Related Mechanisms | Effect of NO | Reference |
|---|---|---|---|---|---|
| Osteoclasts | In vivo and in vitro | High | Reduces the number of osteoclasts and inhibits its spread |
Inhibits mature osteoclasts bone resorption |
Kalyanaraman et al., 2017 [11] |
| Osteoclasts | In vitro | High | Induced by inhibited cGMP-degrading activity of PDE | Inhibits precellular osteoclasts differentiation |
Amano et al., 2019 [12] |
| Osteoclasts | In vitro | Low | Induced by RANKL and produces downstream molecular 8-nitro-cGMP |
Promotes osteoclasts differentiation | Kaneko et al., 2018 [13] |
| Osteoclasts | In vitro | Low | Stimulated by cytokines and other mediators such as PG | Promotes osteoclasts bone resorption | Mentaverri et al., 2003 [14] |
| Osteoblasts | In vitro | Low | Induces osteoblast differentiation factor (Cbfa1) expression |
Promotes osteoblasts differentiation | Gloria et al., 2020 [15] |
| Osteoblasts | In vivo and in vitro | Low | Activates Src, Erk-1/2 and Akt signaling pathway through sGC and PKG2 | Promotes osteoblasts proliferation and anti-apoptotic effects |
Cepeda et al., 2020 [16] Ramdani et al., 2018 [17] |
| Osteoblasts | In vivo and in vitro | Low | Stimulated by estrogen | Promotes osteoblasts growth and development |
Gerbarg et al., 2016 [18] Crescitelli et al., 2019 [19] |
| Osteoblasts | In vitro | Low | Responses to mechanical stimulation |
Promotes osteoblasts proliferation and survival |
Wittkowske et al., 2016 [20] Maycas et al., 2017 [21] |
| Chondrocytes | In vitro | High | Induces caspase expression upregulation | Induces chondrocytes apoptosis | Poderoso et al., 2019 [22] Kamm et al., 2019 [23] |
| Chondrocytes | In vivo and in vitro | High | Stimulated by inflammatory mediators | Affects numerous biomolecular processes in chondrocytes |
Wojdasiewicz et al., 2014 [24] |
| Chondrocytes | In vitro | Low | Stimulated chondrocytes hypertrophy and increased the expression of alkaline phosphatase and type X collagen |
Promotes chondrocytes maturation | Teixeira et al., 2005 [25] Drissi et al., 2005 [26] |
2.1. NO and Osteoclasts
Osteoclasts resorb bone and originate from somatic cells related to the single-core macrophage lineage [27]. Although they have unique bone resorption capabilities, they have various characteristics in common with macrophages, some of which are selectively expressed and regulated. This might be due to the adaptation of osteoclasts to osteoclast physiology, thus developing their specific functions. The similarities and nuances that distinguish osteoclasts from non-bone-resorbing macrophages reflect some specific mechanisms in bone remodeling. The NO/NOS system might be one such mechanism.
Thirty years ago, NO was confirmed to regulate the osteoclasts’ roles. Maclntyre reported that in isolated rat osteoclasts, NO could inhibit the spreading of the cells and bone resorption [28]. Moreover, nitrosyl-cobinamide has been recently reported as an immediate NO release agent, which reduces the number of osteoclasts in intact mice and inhibits the increase in osteoclast numbers in ovariectomized mice [11]. Amano found that during osteoclast formation in mouse bone marrow cells, the osteogenic helioxanthin derivative could inhibit the cyclic guanosine monophosphate (cGMP) degradation activity of phosphodiesterase, promote NO production, and inhibit the differentiation of osteoclasts dose-dependently [12]. Other experiments, such as the mouse skull assay and the rat long bone assay, also demonstrated similar results [29][30]. Taken together, these results indicated the inhibitory effects of high NO concentrations on bone resorption via two types of effects: an immediate inhibitory effect on mature osteoclast bone resorption and a reasonable inhibitory effect on precellular osteoclast differentiation. In this pathway, the effects of NO are cGMP-independent and different from the common pathway in most other systems. Notably, NO can induce mature osteoclasts to get rid of bone and reduce their acid secretion, thereby inhibiting bone resorption [31]. This process appears to be mediated by endogenous NO production and requires cGMP and protein kinase G (PKG).
In addition, lower NO concentrations can also promote osteoclast differentiation and survival. Interleukin (IL)-1β and TNF-α are powerful stimulators of bone resorption; they could appropriately enhance NO formation in the organ cultures of bone as well as bone marrow cultures. The addition of NOS inhibitors resulted in inhibiting the induced bone resorption, demonstrating that the NO/NOS system was stimulated by cytokines and other cytokine-induced mediators, such as prostacyclin (PG), to enhance bone resorption [30][32].
Kaneko et al. found that 8-nitro-cGMP, which is a NO derivate that is formed when NO reacts with cGMP in the presence of reactive oxygen species (ROS), could promote RANKL-induced osteoclast differentiation [13]. Moreover, a study assessed the levels of all three NOS modes in bones as well as isolated osteoblasts and osteoclasts using reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry [33]. Knocking out the nNOS gene increased the relative density of trabecular and cortical bone minerals in mice; the accurate measurement of the bone structure indicated a decrease in the total number of osteoclasts and osteoblasts, with worsened bone remodeling, which was reflected by the low mineral accumulation and bone formation rate [34][35][36]. These major manifestations indicated that nNOS might be essential for the differentiation and/or survival of all normal osteoclasts in vivo.
Moreover, nNOS-deficient bone marrow monocytes could produce poorly functional osteoclasts in vitro. Correspondingly, the iNOS-deficient mice showed no significant bone abnormalities, and their femoral length, trabecular bone volume fraction, bone formation rate, and osteoclast surface were normal [37]; this confirmed that the NO production by iNOS was much larger than that produced by nNOS, resulting in the loss of the promotion function. Therefore, in bone, iNOS might limit osteoclast activity by increasing NO levels, thus preventing excessive bone resorption in inflammatory diseases.
In other words, the high NO concentration can reduce the number of osteoclasts and inhibit their differentiation, demonstrating inhibitory effects on osteoclast bone resorption. On the other hand, the low NO concentration, mainly produced by cNOS, can promote the differentiation and survival of osteoclasts. Thus, the osteoclasts produced in nNOS-deficient individuals can lead to poor function. However, these conclusions are based on only in vivo and in vitro studies and lack the support of clinical data.
2.2. NO and Osteoblasts
Similar to their effects on osteoclasts, they might promote the differentiation and survival of osteoblasts and vice versa. The low NO concentration has been confirmed both in vivo and in vitro. Recent studies on human somatic cells and mouse models showed that the osteoblasts lacked argininosuccinate lyase, an enzyme participating in the synthesis of arginine and contributing to NO production, which resulted in low NO production and failure to differentiate [38]. Another study found that the vascular smooth muscle cells from the renal artery of male Wistar rats treated with aminoguanidine (AG; an iNOS inhibitor) showed a reduced expression of osteoblast differentiation factor (Cbfa1) [15]. Moreover, Wei et al. demonstrated that using the biochemical signaling molecules, such as PGs, NO, and insulin-like growth factor-1 (IGF-1) released by osteocytes, could increase osteogenesis, which showed a guiding significance in contemporary clinical treatment [39]. A study investigating the possible underlying mechanism of action showed that the proliferation of osteoblasts was simulated by cell-permeable cGMP analogs and prevented by pharmacological inhibition of soluble guanylyl cyclase (sGC) or protein kinase 2 (PKG2) or siRNA-mediated PKG2 knockdown; this suggests that the positive effects of low NO concentrations on osteoblast proliferation were mediated by sGC and PKG2 [16]. PKG2 exerts its antiapoptotic and proliferation-promoting effects in osteoblasts by activating Src, extracellularly regulated protein kinases-1/2 (Erk-1/2), and Akt [17]. The activated Akt phosphorylates and inactivates glycogen synthase kinase-3β, thereby stabilizing β-catenin and activating the Wnt pathway genes. The activation of the Wnt signaling pathway is a key factor in the differentiation, proliferation, and survival of (pre)osteoblasts and in driving bone formation [40][41]. In addition, a small dose of NO donors could activate the mRNA expression of osteoblast genes, such as alkaline phosphatase, osteocalcin, and collagen-1, and increase bone matrix synthesis and mineralization, thereby enhancing the osteogenic differentiation of (pre)osteoblasts in vitro [42][43][44].
In addition, based on estrogen stimulation, the moderate cNOS expression in osteoblasts can produce NO, which has a substantial role in the growth and development of osteoblasts as well as cytokine production [18][19].
The initiation of mechanical stimulation is highly important for the growth, development, and remodeling of bone [20][21]. When fluid flows through the bone canalicular system, the resulting shear stress stimulates osteoblasts and osteocytes to enhance their anabolism nonspecifically. With the increase in their proliferation and survival, the bone marrow stromal cells, osteoblasts, and osteocyte-like cells respond to fluid shear stress in vitro. This anabolic reaction requires moderate NO production from calcium-mediated eNOS.
The high NO concentrations resulting from NO donors or proinflammatory cytokines can effectively inhibit the growth and differentiation of osteoblasts [45][46]. These conditions often occur in inflammatory disorders and are related to the inhibitory effects of pro-inflammatory cytokines on bone formation. Based on the animal model of inflammation-mediated osteopenia, the active cytokines are the reason for the reduced osteogenesis [47].
In summary, the high production of NO by iNOS in an inflammatory environment can effectively inhibit the growth and differentiation of osteoblasts. In most physiological conditions, the appropriate NO concentrations can promote osteoblast function, which might be mediated by sGC and PKG2. The initiation of mechanical stimulation can also contribute to the proliferation and survival of osteoblasts through moderate NO production.
2.3. NO and Chondrocytes
The effects of NO on chondrocytes in vivo under normal physiological conditions are hard to observe due to the dominance of iNOS in these bone cells. In other words, a necessary stimulation is required for the chondrocytes to produce NO.
A study using the chick cartilage model reported that the NO metabolites played a role in the maturation and differentiation of chondrocytes [25][26]. All three NOS isoforms are expressed and remain active in the growth plate. At least two NO-mediated functions are important in epiphyseal chondrocytes. After the maturation of chondrocytes, NO and related compounds stimulate chondrocyte hypertrophy via the cGMP-dependent pathway, thereby increasing the expression of alkaline phosphatase and type X collagen (maturation markers). In the late stages, inhibiting NO production can inhibit apoptosis, while exposure to NO donors increases apoptosis, suggesting that NO induces this process. This suggested that based on artificial stimulation, moderate NO production might contribute to chondrocyte proliferation in vitro; however, relevant in vivo data are still lacking.
References
- Subczynski, W.K.; Lomnicka, M.; Hyde, J.S. Permeability of nitric oxide through lipid bilayer membranes. Free Radic. Res. 1996, 24, 343–349.
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012.
- Koppenol, W.H.; Traynham, J.G. Say NO to nitric oxide: Nomenclature for nitrogen- and oxygen-containing compounds. Methods Enzymol 1996, 268, 3–7.
- Davis, K.L.; Martin, E.; Turko, I.V.; Murad, F. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 203–236.
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479.
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073.
- Shirazi, S.; Ravindran, S.; Cooper, L.F. Topography-mediated immunomodulation in osseointegration; Ally or Enemy. Biomaterials 2022, 291, 121903.
- Morimoto, A.; Kikuta, J.; Nishikawa, K.; Sudo, T.; Uenaka, M.; Furuya, M.; Hasegawa, T.; Hashimoto, K.; Tsukazaki, H.; Seno, S.; et al. SLPI is a critical mediator that controls PTH-induced bone formation. Nat. Commun. 2021, 12, 2136.
- Srivastava, R.K.; Sapra, L.; Mishra, P.K. Osteometabolism: Metabolic Alterations in Bone Pathologies. Cells 2022, 11, 3943.
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245.
- Kalyanaraman, H.; Ramdani, G.; Joshua, J.; Schall, N.; Boss, G.R.; Cory, E.; Sah, R.L.; Casteel, D.E.; Pilz, R.B. A Novel, Direct NO Donor Regulates Osteoblast and Osteoclast Functions and Increases Bone Mass in Ovariectomized Mice. J. Bone Miner. Res. 2017, 32, 46–59.
- Amano, H.; Iwaki, F.; Oki, M.; Aoki, K.; Ohba, S. An osteogenic helioxanthin derivative suppresses the formation of bone-resorbing osteoclasts. Regen. Ther. 2019, 11, 290–296.
- Kaneko, K.; Miyamoto, Y.; Tsukuura, R.; Sasa, K.; Akaike, T.; Fujii, S.; Yoshimura, K.; Nagayama, K.; Hoshino, M.; Inoue, S.; et al. 8-Nitro-cGMP is a promoter of osteoclast differentiation induced by RANKL. Nitric Oxide 2018, 72, 46–51.
- Mentaverri, R.; Kamel, S.; Wattel, A.; Prouillet, C.; Sevenet, N.; Petit, J.P.; Tordjmann, T.; Brazier, M. Regulation of bone resorption and osteoclast survival by nitric oxide: Possible involvement of NMDA-receptor. J. Cell Biochem. 2003, 88, 1145–1156.
- Gloria, M.A.D.; Mouro, M.G.; Geraldini, S.; Higa, E.M.S.; Carvalho, A.B. Cbfa1 expression in vascular smooth muscle cells may be elevated by increased nitric oxide/iNOS. J. Bras. Nefrol. 2020, 42, 300–306.
- Cepeda, S.B.; Sandoval, M.J.; Crescitelli, M.C.; Rauschemberger, M.B.; Massheimer, V.L. The isoflavone genistein enhances osteoblastogenesis: Signaling pathways involved. J. Physiol. Biochem. 2020, 76, 99–110.
- Ramdani, G.; Schall, N.; Kalyanaraman, H.; Wahwah, N.; Moheize, S.; Lee, J.J.; Sah, R.L.; Pfeifer, A.; Casteel, D.E.; Pilz, R.B. cGMP-dependent protein kinase-2 regulates bone mass and prevents diabetic bone loss. J. Endocrinol. 2018, 238, 203–219.
- Gerbarg, P.L.; Brown, R.P. Pause menopause with Rhodiola rosea, a natural selective estrogen receptor modulator. Phytomedicine 2016, 23, 763–769.
- Crescitelli, M.C.; Rauschemberger, M.B.; Cepeda, S.; Sandoval, M.; Massheimer, V.L. Role of estrone on the regulation of osteoblastogenesis. Mol. Cell. Endocrinol. 2019, 498, 110582.
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87.
- Maycas, M.; Esbrit, P.; Gortázar, A.R. Molecular mechanisms in bone mechanotransduction. Histol. Histopathol. 2017, 32, 751–760.
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019, 88, 61–72.
- Kamm, A.; Przychodzen, P.; Kuban-Jankowska, A.; Jacewicz, D.; Dabrowska, A.M.; Nussberger, S.; Wozniak, M.; Gorska-Ponikowska, M. Nitric oxide and its derivatives in the cancer battlefield. Nitric Oxide 2019, 93, 102–114.
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459.
- Teixeira, C.C.; Ischiropoulos, H.; Leboy, P.S.; Adams, S.L.; Shapiro, I.M. Nitric oxide-nitric oxide synthase regulates key maturational events during chondrocyte terminal differentiation. Bone 2005, 37, 37–45.
- Drissi, H.; Zuscik, M.; Rosier, R.; O'Keefe, R. Transcriptional regulation of chondrocyte maturation: Potential involvement of transcription factors in OA pathogenesis. Mol. Aspects Med. 2005, 26, 169–179.
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341.
- MacIntyre, I.; Zaidi, M.; Alam, A.S.; Datta, H.K.; Moonga, B.S.; Lidbury, P.S.; Hecker, M.; Vane, J.R. Osteoclastic inhibition: An action of nitric oxide not mediated by cyclic GMP. Proc. Natl. Acad. Sci. USA 1991, 88, 2936–2940.
- Löwik, C.W.; Nibbering, P.H.; van de Ruit, M.; Papapoulos, S.E. Inducible production of nitric oxide in osteoblast-like cells and in fetal mouse bone explants is associated with suppression of osteoclastic bone resorption. J. Clin. Investig. 1994, 93, 1465–1472.
- Ralston, S.H.; Ho, L.P.; Helfrich, M.H.; Grabowski, P.S.; Johnston, P.W.; Benjamin, N. Nitric oxide: A cytokine-induced regulator of bone resorption. J. Bone Miner. Res. 1995, 10, 1040–1049.
- Yaroslavskiy, B.B.; Li, Y.; Ferguson, D.J.; Kalla, S.E.; Oakley, J.I.; Blair, H.C. Autocrine and paracrine nitric oxide regulate attachment of human osteoclasts. J. Cell. Biochem. 2004, 91, 962–972.
- Ralston, S.H.; Grabowski, P.S. Mechanisms of cytokine induced bone resorption: Role of nitric oxide, cyclic guanosine monophosphate, and prostaglandins. Bone 1996, 19, 29–33.
- Helfrich, M.H.; Evans, D.E.; Grabowski, P.S.; Pollock, J.S.; Ohshima, H.; Ralston, S.H. Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J. Bone Miner. Res. 1997, 12, 1108–1115.
- Yan, Q.; Feng, Q.; Beier, F. Endothelial nitric oxide synthase deficiency in mice results in reduced chondrocyte proliferation and endochondral bone growth. Arthritis Rheum. 2010, 62, 2013–2022.
- Hefler, L.A.; Reyes, C.A.; O'Brien, W.E.; Gregg, A.R. Perinatal development of endothelial nitric oxide synthase-deficient mice. Biol. Reprod. 2001, 64, 666–673.
- Afzal, F.; Polak, J.; Buttery, L. Endothelial nitric oxide synthase in the control of osteoblastic mineralizing activity and bone integrity. J. Pathol. 2004, 202, 503–510.
- Watanuki, M.; Sakai, A.; Sakata, T.; Tsurukami, H.; Miwa, M.; Uchida, Y.; Watanabe, K.; Ikeda, K.; Nakamura, T. Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J. Bone Miner. Res. 2002, 17, 1015–1025.
- Liu, H.; Rosen, C.J. Nitric oxide and bone: The phoenix rises again. J. Clin. Investig. 2021, 131, e147072.
- Cao, W.; Helder, M.N.; Bravenboer, N.; Wu, G.; Jin, J.; Ten Bruggenkate, C.M.; Klein-Nulend, J.; Schulten, E. Is There a Governing Role of Osteocytes in Bone Tissue Regeneration? Curr. Osteoporos. Rep. 2020, 18, 541–550.
- Deng, S.; Dai, G.; Chen, S.; Nie, Z.; Zhou, J.; Fang, H.; Peng, H. Dexamethasone induces osteoblast apoptosis through ROS-PI3K/AKT/GSK3β signaling pathway. Biomed. Pharmacother. 2019, 110, 602–608.
- Nishimura, R.; Hata, K.; Kida, J. Regulation of osteoblasts and chondrocytes by Wnt signaling. Clin. Calcium 2019, 29, 299–307.
- Otsuka, E.; Hirano, K.; Matsushita, S.; Inoue, A.; Hirose, S.; Yamaguchi, A.; Hagiwara, H. Effects of nitric oxide from exogenous nitric oxide donors on osteoblastic metabolism. Eur. J. Pharmacol. 1998, 349, 345–350.
- Mancini, L.; Moradi-Bidhendi, N.; Becherini, L.; Martineti, V.; MacIntyre, I. The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem. Biophys. Res. Commun. 2000, 274, 477–481.
- Hikiji, H.; Shin, W.S.; Oida, S.; Takato, T.; Koizumi, T.; Toyo-Oka, T. Direct action of nitric oxide on osteoblastic differentiation. FEBS Lett. 1997, 410, 238–242.
- Damoulis, P.D.; Hauschka, P.V. Cytokines induce nitric oxide production in mouse osteoblasts. Biochem. Biophys. Res. Commun. 1994, 201, 924–931.
- Ralston, S.H.; Todd, D.; Helfrich, M.; Benjamin, N.; Grabowski, P.S. Human osteoblast-like cells produce nitric oxide and express inducible nitric oxide synthase. Endocrinology 1994, 135, 330–336.
- Minne, H.W.; Pfeilschifter, J.; Scharla, S.; Mutschelknauss, S.; Schwarz, A.; Krempien, B.; Ziegler, R. Inflammation-mediated osteopenia in the rat: A new animal model for pathological loss of bone mass. Endocrinology 1984, 115, 50–54.
More
Information
Subjects:
Sport Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Nitric Oxide: Physiology, Pharmacology, and Therapeutic Applications
Revisions:
2 times
(View History)
Update Date:
27 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





