
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federica Curcio | -- | 2314 | 2023-02-23 10:18:23 | | | |
| 2 | Dean Liu | Meta information modification | 2314 | 2023-02-27 02:17:06 | | |
Video Upload Options
Coated stents are defined as innovative stents surrounded by a thin polymer membrane based on polytetrafluoroethylene (PTFE)useful in the treatment of numerous vascular pathologies. Endovascular methodology involves the use of such devices to restore blood flow in small-, medium- and large-calibre arteries, both centrally and peripherally. These membranes cross the stent struts and act as a physical barrier to block the growth of intimal tissue in the lumen, preventing so-called intimal hyperplasia and late stent thrombosis. PTFE for vascular applications is known as expanded polytetrafluoroethylene (e-PTFE) and it can be rolled up to form a thin multilayer membrane expandable by 4 to 5 times its original diameter. This membrane plays an important role in initiating the restenotic process because wrapped graft stent could be used as the treatment option for trauma devices during emergency situations and to treat a number of pathological vascular disease.
1. Introduction
2. e-PTFE Membrane Preparation
2.1. Stretching and Pore-Forming Process
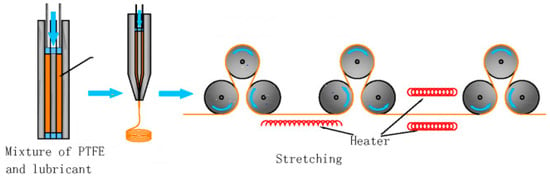
2.2. Sintering Process
2.3. Wrapping Process
2.4. Near-Field Electrospinning Process
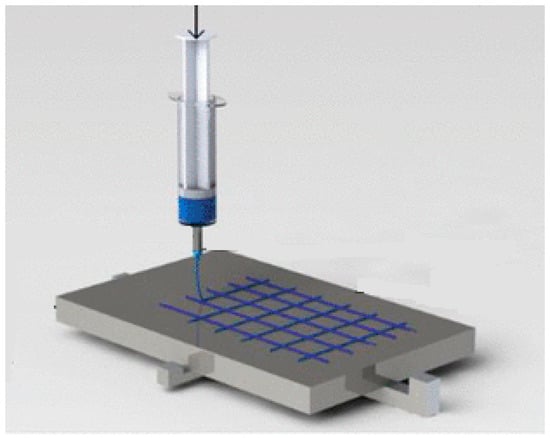
2.5. Electrospinning Process
3. e-PTFE Membrane Used in the Design of Covered Stents
4. The Advantages and Disadvantages of e-PTFE Membranes as Stent Coatings
References
- Roina, Y.; Auber, F.; Hocquet, D.; Herlem, G. ePTFE functionalization for medical applications. Mater. Today Chem. 2021, 20, 100412.
- Aronson, J.K. (Ed.) Polytetrafluoroethylene. In Meyler’s Side Effects of Drugs, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 872–873.
- Huang, H.; Yao, Q.; Zhang, X.; Wang, H. Microporous expanded polytetrafluoroethylene layer functionalized hydrophilic groups for excellent mechanical durability and superior performance in proton exchange membrane fuel cell. J. Power Sources 2022, 526, 231130.
- Weaver, J.D.; Ku, D.N. A study on the effects of covered stents on tissue prolapse. J. Biomech. Eng. 2012, 134, 024505.
- Farhatnia, Y.; Tan, A.; Motiwala, A.; Cousins, B.G.; Seifalian, A.M. Evolution of covered stents in the contemporary era: Clinical application, materials and manufacturing strategies using nanotechnology. Biotechnol. Adv. 2013, 31, 524.
- Soldatos, N.K.; Stylianou, P.; Angelov, N.; Koidou, P.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131.
- Alauddin, M.S.; Ayman, N.; Hayei, A.; Sabarudin, M.A.; Haliza, N.; Baharin, M. Barrier Membrane in Regenerative Therapy: A Narrative Review. Membranes 2022, 12, 444.
- Shiohara, A.; Prieto-Simon, B.; Voelcker, N.H. Porous polymeric membranes: Fabrication techniques and biomedical applications. J. Mater. Chem. B 2021, 9, 2129–2154.
- Jain, R.; Shetty, S.; Yadav, K.S. Unfolding the electrospinning potential of biopolymers for preparation of nanofibers. J. Drug Deliv. Sci. Technol. 2020, 57, 101604.
- Sun, M.; Han, K.; Hu, R.; Liu, D.; Fu, W.; Liu, W. Advances in micro/nanoporous membranes for biomedical engineering. Adv. Healthc. Mater. 2021, 10, 2001545.
- Bottino, A.; Capannelli, G.; Comite, A.; Costaetal, C. Novel polytetrafluoroethylene tubular membranes for membrane distillation. Distill. Water Treat. 2015, 53, 6.
- Guney, A.; Kara, F.; Ozgen, O.; Aksoy, E.A.; Hasirci, V.; Hasirci, N. Surface modification of polymeric biomaterials. Biomater. Surface Sci. 2013, 4, 89–158.
- Weinbaum, J.S.; Haskett, D.G.; Mandelkern, T.F.; Vorp, D.A. Advances in cell seeding of tissue engineered vascular grafts. In Tissue-Engineered Vascular Grafts; Walpoth, B., Bergmeister, H., Bowlin, G., Kong, D., Rotmans, J., Zilla, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 295–319.
- Zhang, L.L.; Ham, S.W.; Weaver, F.A.; Rowe, V.L.; Ziegler, K.R.; Magee, G.A.; Sukgu, M. Performance of Gore Viabahn VBX Compared with Atrium iCast as Bridging Stents During Fenestrated Endovascular Aortic Repairs. J. Vasc. Surg. 2019, 69, e215–e216.
- Patel, N.J.; Agasthi, P.; Mhatre, A.U.; Heuser, R.R. Out of the Mind of Edward B. Diethrich: The Development of the PolytetrafluoroethyleneCovered Coronary Stent. J. Endovasc. Ther. 2020, 27, 157–159.
- Zhou, X.; Qiu, C.; Li, D.; Zhang, H. Treatment of posttraumatic infrapopliteal pseudoaneurysm with a physician modified covered stent. Vasc. Investig. Ther. 2021, 4, 95.
- Patel, D.V. The Value of the GORE® VIABAHN® Endoprosthesis. Supplement to endovascular. 2019.
- Tenorio, E.R.; Oderich, G.S.; Kölbel, T.; Dias, N.V.; Sonesson, B.; Karelis, A.; A Farber, M.; Parodi, F.E.; Timaran, C.H.; Scott, C.K.; et al. Multicenter global early feasibility study to evaluate total endovascular arch repair using three-vessel inner branch stent-grafts for aneurysms and dissections. J. Vasc. Surg. 2021, 74, 1055–1065.
- Jamshidi, P.; Mahmoody, K.; Erne, P. Covered stents: A Review. Int. J. Cardiol. 2008, 130, 28.
- Fenga, S.; Zhonga, Z.; Wanga, Y.; Xinga, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349.
- Tan, X.M.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part II: Production Techniques with Polyethylene, Polydimethylsiloxane, Polypropylene, Polyimide, and Polytetrafluoroethylene. Polymers 2019, 11, 1310.
- Otto, C.; Handge, U.A.; Georgopanos, P.; Aschenbrenner, O.; Kerwitz, J.; Abetz, C.; Metze, A.L.; Abetz, V. Porous UHMWPE membranes and composites filled with carbon nanotubes: Permeability, mechanical, and electrical properties. Macromol. Mater. Eng. 2017, 302, 1600405.
- Remanan, S.; Sharma, M.; Bose, S.; Das, N.C. Recent advances in preparation of porous polymeric membranes by unique techniques and mitigation of fouling through surface modification. ChemistrySelect 2018, 609–633.
- Van Rooyen, L.J.; Bissett, H.; Khoathane, M.C.; Karger-Kocsis, J. Preparation of PTFE/graphene nanocomposites by compression moulding and free sintering: A guideline. J. Appl. Polym. Sci. 2016, 133, 43369.
- Zhang, Y.-Y.; Ge, Q.; Yang, L.-L.; Shi, X.-J.; Li, J.-J.; Yang, D.-Q.; Sacher, E. Durable superhydrophobic PTFE films through the introduction of micro-and nanostructured pores. Appl. Surf. Sci. 2015, 339, 151–157.
- Li, K.; Zhang, Y.; Xu, L.; Zeng, F.; Hou, D.; Wang, J. Optimizing stretching conditions in fabrication of PTFE hollow fber membrane for performance improvement in membrane distillation. J. Membr. Sci. 2018, 550, 126–135.
- Wang, F.; Li, J.; Zhu, H.; Zhang, H.; Tang, H.; Chen, J.; Guo, Y. Effect of the highly asymmetric structure on the membrane characteristics and microfiltration performance of PTFE wrapped hollow fiber membrane. J. Water Proc. Eng. 2015, 7, 36–45.
- He, X.-X.; Zheng, J.; Yu, G.-F.; You, M.-H.; Yu, M.; Ning, X.; Long, Y.-Z. Near-feld electrospinning: Progress and applications. J. Phys. Chem. C 2017, 121, 8663–8678.
- Nazemi, M.M.; Khodabandeh, A.; Hadjizadeh, A. Near-Field Electrospinning: Crucial Parameters, Challenges, and Applications. ACS Appl. Bio Mater. 2022, 5, 394.
- King, W.E.; Bowlin, G.L. Near-Field Electrospinning and Melt Electrowriting of Biomedical Polymers—Progress and Limitations. Polymers 2021, 13, 1097.
- Kang, W.; Li, F.; Zhao, Y.; Qiao, C.; Ju, J.; Cheng, B. Fabrication of porous Fe2O3/PTFE nanofiber membranes and their application as a catalyst for dye degradation. RSC Adv. 2016, 6, 32646.
- Dong, Z.-Q.; Ma, X.-h.; Xu, Z.-L.; You, W.-T.; Li, F. Superhydrophobic PVDF–PTFE electrospun nanofibrous membranes for desalination by vacuum membrane distillation. Desalination 2014, 347, 175.
- Feng, Y.; Xiong, T.; Jiang, S.; Liu, S.; Hou, H. Mechanical properties and chemical resistance of electrospunpolyterafluoroethylenefibres. RSC Adv. 2016, 6, 24250.
- Liu, G.; Gao, C.; Li, X.; Guo, C.; Chen, Y.; Lv, J. Preparation and properties of porous polytetrafluoroethylenehollow fiber membrane through mechanical operations. J. Appl. Polym. Sci. 2015, 132, 1.
- Ghorbani, M.; Griessenauer, C.J.; Shojaei, H.; Wipplinger, C.; Hejazian, E. Endovascular reconstruction of iatrogenic internal carotid artery injury following endonasal surgery: A systematic review. Neurosurg. Rev. 2020, 44, 1797–1804.
- McKavanagh, P.; Zawadowski, G.; Ahmed, N.; Kutryk, M. Expert Review of Cardiovascular The evolution of coronary stents. Therapy 2018, 16, 219–228.
- Stefanini, G.; Byrne, R.A.; Windecker, S.; Kastrati, A. State of the art: Coronary artery stents—Past, present and future. EuroIntervention 2017, 13, 706.
- Sakamoto, A.; Torii, S.; Jinnouchi, H.; Virmani, R.; Finn, A.V. Histopathologic and physiologic effect of overlapping vs single coronary stents: Impact of stent evolution. Expert Rev. Med. Devices 2018, 9, 1–18.
- Weekes, A.; Bartnikowski, N.; Pinto, N.; Jenkins, J.; Meinert, C.; Klein, T.J. Biofabrication of small diameter tissue-engineered vascular grafts. Acta Biomater. 2022, 138, 92–111.
- Uthamaraj, S.; Tefft, B.J.; Jana, S.; Hlinomaz, O.; Kalra, M.; Lerman, A.; Dragomir-Daescu, D.; Sandhu, G.S.J. Fabrication of small caliber stent-grafts using electrospinning and balloon expandable bare metal stents. Vis. Exp. 2016, 116, e54731.
- Dalton, P.D.; Vaquette, C.; Farrugia, B.L.; Dargaville, T.R.; Brown, T.D.; Hutmacher, D.W. Electrospinning and additive manufacturing: Converging technologies. Biomater. Sci. 2013, 1, 171.
- Cronenwett, J.L.; Johnston, K.W. Rutherford’s Vascular Surgery; Elsevier: Amsterdam, The Netherlands, 2014.
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61.
- Gao, F.; Hu, Y.; Li, G.; Liu, S.; Quan, L.; Yang, Z.; Wei, Y.; Pan, C. Layer-by-layer deposition of bioactive layers on magnesium alloy stent materials to improve corrosion resistance and biocompatibility. Bioact. Mater. 2020, 5, 611.
- Labarrere, C.A.; Dabiri, A.E. Thrombogenic and Inflammatory Reactions to Biomaterials in Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 123.
- Lee, K.S.; Kayumov, M.; Emechebe, G.A.; Kim, D.W.; Cho, H.J.; Jeong, Y.J.; Lee, D.W.; Park, J.K.; Park, C.H.; Kim, C.S.; et al. A Comparative Study of an Anti-Thrombotic Small-Diameter Vascular Graft with Commercially Available e-PTFE Graft in a Porcine Carotid Model. Tissue Eng. Regen. Med. 2022, 19, 537–551.
- Ray, P.; Chakraborty, R.; Banik, O.; Banoth, E.; Kumar, P. Surface Engineering of a Bioartificial Membrane for Its Application in Bioengineering Devices. ACS Omega 2023, 8, 3606–3629.
- Sun, W.; Liu, W.; Wu, Z.; Chen, H. Chemical surface modification of polymeric biomaterials for biomedical applications. Macromol. Rapid Commun. 2020, 41, 1900430.
- Gupta, P.; Mandal, B.B. Tissue-engineered vascular grafts: Emerging trends and technologies. Adv. Funct. Mater. 2021.
- Jin, Y.J.; Kang, S.; Park, P.; Choi, D.; Kim, D.W.; Jung, D.; Koh, J.; Jeon, J.; Lee, M.; Ham, J.; et al. Anti-inflammatory and antibacterial effects of covalently attached biomembrane-mimic polymer grafts on gore-teximplants. ACS Appl. Mater. Interfaces 2017, 9, 5–30.
- Lopez-Saucedo, F.; Flores-Rojas, G.; Magarinos, B.; Concheiro, A.; Alvarez, C.; Bucio, E. Radiation grafting of poly (methyl methacrylate) and poly(vinylimidazole) onto polytetrafluoroethylene films and silver immobilization for antimicrobial performance. Appl. Surf. Sci. 2019, 473, 951–959.
- Al Meslmani, B.; Mahmoud, G.; Bakowsky, U. Development of expanded polytetrafluoroethylene cardiovascular graft platform based on immobilization of poly lactic-co-glycolic acid nanoparticles using a wet chemical modification technique. Int. J. Pharm. 2017, 529, 238–244.
- Herten, M.; Idelevich, E.A.; Sielker, S.; Becker, K.; Scherzinger, A.S.; Osada, N.; Torsello, G.B.; Bisdas, T. Vascular graft impregnation with antibiotics: The influence of high concentrations of rifampin, vancomycin, daptomycin, and bacteriophage endolysin HY-133 on viability of vascular cells. Med. Sci. Monit. Basic Res. 2017, 23, 250–257.
- Geelhoed, W.; van der Bogt, K.; Rothuizen, T.; Damanik, F.; Hamming, J.; Mota, C.; van Agen, M.; de Boer, H.; Restrepo, M.T.; Hinz, B.; et al. A novel method for engineering autologous nonthrombogenic in situ tissue-engineered blood vessels for arteriovenous grafting. Biomaterials 2020, 229, 119577.
- Zhang, J.; Wang, Y.; Liu, C.; Feng, F.; Wang, D.; Mo, H.; Si, L.; Wei, G.; Shen, J. Polyurethane/polyurethane nanoparticle-modified expanded poly(tetrafluoroethylene) vascular patches promote endothelialization. J. Biomed. Mater. Res. A 2018, 106, 2131–2140.
- Táborská, J.; Riedelová, Z.; Brynda, E.; Májek, P.; Riedel, T. Endothelialization of an ePTFE vessel prosthesis modified with an antithrombogenic fibrin/heparin coating enriched with bound growth factors. RSC Adv. 2021, 11, 5903–5913.
- Tzchori, I.; Falah, M.; Shteynberg, D.; Ashkenazi, D.L.; Loberman, Z.; Perry, L.; Flugelman, M.Y. Improved patency of ePTFE grafts as a hemodialysis access site by seeding autologous endothelial cells expressing fibulin-5 and VEGF. Mol. Ther. 2018, 26, 1660–1668.
- Cheng, B.; Ishihara, K. Formation of stable polydopamine layer on polytetrafluoroethylene substrate by hybrid process involved plasma treatment and spontaneous chemical reactions. Mater. Today Commun. 2020, 22, 100774.
- Wulff, B.; Stahlhoff, S.; Vonthein, R.; Schmidt, A.; Sigler, M.; Torsello, G.B.; Herten, M. Biomimetic heparan sulfate-like coated ePTFE grafts reduce in-graft neointimal hyperplasia in ovine carotids. Ann. Vasc. Surg. 2017, 40, 274–284.
- Aoki, J.; Tanabe, K. Mechanisms of drug eluting stent restenosis. Cardiovasc. Interv. Ther. 2021, 36, 23–29.
- Gouëffic, Y.; Sauguet, A.; Desgranges, P.; Feugier, P.; Rosset, E.; Ducasse, E.; Kaladji, A.; Salomon du Mont, L.; Pernès, J.M.; Commeau, P.; et al. A Polymer-Free Paclitaxel-Eluting Stent Versus a Bare-Metal Stent for De Novo Femoropopliteal Lesions: The BATTLE Trial. J. Am. Coll. Cardiol. Intv. 2020, 447–457.
- Kuramitsu, S. Drug-eluting stent thrombosis: Current and future perspectives. Cardiovasc. Interv. Ther. 2021, 36, 158–168.
- Gohbara, M.; Sugano, T.; Ishikawa, T.; Tamura, K.; Kimura, K. A case of a coronary covered stent for repeated restenosis at the anastomosis site between saphenous vein graft and graft prosthesis. J. Cardiol. Cases 2021, 25, 110–114.
- Wang, Y.; Li, G.; Yang, L.; Luo, R.; Guo, G. Development of Innovative Biomaterials and Devices for the Treatment of Cardiovascular Disease. Adv. Mater. 2022, 34, 2201971.
- Radu, E.R.; Voicu, S.I.; Thakur, V.K. Polymeric Membranes for Biomedical Applications. Polymers 2023, 15, 619.
- Smelt, H.; Cheng, W.; Tyler, S.; Virmani, R.J. TCT-125 Ultrahigh Molecular Weight Polyethylene Membrane for Use in Vascular Stent Graft Applications—Preliminary Evidence from an Ovine Peripheral Implantation Model. Am. Coll. Cardiol. 2017, 70, B56.




