Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Bevilacqua | -- | 2428 | 2023-02-24 16:11:42 | | | |
| 2 | Sirius Huang | Meta information modification | 2428 | 2023-02-27 06:38:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Altieri, C.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Gut Microbiota in Multiple Sclerosis Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/41644 (accessed on 08 February 2026).
Altieri C, Speranza B, Corbo MR, Sinigaglia M, Bevilacqua A. Gut Microbiota in Multiple Sclerosis Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/41644. Accessed February 08, 2026.
Altieri, Clelia, Barbara Speranza, Maria Rosaria Corbo, Milena Sinigaglia, Antonio Bevilacqua. "Gut Microbiota in Multiple Sclerosis Patients" Encyclopedia, https://encyclopedia.pub/entry/41644 (accessed February 08, 2026).
Altieri, C., Speranza, B., Corbo, M.R., Sinigaglia, M., & Bevilacqua, A. (2023, February 24). Gut Microbiota in Multiple Sclerosis Patients. In Encyclopedia. https://encyclopedia.pub/entry/41644
Altieri, Clelia, et al. "Gut Microbiota in Multiple Sclerosis Patients." Encyclopedia. Web. 24 February, 2023.
Copy Citation
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS), in which autoreactive immune cells attack the myelin sheaths of the neurons. Many scientific studies reveal a significant connection between human intestinal microbiota, eating habits, and the development of chronic-degenerative diseases; therefore, alterations in the composition and function of the microbiota may be accompanied by different chronic inflammatory mechanisms.
gut microbiota
diet
multiple sclerosis
dysbiosis
probiotics
1. Gut-Brain Axis: Role of Microbiota
A gut-brain axis (GBA) represents a dense and complex system of bidirectional communication existing between the gut microbiota and the central nervous system (CNS). This system is divided into 3 components: the neuronal connections, the general neuroendocrine and humoral pathways, and the immune system. The CNS can communicate directly with the intestine via sympathetic or parasympathetic branches of the autonomic nervous system (ANS), particularly via the vagus nerve. Hence, the microbiome can be modulated directly by bioactive molecules released by the enteric nervous system (ENS) or indirectly through other mechanisms that modify the microbial environment, such as gastrointestinal motility, permeability, pH value, or mucus secretion [1].
These adjustments are mostly mediated by the ANS’s secretion of acetylcholine or catecholamines, mediators influencing the circuits of the enteric nervous system.
For example, some studies suggest that acetylcholine is able to suppress the secretion of pro-inflammatory factors such as tumor necrosis factor alpha (TNFα), interleukin 6 (IL-6), and IL-18. Experiments with vagotomized mice illustrated the critical role of the vagus nerve in the dialogue between the gastrointestinal tract and the CNS. Treatment of mice with Lacticaseibacillus rhamnosus reduced the expression of the γ-aminobutyric acid (GABA) receptor in the brain and thus induced anxiolytic and antidepressant effects, that disappeared in mice after vagotomy. Similarly, no anxiolytic and behavioral influence was detected by Bifidobacterium longum in vagotomized mice with chronic colitis, while an attenuation of psychological comorbidities of colitis was observed after administration of Bifidobacterium longum in mice with a nerve signal by intact vagus [2].
The microbial metabolites (propionic acid, butyric acid, and acetic acid) also act as indirect neuromodulators; for example, in the intestine, serotonin is released by enterochromaffin cells (ECC) after their stimulation by the SCFAs [3].
Serotonin, or 5-hydroxytryptamine (5-HT), is synthesized from the amino acid tryptophan (5-HTP). The intestinal microbiota regulates the peripheral availability of Trp and therefore the synthesis of serotonin also at the level of the CNS; in fact, some studies conducted on germ free mice show increased levels of plasma Trp and hippocampal 5-HT after intestinal bacterial colonization [4].
Although the exact mechanisms of peripheral regulation of Trp are unknown, some studies indicate that the microbiota modulates the degradation of Trp along the kynurenine pathway.
Tryptophan can also be metabolized by the intestinal microbiota into indole and its derivatives, such as indole-3-aldehyde (IAld), indole-3-acetic acid (IAA), and indole-3-propionic acid (PAH). Indole formation from tryptophan occurs through activation of the tryptophanase (TnaA) enzyme, which can be found in many gram-negative and gram-positive bacterial species, including Escherichia coli, Clostridium spp., and Bacteroides spp. [4].
Beneficial anti-inflammatory effects are attributed to IPA, IAId, and IAA. These three metabolites of tryptophan would carry out the anti-inflammatory action as ligands of the aryl receptor for hydrocarbons (AHR) [5]. In particular, if these metabolites bind AHR receptors expressed on astrocytes, there is a significant reduction in inflammation of the CNS [4].
The neuroendocrine communication between intestinal microbiota and brain involves the Hypothalamus-Pituitary-Adrenal axis (HPA); in fact, glucocorticoids, mineralocorticoids or catecholamines, released following the activation of the HPA axis, can alter the composition of the microbiota, the permeability of the intestinal epithelium and the immune responses of the intestinal mucosa.
Many scientific studies have demonstrated that, from birth, the development of the immune system depends significantly on the biodiversity of the intestinal microbiota [6].
Contact with microbes is therefore essential for the development of efficient immunity, both at peripheral level and at intestinal level, for the production of GALT (lymphoid tissue associated with the intestine).
Gut microbial metabolites play a key role in inflammatory signaling, interacting both directly and indirectly with the host’s immune cells. SCFAs perform many of their actions by interacting with certain G-protein-coupled receptors (GPRs).
Acetate, a SCFA highly produced by bifidobacteria, regulates intestinal inflammation by stimulating the GPR43 receptor. In particular, when acetate binds to this receptor, it seems to inhibit the secretion of pro-inflammatory IL-18, of which high levels are found in the case of colon cancer [7].
Maslowski et al. [8] found that activation of GPR43 by SCFAs was necessary for normal resolution of some inflammatory responses; in fact, mice deficient in GPR43 (Gpr43 −/−) were shown to be unable to resolve inflammation in models of colitis, arthritis and asthma [9].
2. Multiple Sclerosis and Gut Microbiota
According to Compston and Coles [10], "Multiple sclerosis (MS) is primarily an inflammatory disorder of the brain and spinal cord in which focal lymphocytic infiltration leads to damage of myelin and axons." Autoantibodies cause the formation of circumscribed areas of demyelination in the brain and spinal cord, also called plaques. The pathological features of the plaques are the rupture of the blood-brain barrier (BBB), damage to the myelin sheath, oligodendrocytes, and axons, and an inflammatory infiltrate consisting mainly of lymphocytes and macrophages.
MS has a multifactorial pathogenesis, for which the dysfunctionality of the immune system (IS) has multiple causes, such as genetic predisposition, exposure to viral infectious agents, possible heavy metal poisoning, and pro-inflammatory lifestyles [11][12].
In about 85% of patients, MS occurs in a relapsing-remitting form (RRMS), 4% of people are diagnosed with secondary progressive multiple sclerosis (SPMS), and 10% are diagnosed with primary progressive multiple sclerosis (PPMS). If the RR-MS form is not treated in 50–60% of cases, it evolves towards the primary-progressive form.
Finally, a small percentage of people manifest a form of progressive multiple sclerosis immediately, with occasional relapses (relapsing—progressive multiple sclerosis).
The RRMS type is characterized by periods when symptoms are evident (relapse), followed by periods when symptoms disappear or improve (remission). In the case of SPMS and PPMS, there are no obvious relapses, but there is a gradual worsening of the disability over time.
The risk of developing MS is driven by both genetic factors and environmental exposures. The main genetic risk loci are in the HLA region (risk allele HLA-DRB1*15:01) [13]. However, more than 200 different DNA variants associated with disease susceptibility have been identified [14].
In addition, epigenetics (heritable or non-heritable changes not related to modified DNA sequences and leading to an altered expression or translation of the genome) also plays a major role in MS; the most important mechanisms are DNA methylation, histone modification, and RNA interference [15].
The importance of epigenetics in MS has been elucidated through studies on twins, and the data available suggest that the mechanisms are similar to those found in other autoimmune diseases (DNA methylation, histone modifications, and miRNA-based gene expression regulation); they promote a proinflammatory response, while few data are available on how epigenetics acts on other MS-associated factors (mitochondrial dysfunction, oxidative stress, and axonal degeneration) [15]. Several studies reported either decreased (HERV-W and PAD2) or increased methylation (SHP-1), increased histone citrullination (PAD4), increased (miR-17 and miR-20a), or decreased expression (among others, miR-155) [15]. There is also evidence that epigenetic changes occur in experimental autoimmune encephalomyelitis (EAE) studies [15].
MS is also triggered by environmental risk factors, including smoking, reduced exposure to sunlight, and infection with the Epstein–Barr virus [16]. For example, tobacco smoking is generally associated with an increased risk of MS in a dose-dependent manner [16], probably due to the gene-environment interaction "smoking and HLA-related MS susceptibility" [17], depending on lung irritation due to smoke inhalation and the triggering of T cells in the lungs [18]. Moreover, smoking is probably related to a worse diagnosis and a faster progression of the disease [16]. Another important environmental factor is low sun exposure, which causes a deficiency in vitamin D [19], probably due to the role of this vitamin in the regulation of inflammatory phenomena in auto-immune diseases [20]. Also, obesity and a high BMI index in adolescence increase the risk of MS, probably linked to inactivity, which in turn is responsible for increased peripheral inflammation [21].
According to some studies, there is also an important association between atmospheric pollution and pediatric MS, attributed to prolonged exposure to particulate matter with a diameter of 2.5 μm (PM2.5), carbon monoxide (CO), lead, and sulfur dioxide (SO2) [22]. Januel et al. [23] showed a significant odds ratio between exposure to PM2.5 and recurrences of MS, particularly in patients under the age of 30. The same study describes the mechanism by which PM2.5 would induce the (re)activation of the disease, i.e., the fine particulate reaches the pulmonary alveoli, activating the macrophages and causing the release of pro-inflammatory cytokines that induce the differentiation of T lymphocytes into Th1 lymphocytes. Part of these pro-inflammatory Th1 lymphocytes migrate to the CNS, triggering the inflammatory process in situ and lending credence to the idea of a lung-brain axis. This lung-brain axis would also underline the relationship between MS and exposure to cigarette smoke [24].
3. Dysbiosis in Multiple Sclerosis Patients and Therapeutic Approaches
Dysbiosis may be associated with endotoxemia, intestinal/systemic inflammation, and trigger neuroinflammation, whereas a healthy gut microbiota may dampen the inflammatory processes with the production of antiinflammatory molecules [25][26][27][28].
Recently, intestinal microbiota emerged as an additional potential influencing or triggering factor for MS [29][30], as well as for other autoimmune diseases [31].
There is evidence in the literature that gut bifidobacteria play a role in the onset of MS. A protective role of bifidobacteria in the pathogenesis of MS—and also of Guillain-Barré syndrome (GBS)—was hypothesized by Shi et al. [32]: a lower level of bifidobacteria has been negatively correlated with the levels of IL-17A in cerebrospinal fluid (CSF) and plasma samples of patients. IFN-γ and IL-17 are produced by pro-inflammatory Th1 and Th17 cells, respectively, and have important roles in the pathogenesis of both MS and GBS [33]. Notably, GBS, like MS, is an autoimmune demyelinating disorder, and the coexistence of these two syndromes in a patient has been reported [34].
Studies using experimental models have indicated that multiple sclerosis (MS)-like disease can be triggered in the gut following interactions of brain autoimmune T lymphocytes with local microbiota. When the human-derived microbiota was transferred into transgenic mice expressing a myelin autoantigen-specific T cell receptor, it was found that gut microbiota from MS-affected twins induced central nervous system (CNS)-specific autoimmunity at a higher incidence than microbiota from healthy co-twins. So there was functional evidence that human microbiome components contribute to CNS-specific autoimmunity [35].
Specifically, differences in microbial abundance between MS patients and controls were observed, and it was investigated how particular MS-associated bacteria modulate T lymphocyte responses using both in vitro and in vivo model systems. There is evidence that differences in specific gut bacteria are functionally associated with a shift toward a pro-inflammatory T cell profile, exacerbating autoimmune responses and thus potentially contributing to MS pathogenesis [36].
Berer and collaborators [35] compared the composition of the gut microbiota in 34 pairs of monozygotic twins, which were discordant for MS. There were no differences in the quality and quantity of intestinal microorganisms, except for a higher presence of Akkermansia in the twins with MS not undergoing therapy and a higher relative abundance of Sutturella in healthy twins. In murine transplants, the microbiota of MS patients caused a significantly higher incidence of autoimmunity.
The results showed Akkermansia to be more abundant in mice transplanted with fecal material from the MS twins and Sutterella to be more abundant in mice that received fecal material from healthy twins.
It has been shown that in patients with MS, there is a lower presence of the genera Bacterioides, Parabacteroides, Prevotella, and Lactobacillus; on the other hand, there is a higher abundance of Akkermansia, Ruminococcus, Blautia, and Bifidobacterium (enterotype 3).
Cekanaviciute et al. [36] identified specific bacteria associated with MS and demonstrated that these bacteria regulate T-cell-mediated adaptive immune responses and create a pro-inflammatory environment in vitro and in vivo. The findings of these researchers may suggest “therapeutic” targeting of the microbiota as a treatment for MS. The microbiome of 71 individuals with RR-MS and 71 healthy controls highlighted in the microbiota of subjects affected by MS a greater presence of Akkermansia muciniphila and Acinetobacter calcoaceticus and a lower load of Parabacteroides distasonis.
Figure 1 depicts a synopsis of the gut microbiota and the differences between MS patients and healthy individuals.
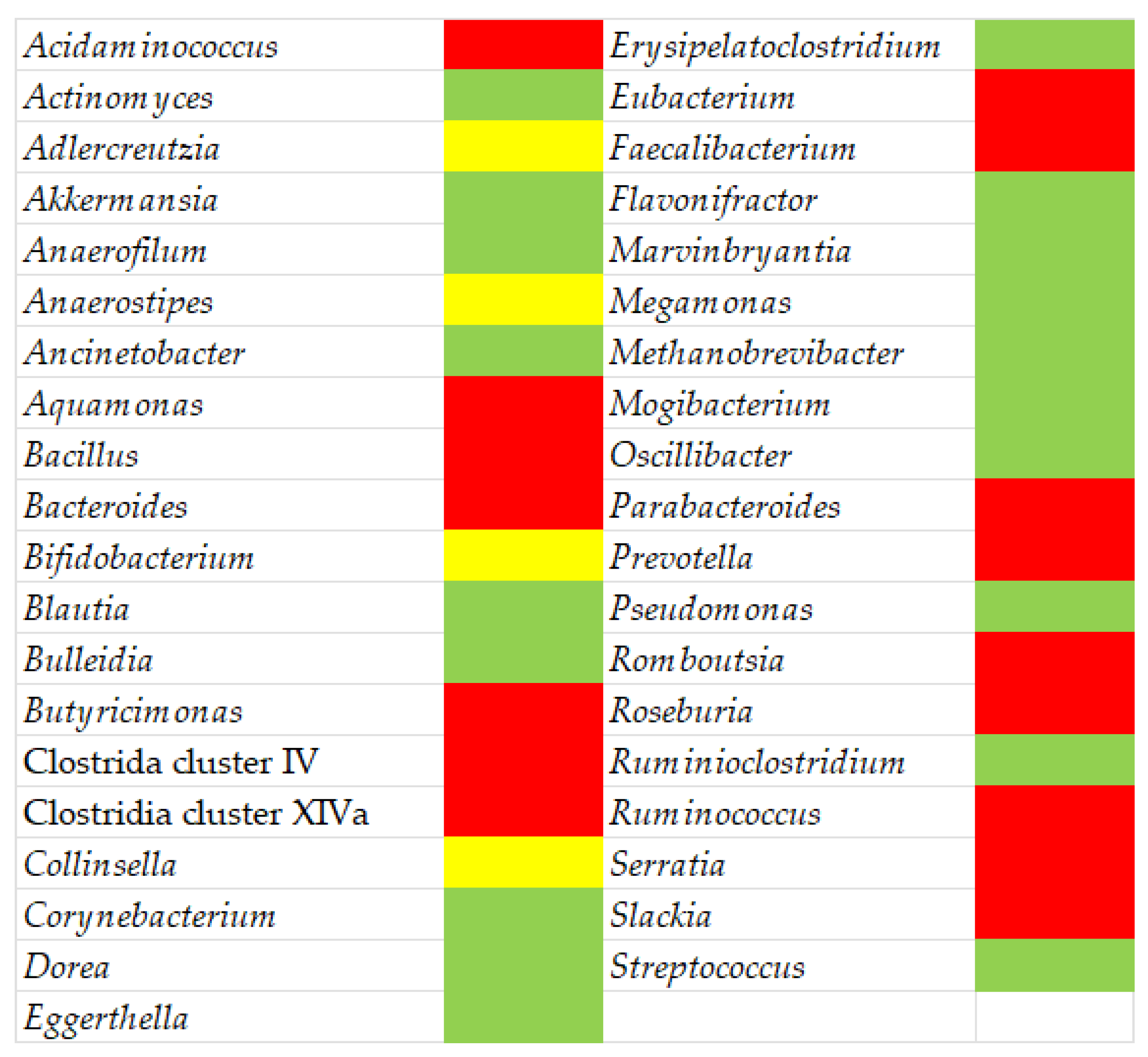
Figure 1. Gut microbiota in MS patients: green, higher abundance compared to healthy subjects; red, decreased abundance; yellow, conflicting results.
Data and evidence available suggest that an action on gut microbiota could contribute to the amelioration and reduction of symptoms and negative effects of MS. In this context, some authors addressed this topic and suggested at least three possible approaches, namely direct manipulation, indirect manipulation, and microbiota replacement [31]. Direct manipulation is generally focused on the supplementation of probiotics. Some studies on probiotic reported positive effects after the supplementation of Limosilactobacillus reuteri, or of a cocktail including several lactobacilli and bifidobacteria (e.g., Lactobacillus acidophilus, Lactiplantibacillus plantarum, Lacticaseibacillus paracasei, Bifidobacterium breve, B. infantis etc.) at least on animal models [37][38][39], and other positive results are reported by some clinical studies [31]. A second approach for direct manipulation is an antibiotic treatment to deplete pro-inflammatory taxa in the gut, but this method has shown conflicting results [31].
Indirect manipulation is focused on the modification of the gut microbiota of MS patients through dietary habits, by promoting butyrate-producing bacteria (for example, through the supplementation of prebiotics) [31]. Finally, microbiota transplantation seems a promising approach, at least based on some pilot studies [40][41].
It is worth mentioning that microbiota modulation has been recognized as a possible approach to halting neurodegenerative symptoms in MS patients, but also as a method to prevent disease appearance by assessing positive biomarkers or as a means to promote active remyelination, considering the positive outcome of butyrate-producing bacteria on animal models [42].
In this context, the discovery of new biomarkers is crucial. Nowadays, there are three main kinds of biomarkers in MS, known as diagnostic biomarkers (to distinguish patients with MS from healthy controls), disease activity biomarkers (to assess inflammation, oxidative stress, or demyelination as well as disease progression), and treatment response biomarkers (to study the effect of a therapy) [43]. A drawback is the lack of a predictive or early-diagnosis biomarker that can identify individuals at risk of developing MS or point out neurologically asymptomatic individuals. An omics approach could be useful through the integration of data and technology from several methodologies (genomics—mainly next-generation sequencing or genome-wide association studies—transcriptomics, lipidomic, and proteomic) [44].
Another promising way is the possibility of developing a new series of non-invasive biomarkers based on saliva or urine due to the promising results of some authors in elucidating the potential of some molecules (human leucocyte antigen-class II; immunoglobulin free light chains; urinary proteins) [45][46][47].
References
- Wang, S.Z.; Yu, Y.J.; Adeli, K. Role of Gut Microbiota in Neuroendocrine Regulation of Carbohydrate and Lipid Metabolism via the Microbiota-Gut-Brain-Liver Axis. Microorganisms 2020, 8, 527.
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441.
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes 2022, 14, 2102878.
- Haase, S.; Linker, R.A. Inflammation in multiple sclerosis. Therap. Adv. Neurol. Disord. 2021, 14, 17562864211007687.
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34.
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The role of microbiota in infant health: From early life to adulthood. Front. Immunol. 2021, 12, 708472.
- Li, Y.; Zhang, X.; Zhao, C. Guillain-Barré Syndrome-Like Polyneuropathy Associated with Immune Checkpoint Inhibitors: A Systematic Review of 33 Cases. BioMed Res. Int. 2021, 2021, 9800488.
- Maslowski, K.; Vieira, A.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587.
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517.
- Riccio, P.; Rossano, R. Diet, Gut Microbiota, and Vitamins D + A in Multiple Sclerosis. Neurotherapeutics 2018, 15, 75–91.
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple sclerosis: Pathogenesis, symptoms, diagnoses, and cell based therapy. Cell J. 2017, 19, 1–10.
- Miller, D.H.; Hornabrook, R.W.; Dagger, J.; Fong, R. Class II HLA antigens in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1989, 52, 575–577.
- Sawcer, S.; Franklin, R.J.; Ban, M. Multiple sclerosis genetics. Lancet Neurol. 2014, 13, 700–709.
- Küçükali, C.; Kürtüncü, M.; Çoban, A.; Çebi, M.; Tüzün, E. Epigenetics of multiple sclerosis: An update review. Neuromol. Med. 2015, 17, 83–96.
- Huang, J.; Kockum, I.; Stridh, P. Trends in the environmental risks associated with earlier onset in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 68, 104250.
- Hedstrom, A.K.; Katsoulis, M.; Hossjer, O.; Bomfim, I.L.; Oturai, A.; Sondergaard, H.B.; Sellebjerg, F.; Ullum, H.; Thorner, L.W.; Gustavsen, M.W.; et al. The interaction between smoking and HLA genes in multiple sclerosis: Replication and refinement. Eur. J. Epidemiol. 2017, 32, 909–919.
- Odoardi, F.; Sie, C.; Streyl, K.; Ulaganathan, V.K.; Schlager, C.; Lodygin, D.; Heckelsmiller, K.; Nietfeld, W.; Ellwart, J.; Klinkert, W.E.; et al. T cells become licensed in the lung to enter the central nervous system. Nature 2012, 488, 675–679.
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann. Neurol. 2007, 61, 504–513.
- Cantorna, M.T.; Hayes, C.E.; DeLuca, H.F. 1,25-dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 1996, 93, 7861–7864.
- Cortese, M.; Riise, T.; Bjornevik, K.; Myhr, K.M. Multiple Sclerosis Conscript Service Database Study, G. Body size and physical exercise, and the risk of multiple sclerosis. Mult. Scler. 2018, 24, 270–278.
- Hardy, D.; Chitnis, T.; Waubant, E.; Banwell, B. Preventing Multiple Sclerosis: The Pediatric Perspective. Front. Neurol. 2022, 13, 802380.
- Januel, E.; Dessimond, B.; Colette, A.; Annesi-Maesano, I.; Stankoff, B. Fine Particulate Matter Related to Multiple Sclerosis Relapse in Young Patient. Front. Neurol. 2021, 12, 651084.
- Lavery, A.M.; Collins, B.N.; Waldman, A.T.; Hart, C.N.; Bar-Or, A.; Marrie, R.A.; Arnold, D.; O’Mahony, J.; Banwell, B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult. Scler. 2018, 25, 515–522.
- Mirza, A.; Forbes, J.B.; Zhu, F.; Bernstein, C.N.; Van Domselaar, G.; Graham, M.; Waubant, E.; Tremlett, H. The multiple sclerosis gut microbiota: A systematic review. Mult. Scler. Relat. Disord. 2020, 37, 101427.
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in Multiple Sclerosis; Where Are We, What We Know and Do Not Know. Brain Sci. 2020, 10, 234.
- Rea, K.; Dinan, T.G.; Cryan, J.F. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 2016, 4, 23–33.
- Daisuke, N.; Miyake, S. Gut dysbiosis and multiple sclerosis. Clin. Immunol. 2022, 235, 108380.
- Berer, K.; Krishnamoorthy, G. Microbial view of central nervous system autoimmunity. FEBS Lett. 2014, 588, 4207–4213.
- Wekerle, H.; Hohlfeld, R. Gut Microbiota in Multiple Sclerosis: A Bioreactor Driving Brain Autoimmunity. In Translational Neuroimmunology in Multiple Sclerosis. From Disease Mechanisms to Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2016; Volume 9, pp. 113–125.
- Miyahuchi, E.; Shimokawa, C.; Steimle, A.; Desai, M.S.; Ohno, H. The impact of gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 2023, 23, 9–23.
- Shi, P.; Qu, H.; Nian, D.; Chen, Y.; Liu, X.; Li, Q.; Wang, C.; Ye, M.; Ma, B. Treatment of Guillain-Barré syndrome with Bifidobacterium infantis through regulation of T helper cells subsets. Int. Immunopharmacol. 2018, 61, 290–296.
- Li, S.; Jin, T.; Zhang, H.L.; Yu, H.; Meng, F.; Quezada, H.C.; Zhu, J. Circulating Th17, Th22, and Th1 Cells Are Elevated in the Guillain-Barré Syndrome and Downregulated by IVIg Treatments. Mediat. Inflamm. 2014, 4, 1–10.
- Etemadifar, M.; Roomizadeh, P.; Abtahi, S.H.; Sajjadi, S.; Abedini, A.; Golabbakhsh, A.; Fereidan-Esfahani, M.; Akbari, M. Linkage of multiple sclerosis and guillain-barre syndrome: A population-based survey in Isfahan, Iran. Autoimmune Dis. 2012, 2012, 232139.
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jiac, X.; Xiao, L.; Xia, Z.; Liud, C.; Klotze, L.; Staufferf, U.; Baranzinic, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724.
- Cekanaviciute, E.; Yoob, B.B.; Runiaa, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718.
- Calvo- Barreiro, L.; Eixarch, H.A.; Ponce-Alonso, M.; Castillo, M.; Lebrón-Galán, R.; Mestre, L.; Guaza, C.; Clemente, D.; Del Campo, R.; Montalban, X.; et al. A commercial probiotic induces tolerogenic and reduces pathogenic responses in experimental autoimmune encephalomyelitis. Cells 2020, 9, 906.
- He, B.; Hoanh, T.K.; Tian, X.; Taylor, C.M.; Blanchard, E.; Luo, M.; Bhattacharjee, M.B.; Freeborn, J.; Park, S.; Couturier, J.; et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front. Immunol. 2019, 10, 385.
- Kwon, H.K.; Kim, G.-C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.-E.; Nam, J.H.; Im, S.-H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227.
- Makkawi, S.; Camara- Lemarroy, C.; Metz, L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e459.
- Borody, T.; Leis, S.; Campbell, J.; Margaux, T.; Anna, N. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Am. J. Gastoenterol. 2011, 106, S352.
- Chen, T.; Noto, D.; Hoshino, Y.; Mizuno, M.; Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J. Neuroinflamm. 2019, 16, 165.
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkets in multiple sclerosis. Cold. Spring Harb. Perspect. Med. 2019, 9, a029058.
- Lorefice, L.; Pitzalis, M.; Murgia, F.; Fenu, G.; Atzori, L.; Cocco, E. Omics approaches to understanding the efficacy and safety of disease-modifying treatments in multiple sclerosis. Front. Genet. 2023, 14, 1076421.
- Farah, M.; Haraty, H.; Salame, Z.; Fares, Y.; Ojcius, D.M.; Sadier, N.S. Salivary biomarkers for the diagnosis and monitoring of neurological disease. Biomed. J. 2018, 41, 63–87.
- Lotan, I.; Ganelin-Cohen, E.; Tartakovsky, E.; Khasminsky, V.; Hellmann, M.A.; Steiner, I.; Ben-Zvi, I.; Livneh, A.; Golderman, S.; Kaplan, B. Saliva immunoglobulin free light chain analysis for monitoring disease activity and response to treatment in multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 44, 102339.
- Ahn, J.A.; Kang, C.-K.; Kim, E.-M.; Kim, A.R.; Kim, A. Proteomics of early detection of non-muscle invasive bladder cancer: Clinically useful urine protein biomarkers. Life 2022, 12, 395.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
669
Revisions:
2 times
(View History)
Update Date:
27 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




