Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bernhard Biersack | -- | 6466 | 2023-02-24 14:34:49 | | | |
| 2 | Jessie Wu | -2218 word(s) | 4248 | 2023-02-27 02:00:25 | | | | |
| 3 | Jessie Wu | Meta information modification | 4248 | 2023-02-27 02:03:03 | | | | |
| 4 | Jessie Wu | + 3 word(s) | 4251 | 2023-02-27 02:04:56 | | | | |
| 5 | Jessie Wu | + 3 word(s) | 4254 | 2023-02-27 02:06:20 | | | | |
| 6 | Jessie Wu | + 6 word(s) | 4260 | 2023-02-27 02:12:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nitzsche, B.; Höpfner, M.; Biersack, B. Heat Shock Protein 70 Inhibitors. Encyclopedia. Available online: https://encyclopedia.pub/entry/41640 (accessed on 07 February 2026).
Nitzsche B, Höpfner M, Biersack B. Heat Shock Protein 70 Inhibitors. Encyclopedia. Available at: https://encyclopedia.pub/entry/41640. Accessed February 07, 2026.
Nitzsche, Bianca, Michael Höpfner, Bernhard Biersack. "Heat Shock Protein 70 Inhibitors" Encyclopedia, https://encyclopedia.pub/entry/41640 (accessed February 07, 2026).
Nitzsche, B., Höpfner, M., & Biersack, B. (2023, February 24). Heat Shock Protein 70 Inhibitors. In Encyclopedia. https://encyclopedia.pub/entry/41640
Nitzsche, Bianca, et al. "Heat Shock Protein 70 Inhibitors." Encyclopedia. Web. 24 February, 2023.
Copy Citation
A class of chaperones dubbed heat shock protein 70 (Hsp70) possesses high relevance in cancer diseases due to its cooperative activity with the well-established anticancer target Hsp90. However, Hsp70 is closely connected with a smaller heat shock protein, Hsp40, forming a formidable Hsp70-Hsp40 axis in various cancers, which serves as a suitable target for anticancer drug design.
Hsp70
heat shock proteins
cellular
Hsp40
anticancer agents
1. Introduction
Stress factors such as heat lead to defensive and protective cellular responses, enabling the cell to cope with the stress [1]. The emergence of chromosomal puffs as a defined heat shock response in Drosophila flies was observed for the first time in 1962 by Ferruccio Ritossa [2]. In 1974, the increased expression of certain heat shock proteins in response to heat and other stress factors was discovered [3]. These heat shock proteins (Hsps) are classified by their molecular weights in kDa (e.g., Hsp90, heat shock protein 70 (Hsp70), etc.) and function as chaperones in order to protect important proteins from degradation, to control the quality of protein folding, and to deliver misfolded or damaged proteins to the proteasome for disposal (protein triage), thus vouchsafing cell viability under these conditions [4]. This multi-chaperone system (“epichaperome”) plays an important role in various cancer diseases [5]. Canonical functions of chaperones are linked with the ubiquitin-proteasome system (UPS) and chaperone-mediated autophagy (CMA), while non-canonical functions affect the immune system, including inflammatory and autoimmune mechanisms [6]. Thus, targeting heat shock proteins is a promising strategy to combat cancer.
The heat shock protein (Hsp) family is subdivided into Hsp110 (HSPH), Hsp90 (HSPC), Hsp70 (HSPA), Hsp40 (DNAJ), small Hsps (HSPB), and the chaperonin family proteins Hsp60/Hsp10 (HSPD/E) and TRiC (CCT) [7]. Hsp90 has already become a valuable chaperone drug target. Prominent examples of anticancer active Hsp90 inhibitors are the natural products (geldanamycin and radicicol) as well as their (semi-)synthetic derivatives, 17-AAG/tanespimycin and ganetespib, the latter have reached clinical trials [8]. Hsp90 regulates the activity and stability of crucial transcription factors such as the tumor suppressor p53 and the androgen receptor (AR), while Hsp90 activity itself is regulated by posttranslational modifications (e.g., lysine acetylation under control by HDAC6) and by other heat shock proteins such as Hsp70 [9]. The protein folding by Hsp90 and Hsp70 is ATP-dependent, while Hsp70 has a crucial function in the protection of cells against various stress factors, including enhanced cell survival. In addition, Hsp70 forms complexes with Hsp90 with the help of HOP (Hsp70–Hsp90 organizing protein) in order to exert its housekeeping activities [10][11][12]. There is growing evidence that Hsp70 inhibitors have the potential to overcome Hsp90 inhibitor resistance [13]. The organelle-specific members of the Hsp70 protein family, such as mitochondrial mortalin (mtHsp70, Grp75) and endoplasmic reticulum/ER-based Grp78, were also identified as possible anticancer drug targets due to their crucial roles in cell proliferation and survival in various cancers [14][15]. In addition, extracellular Hsps attracted increased attention as cancer targets and biomarkers [16].
Client polypeptides are transferred to Hsp70 by the smaller co-chaperone, Hsp40. Hsp40 prevents the aggregation of unfolded polypeptides, but there are also folded proteins among the clients of Hsp40. There are numerous Hsp40 isoforms, also dubbed J-domain proteins (DNAJs), that are structurally different from Hsp70 and Hsp90 proteins [17]. Nevertheless, DNAJ/Hsp40 and Hsp70 proteins form a tight Hsp70/Hsp40 complex in order to fulfill their protein-folding functions [17][18]. Hsp40 binds to the nucleotide-binding domain (NBD) of Hsp70 and accelerates the ATPase activity of Hsp70 enormously [18]. Thus, small-molecule inhibitors of the Hsp70-Hsp40 axis have considerable potential as anticancer drug candidates.
2. Inducible Heat Shock Protein 70 and Heat Shock Cognate 71 Kilodalton Protein Inhibitors
Inducible Hsp70 (also abbreviated as Hsp70i) and consecutive Hsc70 inhibitors can be classified according to their binding mode into N-terminal nucleotide binding domain/NBD-targeting inhibitors, C-terminal substrate binding domain/SBD-targeting inhibitors, and allosteric inhibitors (Table 1).
In 2009, the adenosine-derivative 1 (Figure 1, VER-155008) was identified as a selective NBD-targeting Hsc70/Hsp70 inhibitor (IC50 = 0.5 µM), which showed antiproliferative effects on HCT-116 colon carcinoma cells (GI50 = 5.0 µM), reduced Raf-1 and Her2 protein levels, and enhanced the apoptosis induction by the Hsp90 inhibitors 17-AAG and VER-82160 in HCT-116 cells [19][20]. Compound 1 also revealed promising effects on non-small cell lung cancer (NSCLC), such as inhibition of NSCLC proliferation and cell cycle arrest (increased G0/G1 cell percentage) [21]. In addition, compound 1 inhibited pleural mesothelioma cell proliferation and colony formation, which are associated with G1 cell cycle arrest, suppressed phospho-Akt, and induction of macroautophagy [22]. In LNCaP95 prostate cancer cells, the Hsp70 inhibitor 1 induced apoptosis and suppressed the expression of the full-length androgen receptor (AR-FL) and of the androgen receptor splice variant 7 (AR-V7), which are associated with castration-resistant prostate cancer (CRPC) [23]. These AR-suppressing effects were correlated with an inhibition of YB-1 phosphorylation by compound 1, followed by reduced nuclear translocation of YB-1. Another study using the AR-positive LNCaP and the AR-negative PC-3 prostate cancer cell lines showed that compound 1 was antiproliferative and pro-apoptotic in both cell lines, albeit the pro-apoptotic effects were higher in the AR-positive cells [24]. Compound 1 downregulated AR expression and induced G1 cell cycle arrest in the LNCaP cells. Moreover, a distinct suppression was observed for Hsp27 in PC-3 cells and HOP and Hsp90β in both cell lines treated with compound 1. In MCF-7 breast cancer cells, compound 1 induced apoptosis associated with mitochondrial damage, and the anticancer activity of compound 1 was reduced by heat shock [25]. Anaplastic thyroid carcinoma (APC) is the most lethal thyroid cancer with high drug resistance, but compound 1 was able to induce paraptosis in APC cells dependent on de novo protein synthesis [26]. In a panel of glioma cells (1321N1, GOS-3, and U87-MG), compound 1 showed higher antiproliferative activities (IC50 = 12–13 µM) than the approved drug temozolomide (IC50 = 135–180 µM) associated with downregulation of Akt kinase activity and the modulation of certain miRNAs, e.g., the upregulation of miR-215 and miR-194-5p [27]. Compound 1 was evaluated in muscle-invasive bladder cancer (MIBC) models and induced apoptosis along with inhibition of MIBC cell proliferation and migration [28]. The activity of compound 1 against MIBC was associated with the suppression of protein members of p53/Rb, PI3K, and SWI/SFW signaling. Especially strong degrading effects of compound 1 were observed on the demethylase KDMA6 and the histone acetyltransferase EP300, both members of the histone modification pathway.
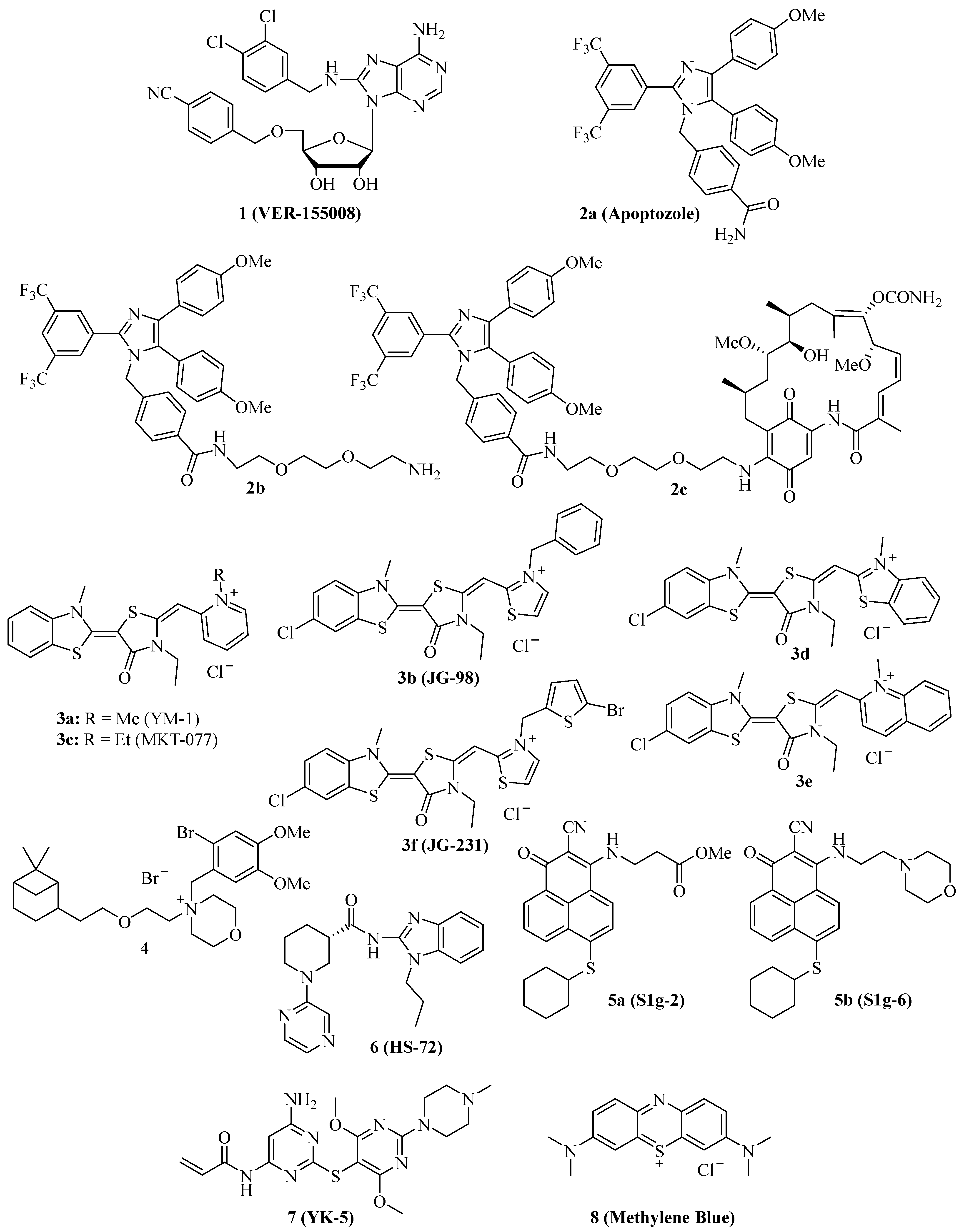
Figure 1. Structures of NBD-binding and interfering compounds 1–8.
Due to these promising anticancer effects of compound 1, its combination with various other anticancer drugs was investigated. In combination with the Hsp90 inhibitor radicicol, compound 1 was able to enhance the APC cell killing activity of radicicol, accompanied by suppressed heat shock cognate 70/Hsc70, Akt, and survival [29]. The combination of compound 1 with the Hsp90 inhibitor STA9090 was evaluated in MIBC models and was more efficient than single compound therapy [28]. However, the combination of compound 1 with doxorubicin in canine osteosarcoma (OSA) cells showed no improvements. Already, compound 1 alone displayed strong apoptosis induction, inhibition of colony formation, and antiproliferative activities against OSA cells based on Hsp70 inhibition as well as Akt suppression and BAG1 degradation [30]. In contrast to that, compound 1 showed synergy effects in combination with the Hsp90 inhibitor 17-AAD and sensitized A549 NSCLC cells to radiation therapy [21]. The combination of compound 1 with manumycin A, an anticancer active antibiotic that upregulates Hsp70 in cancer cells, sensitized lung tumor cells to manumycin A treatment [31]. Micelles of compound 1 together with gold nanorods were successfully tested as mild-temperature photothermal therapy in colon cancer, leading to strong colon tumor growth in vivo at a temperature of 45 °C [32]. Compound 1 exhibited considerable activity against multiple myeloma (MM) cells (IC50 = 1.7 µM for OPM2, 3.0 µM for RPMI 8226, and 6.5 µM for MM.1S cells), and the combination of compound 1 with the proteasome inhibitor bortezomib displayed synergy effects in terms of apoptosis induction in MM cells, which was associated with suppression of anti-apoptotic Bcl-2, Bcl-xL, and Mcl-1 and upregulation of pro-apoptotic NOXA and Bim [33]. In addition, the ER stress marker CHOP (CCAAT-enhancer binding protein homologous protein) was induced by this combination treatment. The natural product shikonin was described as a proteasome inhibitor and necroptosis inducer in MM cells while it also upregulated Hsp70, and, thus, the combination with compound 1 enhanced shikonin-induced MM cell death [34]. Compound 1 also exhibited promising effects on acute myeloid leukemia (AML) cells alone (induction of apoptosis, inhibition of cell proliferation, and colony formation) and in combination with the Hsp90 inhibitor 17-DMAG (additive antiproliferative and pro-apoptotic activity) [35]. The release of AML cell growth factors and regulators such as TNF-α, VEGF, IL-3, IL-1β, and IL-1 receptor antagonist was strongly suppressed upon treatment with compound 1. A new structurally related 6,8,9-trisubstituted purine derivative was recently disclosed that induced apoptosis and senescence in luminal A subtype MCF-7 breast carcinoma cells [36].
The substituted imidazole derivative 2a (Figure 1, apoptozole) was discovered as a pro-apoptotic inhibitor of Hsc70 (KD = 210 nM) and Hsp72 (KD = 140 nM) in 2008, which showed high tumor cell growth inhibitory activities with GI50 values in the nanomolar concentration range (GI50 = 220 nM for SK-OV-3, 250 nM for HCT-15, and 130 nM for A549 cells) [37]. Compound 2a was identified as an inhibitor of the Hsp70 ATPase by affinity chromatography upon conjugation of the amino-ethyloxy modified apoptozole derivative 2b (Figure 1) with a resin, leading to a reduced interaction of Hsp70 with APAF-1, while no affinity to Hsp40, Hsp60, or Hsp90 was observed [38]. Time-dependent antiproliferative activity was checked, and amenable IC50 values (0.8 µM for A549, 0.8 µM for HeLa, 0.7 µM for MDA-MB-231, and 0.7 µM for HepG2 cells) were obtained after 72 h of incubation, while the combination with doxorubicin led to a sensitization of A549 and HeLa cells to doxorubicin treatment. At doses of 4 mg/kg/day (i.p.) given for two weeks, compound 2b reduced the tumor growth of A549, RKO, and HeLa tumor xenografts by 61%, 65%, and 68%, respectively; in the latter case, the combination with doxorubicin led to more pronounced tumor growth inhibition (81% tumor growth reduction). The anti-leukemia properties of compound 2b and its hybrid molecules, such as compound 2c (Figure 1), with the Hsp90 inhibitor geldanamycin (2b and geldanamycin connected by ethylene glycol-based linker systems) were studied [39]. Compounds 2b, hybrid 2c, and other related hybrids induced apoptosis in a caspase-dependent way, however, the hybrids were more active against leukemia cells than their parent compounds 2b and geldanamycin. Compound 2c inhibited autophagy, but it also induced apoptosis in HeLa cancer cells based on its selective inhibition of the lysosomal Hsp70 and the degradation of lysosomal membranes associated with cathepsin release followed by caspase activation [40].
Several synthetic allosteric inhibitors of the Hsp70 ATPase domain were described. The rhodacyanine class of the Hsp70-inhibitory dye compounds was established by the discovery of compound 3a (Figure 1, YM-1), in particular, by the blocking of the Hsp70 interaction with the nucleotide exchange factor (NEF) Bag3 by compound 3a (IC50 = 4.8 µM) [41]. 3a binds to an allosteric binding site, stabilizing ADP-bound Hsp70 with a weak Bag3 affinity. Consequently, compound 3a suppressed FoxM1 and HIF1α pathways in MCF-7 and HeLa cells, which is unique for Hsp70-Bag3 inhibition since other Hsp70 inhibitors (e.g., the natural flavonoid myricetin) did not show such mechanistic effects. In MCF-7 breast carcinoma xenografts, compound 3a (25 mg/kg every second day for 6 days, i.p.) inhibited tumor growth associated with induction of p21 and suppression of FoxM1 and survivin. The activity of compound 3a was also investigated in glioma models, and it sensitized U251 and U343 glioma cells to treatment with the Bcl-2 inhibitors (-)-gossypol (AT-101) and ABT-737 [42]. Analogously, 3a sensitized apoptosis-resistant and chemo-resistant breast cancer cells to drug treatment [43]. In doxorubicin-resistant BT-549rDOX cells, compound 3a suppressed Mcl-1 and showed synergistic antiproliferative effects in combination with doxorubicin. In addition to compound 3a, further close Hsp70-inhibitory analogs such as compound 3b (Figure 1, JG-98) with thiazolium moieties were described, which were discovered during the search for optimized Hsp70 inhibitors derived from compound 3a and the mortalin (mitochondrial Hsp70) inhibitor MKT-077 (Figure 1, compound 3c, see below). Compound 3b exhibited prolonged microsomal half-lives and at least 3-fold higher antiproliferative activities against MDA-MB-231 triple-negative breast cancer (TNBC; EC50 = 0.4 µM) and hormone-sensitive MCF-7 breast cancer cells (EC50 = 0.7 µM) than compounds 3a (EC50 = 2.0 for MDA-MB-231 and 5.2 for MCF-7 cells) and 3c (EC50 = 1.4 for MDA-MB-231 and 2.2 for MCF-7 cells) [44]. Compound 3b induced apoptosis in MDA-MB-231 cells by caspase activation and enhanced p62 oligomerization as a hint at forming autophagosomes. Compound 3b also inhibited the Hsp70-Bag3 interaction, which was accompanied by FoxM1 suppression and upregulation of p21 and p27 in treated MCF-7 cells [45]. Doses of 3 mg/kg (every second day for six days) of compound 3b inhibited the growth of MCF-7 breast carcinoma xenografts in vivo. In addition to breast cancer cell lines MCF-7 and MDA-MB-231, further cancer cell lines sensitive to compound 3b were identified (with EC50 < 1 µM), such as HeLa, A375, HT-29, SKOV-3, Jurkat, MM1.R, INA6, RPMI-8226, JJN-3, and U266. Mechanistically, compound 3b exerted its antiproliferative activities against breast cancers both in Bag3-dependent (via ERK activation) and Bag3-independent ways (via suppressed Akt and c-Myc) [46]. Combinations with the proteasome inhibitors MG132 and bortezomib enhanced the antitumor activity of compound 3b in breast cancer models both in vitro and in vivo (5 mg/kg of compound 3b plus 1 mg/kg of bortezomib in MDA-MB-231 TNBC xenografts). Synergy effects in breast cancer cells were also observed in combination with α-amanitin (RNA-polymerase II inhibitor), LY294002 (Akt inhibitor), and sunitinib (RTK inhibitor). In addition, the infiltration of tumor bodies by tumor-associated macrophages (TAMs) was inhibited by compound 3b based on the inactivation of tumor stromal cell Hsp70 proteins [47]. Similar to compound 1, compound 3b also sensitized lung cancer cells to treatment with manumycin A [31]. In a recent effort, new rhodacyanine analogs of compound 3b with benzo-fused N-heterocycle moieties were described, with benzothiazolium compound 3d and quinolinium compound 3e (Figure 1) as the most promising compounds exhibiting high antiproliferative activity against TNBC cells (IC50 = 0.24 µM for 3d and 0.37 µM for 3e in MDA-MB-231 cells), accompanied by a considerable selectivity since non-malignant MCF-10A cells with low levels of Hsp70 were much less sensitive to treatment with 3d [48]. Both compounds are very stable, with half-lives of more than 2 h in microsomes. Apoptosis upon caspase-activation was induced by compounds 3d and 3e in MDA-MB-468 breast cancer cells, while both compounds led to autophagy both in MDA-MB-231 and MDA-MB-468 cells. In addition to FoxM1, survivin, HuR, and Akt suppression, compounds 3d and 3e also degraded KRAS in MDA-MB-231 and MDA-MB-468 cells. Further 3b/JG-98-derived benzothiazole rhodacyanines were described, culminating in the discovery of the bromothienyl analog 3f (JG-231), which showed high activity against MCF-7 (IC50 = 0.12 µM) and MDA-MB-231 breast cancer cells (IC50 = 0.25 µM), disruption of Bag3 interaction, amenable microsomal stability (half-life of more than 60 min), degradation of Akt and HuR in MCF-7 xenografts, amenable in vivo pharmacokinetics parameters and in vivo tumor growth inhibition of MDA-MB-231 xenografts at doses of 4 mg/kg (i.p.) [49].
The cationic spasmolytic drug pinaverium bromide (Figure 1, 4) was repurposed as an inhibitor of constitutively activated Hsc70. Compound 4 inhibited cell proliferation (IC50 of ca. 10 µM) of A2058 melanoma cells and induced apoptosis in these cells [50]. Its binding site was located at the NBD and linker domains of Hsc70.
S1g-2 (Figure 1, 5a) was identified as an inhibitor of Hsp70-Bim interaction (IC50 = 0.4 µM) in CML cells by screening a Bcl-2 inhibitor library [51]. Its allosteric Hsp70-binding site is near the binding site of the rhodacyanines 3. Compound 5a selectively induces apoptosis in CML cells by suppressing oncoprotein clients such as Akt, Raf-1, eIF4E, and RPS16. Hsp70-Bim interaction protected BCR-ABL-independent TKI-resistant CML cells from apoptosis, and, thus, treatment with compound 5a can overcome the resistance of a highly problematic CML type. The close analog compound 5b (Figure 1, S1g-6), which has a morpholino side chain replacing the unstable ester side chain of 5a, was recently described as a new sub-micromolar Hsp70-Bim interaction inhibitor [52]. Compound 5b also induced apoptosis in cancer cells and suppressed Akt and Raf-1.
The benzimidazole derivative 6 (Figure 1, HS-72) selectively inhibits inducible Hsp70 (Hsp70i), which is in stark contrast to its low affinity for the closely related constitutively activated Hsc70 [53]. At doses of 25 and 50 µM, compound 6 inhibited the proliferation of BT474, MCF-7, and SkBr3 breast cancer cells, and the proteins Her2 and Akt were degraded by compound 6 in BT474 and MCF-7 breast cancer cells. In the Her2-overexpressing MMTV-neu spontaneous breast tumor mouse model, compound 6 (20 mg/kg biweekly for 21 days, i.p.) was well tolerated and inhibited breast tumor growth, leading to prolonged survival of treated mice.
The 2,5′-thiodipyrimidine 7 (Figure 1, YK-5) was identified as an irreversible inhibitor of a new allosteric site of the Hsp70 NBD domain and binds covalently to a cysteine residue of the binding site via its reactive acrylamide moiety [54]. It is highly active against Kasumi-1 AML cells (IC50 = 0.9 µM) and SkBr3 breast cancer cells (IC50 = 0.8 µM) and a potent apoptosis inducer by caspase-3/7 activation (IC50 = 1.2 µM) in MOLM13 AML cells. 7 degraded Her2 and Raf-1 in SkBr3 breast cancer cells as a consequence of Hsp70 inhibition.
The dye methylene blue (Figure 1, 8) showed manifold biological activities, and, thus, it was also identified as an inhibitor of the Hsp70 ATPase, leading to a rapid suppression of Tau protein in neurodegenerative cell models [55]. In HeLa cervix carcinoma cells expressing poly-glutaminylated AR (AR112Q), compound 8 inhibited the Hsp70-mediated degradation of AR112Q [56]. In A375 and G361 metastatic melanoma cells, compound 8 suppressed the heat shock response (downregulation of Hsp27, Hsp70, and Hsc70), induced ROS formation, and caused glutathione depletion at a concentration of 10 µM [57]. Geldanamycin is an Hsp90 inhibitor, which increases Hsp70 expression, but compound 8 (10 µM) was able to suppress geldanamycin-induced Hsp70 expression in A375 melanoma cells. Hence, 10 µM of compound 8 also sensitized A375 melanoma cells to geldanamycin treatment, but it also sensitized these cells to treatment with etoposide and doxorubicin. Compound 8 possessed antiproliferative properties against A549 NSCLC cells and induced early apoptosis, yet it enhanced the degradation of N-terminal AR fragments as well as autophagy [58]. In mice, compound 8 inhibited benzo[a]pyrene induced lung carcinogenesis and suppressed Hsp70 as well as the tumor biomarkers ADA and LDH.
The structures of competitive and allosteric NBD binders are shown in Figure 1.
Table 1. Effects of NBF-targeting Hsp70 inhibitors on cancers.
| Compound | Cancer Model(s) | Effects |
|---|---|---|
| 1 (VER-155008) | HCT-116 colon carcinoma, A549 NSCLC, pleural mesothelioma, LNCaP95 prostate carcinoma, anaplastic thyroid carcinoma, glioma (1321N1, GOS-3, U87-MG), muscle-invasive bladder cancer, osteosarcoma, multiple myeloma, acute myeloid leukemia | Selective Hsp70/Hsc70 inhibition, antiproliferative, suppression of Her2 and Raf-1, G1 cell cycle arrest, sensitization to 17-AAG and radiation, suppression of Akt and phospho-Akt, macroautophagy induction, suppression of AR-FL and ARV7, apoptosis and paraptosis induction, upregulation of miR-215 and miR-194-5p, degradation of KDMA6 and EP300, degradation of BAG1, upregulation of CHOP, suppression of VEGF release by leukemia cells, synergy effects with drugs (manumycin A, bortezomib, shikonin, 17-DMAG) and PDT [19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35] |
| 2a (Apoptozole) | SK-OV-3 ovarian carcinoma, HCT-15 colon carcinoma, A549 NSCLC | Suppression of Hsp70-APAF-1, antiproliferative, pro-apoptotic [37] |
| 2b, 2c | HeLa cervix carcinoma, MDA-MB-231 breast carcinoma, HepG2 hepatoma, A549 NSCLC, RKO colon carcinoma, leukemia | Suppression of Hsp70-APAF-1, antiproliferative, sensitization to doxorubicin, in vivo tumor growth inhibition of A549, RKO, and HeLa xenografts, apoptosis induction, autophagy inhibition, cathepsin release [38][39][40] |
| 3a (YM-1) | MCF-7 breast carcinoma, HeLa cervix carcinoma, U251 and U343 glioma, doxorubicin-resistant BT-549rDOX breast carcinoma | Inhibition of Hsp70-Bag3, suppression of FoxM1 and HIF1α pathways, in vivo inhibition of MCF-7 tumor growth, induction of p21, suppression of FoxM1 and surviving, sensitization of glioma to (−)-gossypol (AT-101) and ABT-737, suppression of Mcl-1, synergistic antiproliferative effects with doxorubicin [41][42][43] |
| 3b (JG-98) | Triple-negative MDA-MB-231 and hormone sensitive MCF-7 breast cancer, lung cancer, miscellaneous | Inhibition of Hsp70-Bag3, FoxM1 suppression, upregulation of p21 and p27, sensitization of breast cancer to bortezomib in vivo, inhibition of TAM infiltration, sensitization of lung cancer to manumycin A [31][44][45][46][47] |
| 3d, 3e | Triple-negative breast cancer (e.g., MDA-MB-231, MDA-MB-468) | Stable, antiproliferative, tumor-selective, induction of apoptosis and autophagy, degradation of KRAS, suppression of FoxM1, survivin, HuR, and Akt [48] |
| 3f (JG-231) | MCF-7 and MDA-MB-231 breast cancer | Stable, antiproliferative, inhibition of Hsp70-Bag3, degradation of Akt and HuR, tumor growth inhibition in vivo [49] |
| 4 (Pinaverium bromide) | A2058 melanoma | Apoptosis induction [50] |
| 5a (S1g-2) | CML | Inhibition of Hsp70-Bim, apoptosis induction, suppression of Akt, Raf-1, eIF4E and RPS16 [51] |
| 5b (S1g-6) | Miscellaneous | Inhibition of Hsp70-Bim, apoptosis induction, degradation of Akt and Raf-1 [52] |
| 6 (HS-72) | BT474, MCF-7 and SkBr3 breast carcinoma, Her2-overexpressing MMTV-neu spontaneous breast tumor mouse model | Selective Hsp70i inhibition, antiproliferative, Her2 and Akt degradation, tumor growth inhibition and prolonged survival in vivo [53] |
| 7 (YK-5) | Kasumi-1 AML, SkBr3 breast carcinoma, MOLM13 AML | Antiproliferative, apoptosis induction, Her2 and Raf-1 degradation [54] |
| 8 (Methylene Blue) | AR112Q-expressing HeLa cervix carcinoma, A375 and G361 melanoma, A549 NSCLC | Heat shock response suppression, ROS formation, glutathione depletion, suppressed geldanamycin-induced Hsp70, sensitization of cancer cells to geldanamycin, etoposide and doxorubicin, apoptosis induction, inhibition of lung carcinogenesis in vivo [56][57][58] |
Some Hsp70 inhibitors with SBD-targeting properties were described (Figure 2). Compound 9a (Figure 2, 2-phenylethynesulfonamide, PES, pifithrin-µ) is a prominent example, which was thoroughly studied for its Hsp70-related effects in various cancers. Compound 9a showed antiproliferative activities against various osteosarcoma, breast, and pancreatic carcinoma cell lines at IC50 values of 5–10 µM independent of the p53-state of the tumor cells, induced cell death independent of caspase activation, and led to dysfunctional autophagy by the formation of p62-oligomers/aggregates [59]. It decreased the interaction of Hsp70 with APAF1, p53, and the co-chaperones Hsp40, CHIP, and BAG-1M and suppressed NF-κB signaling and activity. In vivo, compound 9a (40 mg/kg, i.p., every five days for 30 days) blocked Myc-based lymphopathogenesis and led to prolonged survival in Eµ-Myc transgenic mice. Compound 9a exhibited considerable antiproliferative activity against acute leukemia (AML and ALL) cells (IC50 = 2.5–12.7 µM), induced apoptosis in these cells by caspase activation, and led to a degradation of Akt and ERK1/2 [60]. In addition, compound 9a sensitized acute leukemia cells to treatment with cytarabine (an antimetabolite), 17-AAG (a Hsp90 inhibitor), vorinostat (a HDAC inhibitor), and sorafenib (a RTK inhibitor). In primary effusion lymphoma (PEL), compound 9a exerted cytotoxic effects on BC3 and BCBL1 cells by apoptosis and another cell death mechanism, which was associated with immunogenic activity such as activation of dendritic cells [61]. Compound 9a increased lysosome permeabilization and cathepsin D release in PEL cells, accompanied by Bid cleavage, outer mitochondrial depolarization, and AIF translocation to the nucleus. Moreover, compound 9a sensitized PEL cells to bortezomib treatment. The combination of compound 9a with DNA-targeting platinum complexes such as cisplatin and oxaliplatin also revealed synergy effects in HT-29 colon carcinoma and PC-3 prostate carcinoma cells [62]. In prostate cancer cells, compound 9a also increased the antitumor effects of hyperthermia (HT, 43 °C), best when given immediately before HT started, which was accompanied by upregulation of p21 and suppression of c-Myc and cyclin D1 [63]. The combination of compound 9a (100 µg in 50 µL) and HT (43 °C for 1 h, twice on days 0 and 4), led to significant PC-3 prostate carcinoma xenograft growth inhibition. Its modified analog compound 9b (Figure 2, PES-Cl) was antiproliferative against a panel of BRAF-V600E mutant melanoma (IC50 values between 2–5 µM, while inactive against melanocytes) and showed higher antiproliferative activity than compound 9a against SkBr3 breast carcinoma, FaDu head and neck squamous cell carcinoma, and H1299 lung adenocarcinoma cells [64]. The cytotoxic activity of compound 9b is based on apoptosis induction (caspase activation) and inhibition of autophagy (p62 accumulation), while the HeLa cell cycle was arrested in the G2-M phase by compounds 9a and 9b associated with cyclin B1 degradation. At doses of 20 mg/kg (i.p., once per week), compound 9b led to a much higher survival rate of Eµ-Myc mice (71.4% survival) than compound 9a (35% survival) after 210 days. Compound 9b also induced apoptosis in A375 melanoma cells, accompanied by Her2 degradation in these cells [65]. In contrast to compounds 1 and 3c, only compound 9b led to G2-M arrest in H1299 and A375 cells based on cyclin B1 degradation.
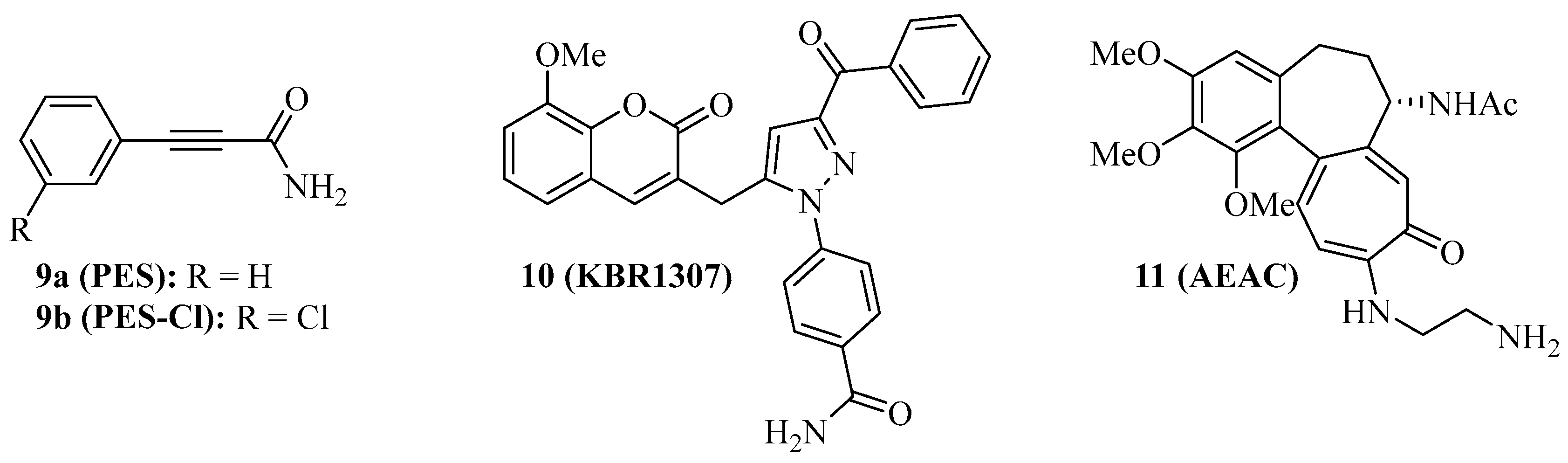
Figure 2. Structures of SBD-interacting Hsp70 inhibitors 9–11.
Compound 9a does not interact with Hsp70 when no nucleotide is bound to the protein. The coumarin-pyrazole hybrid, compound 10 (Figure 2, KBR1307) was designed, which binds Hsp70 in the presence and absence of nucleotides [66]. Both compounds 9a and 10 reduce the activity of Hsp70 ATPase significantly, but compound 10 was more active against MCF-7 cells than compound 9a. Similar coumarin-thiazole hybrids were described by the same group before as binders to the C-terminus of Hsp70 with activity against DLD-1 colon carcinoma and HepG2 hepatoma cells [67].
The screening of the InterBioScreen compound library for Hsp70 inhibitors revealed that the semi-synthetic colchicine derivative, compound 11 (Figure 2, N-aminoethylaminocolchicine, AEAC), interferes with substrate binding and refolding functions of Hsp70, based on a nanomolar affinity for Hsp70 (KD = 149 nM) [68]. Although the antiproliferative and cytotoxic activities of compound 11 are low, it sensitized C6 rat glioblastoma and B16 mouse melanoma cells to doxorubicin treatment. The combination of compound 11 (2 mg/kg) and doxorubicin (1 mg/kg) inhibited in vivo B16 tumor growth by 71% and increased the lifespan of treated mice by ca. 15 days when compared with untreated mice.
The structures of SBD-interacting compounds are shown in Figure 2. Table 2 summarizes the anticancer effects of the described SBD-domain interacting Hsp70 inhibitors.
Table 2. SBD-interacting Hsp70 inhibitors and their effects on cancer model(s).
| Compound | Cancer Model(s) | Effects |
|---|---|---|
| 9a (PES) | Colon, breast, prostate and pancreas carcinoma, osteosarcoma, lymphoma, acute leukemia | Antiprolierative independent from p53-state, caspase activation, dysfunctional autophagy, prolonged survival of Eµ-Myc mice, NF-κB suppression, degradation of Akt and ERK1/2, immunogenic activity, sensitization of cancers to drugs and hyperthermia [59][60][61][62][63] |
| 9b (PES-Cl) | BRAF-V600E mutant melanoma, SkBr3 breast carcinoma, FaDu head and neck squamous cell carcinoma, H1299 lung adenocarcinoma, lymphoma | More antiproliferative than 9a, apoptosis induction, autophagy inhibition, G2-M phase arrest, degradation of cyclin B1 and Her2, prolonged survival of Eµ-Myc mice [64][65] |
| 10 (KBR1307) | MCF-7 breast carcinoma | More antiproliferative than 9a, binds Hsp70 in absence of nucleotide [66] |
| 11 (AEAC) | C6 rat glioblastoma, B16 mouse melanoma | Increased doxorubicin activity in vitro and in vivo, tumor growth inhibition (71%) and prolonged survival in B16 mice [68] |
References
- Santoro, M.G. Heat shock factors and the control of stress response. Biochem. Pharmacol. 2000, 59, 55–63.
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962, 18, 571–573.
- Tissiéres, A.; Mitchell, H.K.; Tracy, U.M. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. J. Mol. Biol. 1974, 84, 389–398.
- Hasday, J.D.; Singh, I.S. Fever and the heat shock response: Distinct, partially overlapping processes. Cell Stress Chaperones 2000, 5, 471–480.
- Rodina, A.; Wang, T.; Yan, P.; Gomes, E.D.; Dunphy, M.P.S.; Pillarsetty, N.; Koren, J.; Gerecitano, J.F.; Taldone, T.; Zong, H.; et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 2016, 538, 397–401.
- Macario, A.J.L.; Conway de Macario, E. Chaperone Proteins and Chaperonopathies. In Stress: Physiology, Biochemistry, and Pathology Handbook of Stress Series; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 3, pp. 135–152.
- Kampinga, H.H.; Hageman, J.; Vos, V.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111.
- Li, L.; Wang, L.; You, Q.-D.; Xu, X.-L. Heat shock protein 90 inhibitors: An update on achievements, challenges, and future directions. J. Med. Chem. 2020, 63, 1798–1822.
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of Hsp90 in cancer. Int. J. Mol. Sci. 2021, 22, 10317.
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680.
- Albakova, Z.; Armeev, G.A.; Kanevsky, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. Hsp70 multi-functionality in cancer. Cells 2020, 9, 587.
- Fu, X.; Liu, J.; DiSanto, M.E.; Zhang, X. Heat shock protein 70 and 90 family in prostate cancer. Life 2022, 12, 1489.
- Yang, S.; Xiao, H.; Cao, L. Recent advance in heat shock proteins in cancer diagnosis, prognosis, metabolism and treatment. Biomed. Pharmacother. 2021, 142, 112074.
- Elfiky, A.A.; Baghdady, A.M.; Ali, S.A.; Ahmed, M.I. GRP78 targeting: Hitting two birds with a stone. Life Sci. 2020, 260, 118317.
- Elwakeel, A. Abrogating the interaction between p53 and mortalin (Grp75/HSPA9/mtHsp70) for cancer therapy: The story so far. Front. Cell Dev. Biol. 2022, 10, 879632.
- Albakova, Z.; Siam, M.K.S.; Sacitharan, P.K.; Ziganshin, R.H.; Ryazantsev, D.Y.; Sapozhnikov, A.M. Extracellular heat shock proteins and cancer: New perspectives. Transl. Oncol. 2021, 14, 100995.
- Li, J.; Qian, X.; Sha, B. Heat shock protein 40: Structural studies and their functional implications. Protein Pept. Lett. 2009, 16, 606–612.
- Liu, Q.; Liang, C.; Zhou, L. Structural and functional analysis of the Hsp70/Hsp40 chaperone system. Protein Sci. 2020, 29, 378–390.
- Williamson, D.S.; Borgognoni, J.; Clay, A.; Daniels, Z.; Dokurno, P.; Drysdale, M.J.; Foloppe, N.; Francis, G.L.; Graham, C.J.; Howes, R.; et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J. Med. Chem. 2009, 52, 1510–1513.
- Massey, A.J.; Williamson, D.S.; Browne, H.; Murray, J.B.; Dokurno, P.; Shaw, T.; Macias, A.T.; Daniels, Z.; GEoffroy, S.; Dopson, M.; et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis. Cancer Chemother. Pharmacol. 2010, 66, 535–545.
- Wen, W.; Liu, W.; Shao, Y.; Chen, L. VER-155008, a small molecule inhibitor of HSP70 with potent antio-cancer activity on lung cancer cell lines. Exp. Biol. Med. 2014, 239, 638–645.
- Sakai, K.; Inoue, M.; Mikami, S.; Nishimura, H.; Kuwabara, Y.; Kojima, A.; Toda, M.; Ogawa-Kobayashi, Y.; Kikuchi, S.; Hirata, Y.; et al. Functional inhibition of heat shock protein 70 by VER-155008 suppresses pleural mesothelioma cell proliferation via an autophagy mechanism. Thoracic Cancer 2021, 12, 491–503.
- Kita, K.; Shiota, M.; Tanaka, M.; Otsuka, A.; Matsumoto, M.; Kato, M.; Tamada, S.; Iwao, H.; Miura, K.; Nakatani, T.; et al. Heat shock protein 70 inhibitors suppress androgen receptor expression in LNCAP95 prostate cancer cells. Cancer Sci. 2017, 108, 1820–1827.
- Brünnert, D.; Langer, C.; Zimmermann, L.; Bargou, R.C.; Burchardt, M.; Chatterjee, M.; Stope, M.B. The heat shock protein 70 inhibitor VER155008 suppresses the expression of HSP27, HOP and HSP90β and the androgen receptor, induces apoptosis, and attenuates prostate cancer cell growth. J. Cell. Biochem. 2020, 121, 407–417.
- Yu, B.; Yang, H.; Zhang, X.; Li, H. Visualizing and quantifying the effect of the inhibition of HSP70 on breast cancer cells based on laser scanning microscopy. Technol. Cancer Res. Treat. 2018, 17, 1–7.
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.-H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. The hsp70 inhibitor VER155008 induces paraptosis requiring de novo protein synthesis in anaplastic thyroid carcinoma cells. Biochem. Biophys. Res. Commun. 2014, 454, 36–41.
- Shervington, L.; Patil, H.; Shervington, A. Could the anti-chaperone VER155008 replace temozolomide for glioma treatment. J. Cancer 2015, 6, 786–794.
- Cavanaugh, A.; Juengst, B.; Sheridan, K.; Danella, J.F.; Williams, H. Combined inhibition of heat shock proteins 90 and 70 leads to simultaneous degradation of the oncogenic signaling proteins involved in muscle invasive bladder cancer. Oncotarget 2015, 6, 39821–39838.
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.-H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. Hsp70 inhibition potentiates radicicol-induced cell death in anaplastic thyroid carcinoma cells. Anticancer Res. 2014, 34, 4829–4838.
- Asling, J.; Morrison, J.; Mutsaers, A.J. Targeting HSP70 and GRP78 in canine osteosarcoma cells in combination with doxorubicin chemotherapy. Cell Stress Chaperones 2016, 21, 1065–1076.
- Sojka, D.R.; Hastorek, S.; Vydra, N.; Toma-Jonik, A.; Wieczorek, A.; Gogler-Piglowska, A.; Scieglinska, D. Inhibition of the heat shock protein A (HSPA) family potentiates the anticancer effects of manumycin A. Cells 2021, 10, 1418.
- Tang, X.; Tan, L.; Shi, K.; Peng, J.; Xiao, Y.; Li, W.; Chen, L.; Yang, Q.; Qian, Z. Gold nanorods together with HSP inhibitor-VER-155008 micelles for colon cancer mild-temperature photothermal therapy. Acta Pharmaceut. Sin. 2018, 8, 587–601.
- Huang, L.; Wang, Y.; Bai, J.; Yang, Y.; Wang, F.; Feng, Y.; Zhang, R.; Li, F.; Zhang, P.; Lv, N.; et al. Blockade of HSP70 by VER-155008 synergistically enhances bortezomib-induced cytotoxicity in multiple myeloma. Cell Stress Chaperones 2020, 25, 357–367.
- Wada, N.; Kawano, Y.; Fujiwara, S.; Kikukawa, Y.; Okuno, Y.; Tasaki, M.; Ueda, M.; Ando, Y.; Yoshinaga, K.; Ri, M.; et al. Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells. Int. J. Oncol. 2015, 46, 963–972.
- Reikyam, H.; Nepstad, I.; Sulen, A.; Gjertsen, B.T.; Hatfield, K.J.; Bruserud, O. Increased antileukemic effects in human acute myeloid leukemia by combining HSP70 and HSP90 inhibitors. Exp. Opin. Investig. Drugs 2013, 22, 551–563.
- Kul, P.; Tuncbilek, M.; Ergul, M.; Tunoglu, E.N.Y.; Tutar, Y. A novel 6,8,9.trisubstituted purine analogue drives breast cancer luminal A subtype MCF-7 to apoptosis and senescence through Hsp70 inhibition. Anticancer Agents Med. Chem. 2023, 23, 585–598.
- Williams, D.R.; Ko, S.-K.; Park, S.; Lee, M.-R.; Shin, I. An apoptosis-inducing small molecule that binds to heat shock protein 70. Angew. Chem. Int. Ed. 2008, 47, 7466–7469.
- Ko, S.-K.; Kim, J.; Na, D.C.; Park, S.; Park, S.-H.; Hyun, J.Y.; Baek, K.-H.; Kim, N.D.; Kim, N.-K.; Park, Y.N.; et al. A small molecule inhibitor of ATPase activity of HSP70 induces apoptosis and has antitumor activities. Chem. Biol. 2015, 22, 391–403.
- Park, S.-H.; Kim, W.-J.; Li, H.; Seo, W.; Park, S.-H.; Kim, H.; Shin, S.C.; Zuiderweg, E.R.P.; Kim, E.E.; Sim, T.; et al. Anti-leukemia activity of a Hsp70 inhibitor and its hybrid molecules. Sci. Rep. 2017, 7, 3537.
- Park, S.-H.; Baek, K.-H.; Shin, I.; Shin, I. Subcellular Hsp70 inhibitors promote cancer cell death via different mechanisms. Cell Chem. Biol. 2018, 25, 1242–1254.
- Colvin, T.A.; Gabai, V.L.; Calderwood, S.K.; Li, H.; Gummuluru, S.; Matchuk, O.N.; Smirnova, S.G.; Orlova, N.V.; Zamulaeva, I.A.; Garcia-Marcos, M.; et al. Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Res. 2014, 74, 4731–4740.
- Antonietti, P.; Linder, B.; Hehlgans, S.; Mildenberger, I.C.; Burger, M.C.; Fulda, S.; Steinbach, J.P.; Gessler, F.; Rödel, F.; Mittelbronn, M.; et al. Interference with the HSF1/HSP70/BAG3 pathway primes glioma cells to matrix detachment and BH3 mimetic-induced apoptosis. Mol. Cancer Ther. 2016, 16, 156–168.
- Das, C.K.; Linder, B.; Bonn, F.; Rothweiler, F.; Dikic, I.; Michaelis, M.; Cinatl, J.; Mandal, M.; Kögel, D. BAG3 overexpression and cytoprotective autophagy mediate apoptosis resistance in chemoresistant breast cancer cells. Neoplasia 2018, 20, 263–279.
- Li, X.; Srinivasan, S.R.; Connarn, J.; Ahmad, A.; Young, Z.T.; Kabza, A.M.; Zuiderweg, E.R.P.; Sun, D.; Gestwicki, J.E. Analogues of the allosteric heat shock protein 70 (Hsp70) inhibitor, MKT-077, as anti-cancer agents. ACS Med. Chem. Lett. 2013, 4, 1042–1047.
- Li, X.; Colvin, T.; Rauch, J.N.; Acosta-Alvear, D.; Kampmann, M.; Dunyak, B.; Hann, B.; Aftab, B.T.; Murnane, M.; Cho, M.; et al. Validation of the Hsp70-Bag3 protein-protein interaction as a potential therapeutic target in cancer. Mol. Cancer Ther. 2015, 14, 642–648.
- Yaglom, J.A.; Wang, Y.; Li, A.; Li, Z.; Monti, S.; Alexandrov, I.; Lu, X.; Sherman, M.Y. Cancer cell responses to Hsp70 inhibitor JG-98: Comparison with Hsp90 inhibitors and finding synergistic drug combinations. Sci. Rep. 2018, 8, 3010.
- Gabai, V.L.; Yaglom, J.A.; Wang, Y.; Meng, L.; Shao, H.; Kim, G.; Colvin, T.; Gestwicki, J.; Sherman, M.Y. Anticancer effects of targeting Hsp70 in tumor stromal cells. Cancer Res. 2016, 76, 5926–5932.
- Chang, C.-S.; Kumar, V.; Lee, D.-Y.; Chen, Y.; Wu, Y.-C.; Gao, J.-Y.; Chu, P.-C. Development of novel rhodacyanine-based heat shock 70 inhibitors. Curr. Med. Chem. 2021, 28, 5431–5446.
- Shao, H.; Li, X.; Moses, M.A.; Gilbert, L.A.; Kalyanaraman, C.; Young, Z.T.; Chernova, M.; Journey, S.N.; Weissman, J.S.; Hann, B.; et al. Exploration of benzothiazole rhodacyanines as allosteric inhibitors of protein-protein interactions with heat shock protein 70 (Hsp70). J. Med. Chem. 2018, 61, 6163–6177.
- Dublang, L.; Underhaug, J.; Flydal, M.I.; Velasco-Carneros, L.; Maréchal, J.-D.; Moro, F.; Boyano, M.D.; Martinez, A.; Muga, A. Inhibition of the humN Hac/0 system by small ligands as a potential anticancer approach. Cancers 2021, 13, 2936.
- Song, T.; Guo, Y.; Xue, Z.; Guo, Z.; Wang, Z.; Lin, D.; Zhang, H.; Pan, H.; Zhang, X.; Yin, F.; et al. Small-molecule inhibitor targeting the Hsp70-Bim protein-protein interaction in CML cells overcomes BCR-ABL-independent TKI resistance. Leukemia 2021, 35, 2862–2874.
- Wang, Z.; Song, T.; Guo, Z.; Uwituze, L.B.; Guo, Y.; Zhang, H.; Wang, H.; Zhang, X.; Pan, H.; Ji, T.; et al. A novel Hsp70 inhibitor specifically targeting the cancer-related Hsp70-Bim protein-protein interaction. Eur. J. Med. Chem. 2021, 220, 113452.
- Howe, M.K.; Bodoor, K.; Carlson, D.A.; Hughes, P.F.; Alwarawrah, Y.; Loiselle, D.R.; Jaeger, A.M.; Darr, D.B.; Jordan, J.L.; Hunter, L.M.; et al. Identification of an allosteric small-molecule inhibitor selective for the inducible form of heat shock protein 70. Chem. Biol. 2014, 21, 1648–1659.
- Kang, Y.; Taldone, T.; Patel, H.J.; Patel, P.D.; Rodina, A.; Gozman, A.; Maharaj, R.; Clement, C.C.; Patel, M.R.; Brodsky, J.L.; et al. Heat shock protein 70 inhibitors. 1. 2,5′-Thiopyrimidine and 5-(phenylthio)pyrimidine acrylamides as irreversible binders to an allosteric site on heat shock protein 70. J. Med. Chem. 2014, 57, 1188–1207.
- Jinwal, U.K.; Miyata, Y.; Koren III, J.; Jones, J.R.; Trotter, J.H.; Chang, L.; O’Leary, J.; Morgan, D.; Lee, D.C.; Shults, C.L.; et al. Chemical manipulation of Hsp70 ATPase activity regulates Tau stability. J. Neurosci. 2009, 29, 12079–12088.
- Wang, A.M.; Morishima, Y.; Clapp, K.M.; Peng, H.-M.; Pratt, W.B.; Gestwicki, J.E.; Osawa, Y.; Lieberman, A.P. Inhibition of Hsp70 by methylene blue affects signaling protein function and ubiquitination and modulates polyglutamine protein degradation. J. Biol. Chem. 2010, 285, 15714–15723.
- Davis, A.L.; Cabello, M.C.; Qiao, S.; Azimian, S.; Wondrak, G.T. Phenotypic identification of the redox dye methylene blue as an antagonist of heat shock response gene expression in metastatic melanoma cells. Int. J. Mol. Sci. 2013, 14, 4185–4202.
- Sanchala, D.; Bhatt, L.K.; Pethe, P.; Shelat, R.; Kulkarni, Y.A. Anticancer activity of methylene blue via inhibition of heat shock protein 70. Biomed. Pharmacother. 2018, 107, 1037–1045.
- Leu, J.I.; Pimkina, J.; Frank, A.; Murphy, M.E.; George, D.L. A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell 2009, 36, 15–27.
- Kaiser, M.; Kühnl, A.; Reins, J.; Fischer, S.; Ortiz-Tanchez, J.; Schlee, C.; Mochmann, L.H.; Heesch, S.; Benlasfer, O.; Hofmann, W.-K.; et al. Antileukemic activity of the HSP70 inhibitor pifithrin-µ in acute leukemia. Blood Cancer J. 2011, 1, e28.
- Granato, M.; Lacconi, V.; Peddis, M.; Lotti, L.V.; Di Renzo, L.; Gonnella, R.; Santarelli, R.; Trivedi, P.; Frati, L.; D’Orazi, G.; et al. HSP70 inhibition by 2-phenylethynesulfonamide induces lysosomal cathepsin D release and immunogenic cell death in primary effusion lymphoma. Cell Death Dis. 2013, 4, e730.
- McKeon, A.M.; Egan, A.; Chandanshive, J.; McMahon, H.; Griffith, D.M. Novel improved synthesis of HSP70 inhibitor, Pifithrin-µ. In vitro synergy quantification of Pifithrin-µ combined with Pt drugs in prostate and colorectal cancer cells. Molecules 2016, 21, 949.
- Sekihara, K.; Harashima, N.; Tongu, M.; Tamaki, Y.; Uchida, N.; Inomata, T.; Harada, M. Pifithrin-µ, and inhibitor of heat-shock protein 70, can increase the antitumor effects of hyperthermia against human prostate cancer cells. PLoS ONE 2013, 8, e78772.
- Balaburski, G.M.; Leu, J.I.; Beeharry, N.; Hayik, S.; Andrake, M.D.; Zhang, G.; Herlyn, M.; Villanueva, J.; Dunbrack, R.L., Jr.; Yen, T.; et al. A modified HSP70 inhibitor shows broad activity as an anticancer agent. Mol. Cancer Ther. 2013, 11, 219–229.
- Budina-Kolomets, A.; Balaburski, G.M.; Bondar, A.; Beeharry, N.; Yen, T.; Murphy, M.E. Comparison of the activity of three different HSP70 inhibitors on apoptosis, cell cycle arrest, autophagy inhibition, and HSP90 inhibition. Cancer Biol. Ther. 2014, 15, 194–199.
- Coskun, K.A.; Koca, I.; Gümüs, M.; Tutar, Y. Designing specific HSP70 substrate binding domain inhibitor for perturbing protein folding pathways to inhibit cancer mechanism. Anticancer Agents Med. Chem. 2021, 21, 1472–1480.
- Kocam, I.; Gümüs, M.; Özgür, A.; Disli, A.; Tutar, Y. A noverl approach to inhibit heat shock response as anticancer strategy by coumarine compounds containing thiazole skeleton. Anticancer Agents Med. Chem. 2015, 15, 916–930.
- Lazarev, V.F.; Sverchinsky, D.V.; Mikhaylova, E.R.; Semenyuk, P.I.; Komarova, E.Y.; Niskanen, S.A.; Nikotina, A.D.; Burakov, A.V.; Kartsev, V.G.; Guzhova, I.V.; et al. Sensitizing tumor cells to conventional drugs: HSP70 chaperone inhibitors, their selection and application in cancer models. Cell Death Dis. 2018, 9, 41.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
6 times
(View History)
Update Date:
27 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




