Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander Kruchinin | -- | 1758 | 2023-02-21 19:14:27 | | | |
| 2 | Catherine Yang | Meta information modification | 1758 | 2023-02-22 01:55:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kruchinin, A.A.; Makarova, A.V. Pol θ as a Central Player in TMEJ. Encyclopedia. Available online: https://encyclopedia.pub/entry/41497 (accessed on 07 February 2026).
Kruchinin AA, Makarova AV. Pol θ as a Central Player in TMEJ. Encyclopedia. Available at: https://encyclopedia.pub/entry/41497. Accessed February 07, 2026.
Kruchinin, Alexander A., Alena V. Makarova. "Pol θ as a Central Player in TMEJ" Encyclopedia, https://encyclopedia.pub/entry/41497 (accessed February 07, 2026).
Kruchinin, A.A., & Makarova, A.V. (2023, February 21). Pol θ as a Central Player in TMEJ. In Encyclopedia. https://encyclopedia.pub/entry/41497
Kruchinin, Alexander A. and Alena V. Makarova. "Pol θ as a Central Player in TMEJ." Encyclopedia. Web. 21 February, 2023.
Copy Citation
DNA polymerase θ belongs to the A family of DNA polymerases and plays a key role in DNA repair and damage tolerance, including double-strand break (DSB) repair and DNA translesion synthesis. During Pol θ-mediated end joining (TMEJ), Pol θ aligns resected 3′-single-stranded DNA ends based on microhomology, fills DNA gaps and generates repair products with deletions of nonhomologous sequences flanking the DSB site.
DNA polymerase θ
double-strand break repair
DNA translesion synthesis
1. Discovery of TMEJ
DSBs arising under various conditions (oxidative and genotoxic stress, ionizing and UV radiation) and accompanied by genomic instability are extremely dangerous to cells. DSBs can lead to malignant cell transformation, cell death, and the development of various pathologies. To withstand this type of damage, the cell relies on at least four major types of DSB repair. The non-homologous end joining (which is now often referred to as canonical NHEJ, or c-NHEJ) does not require a homologous chromosome and directly ligates the break ends. NHEJ is typically guided by very short (1–4 bp) terminal microhomologies but can be realized even without microhomology [1].
Other types of DSB repair pathways—single-strand annealing (SSA), homologous recombination (HR) and alternative end joining (a-EJ)—are based on different principles, which require 5′ to 3′ nucleolytic resection of broken ends to generate 3′-ssDNA tails at the DSB site and more extensive homology to anneal complementary sequences.
At present, the importance of Pol θ for DSB repair is well-established. It took more than a decade to uncover the molecular basis underlying the role of Pol θ in DSB repair. The phenotype-based screening of mutant mice showed that the missense mutant allele chaos1 (a C>T substitution at nucleotide 5794 in the coding region of exon 19) of the Polq gene leads to a spontaneous and radioactivity-induced increase in micronuclei number in erythrocytes. The analysis of Polqchaos1/Polqchaos1 mice with an ATM (ataxia telangiectasia mutated) deficiency allowed the suggestion that Pol θ might be involved in an alternative DSB repair pathway distinctive from the major HR ATM-dependent pathway [2]. About 90% of chaos1 homozygous mice with an ATM-deficient background died during the neonatal period and surviving animals exhibited severe growth retardation and chromosome instability which was synergistic to the ATM-deficiency phenotype. A study by J. Goff et al., demonstrated the sensitivity of POLQ-defective bone-marrow cells to γ-radiation and bleomycin, suggesting the role of Pol θ in DSB repair [3]. Finally, experiments by S.H. Chan et al., with the P-element of D. melanogaster revealed that Pol θ is a critical factor of Lig4 (DNA ligase 4)-independent alternative end joining (a-EJ) [4].
As discussed in [5] by Ramsden et al., previously, a-EJ was considered as a DSB repair pathway which does not depend on LIG4. Currently, the term “a-EJ” is applied to a group of end-joining pathways that do not require at least one of the c-NHEJ factors: Ku, XRCC4 or LIG4. a-EJ is often associated with microhomology-mediated end joining (MMEJ) and DNA polymerase theta (Pol θ)-mediated end joining (TMEJ). Definitions of a-EJ and TMEJ are not equivalent. In fungi, end joining proceeds in a Pol θ-independent manner. Thus, a-EJ can be divided into two subgroups: TMEJ and Pol θ-independent a-EJ (the definition is given based on a key factor involved). In turn, MMEJ is an umbrella term for end joining realized by the mechanism of broken DNA strand alignment based on microhomologous sequences [5].
2. Mechanism and Regulation of TMEJ in Cells
Several factors favoring the cell choice of TMEJ have been defined. The main regulation is most likely realized during the first step—resection, when the 3′-ssDNA overhangs are generated by the MRE11–RAD50–NBS1 (MRN) complex and CtBP-interacting protein (CtIP) [5]. The formation of such intermediates, which are unsuitable substrates for the c-NHEJ Ku factors, antagonizes this pathway and favors HR and TMEJ. The phase of the cell cycle, the presence of some regulatory and cell-signaling proteins, and the length and degree of homology of the processed 3′-overhangs appear to be the main factors in the Pol θ recruitment and cell’s choice of TMEJ over c-NHEJ, HR and SSA (Figure 1) [6][7][8][9][10][11][12]. TMEJ, together with HR and SSA, are limited to the S and G2 stages, while c-NHEJ operates predominantly in the G0/G1 phases [12].
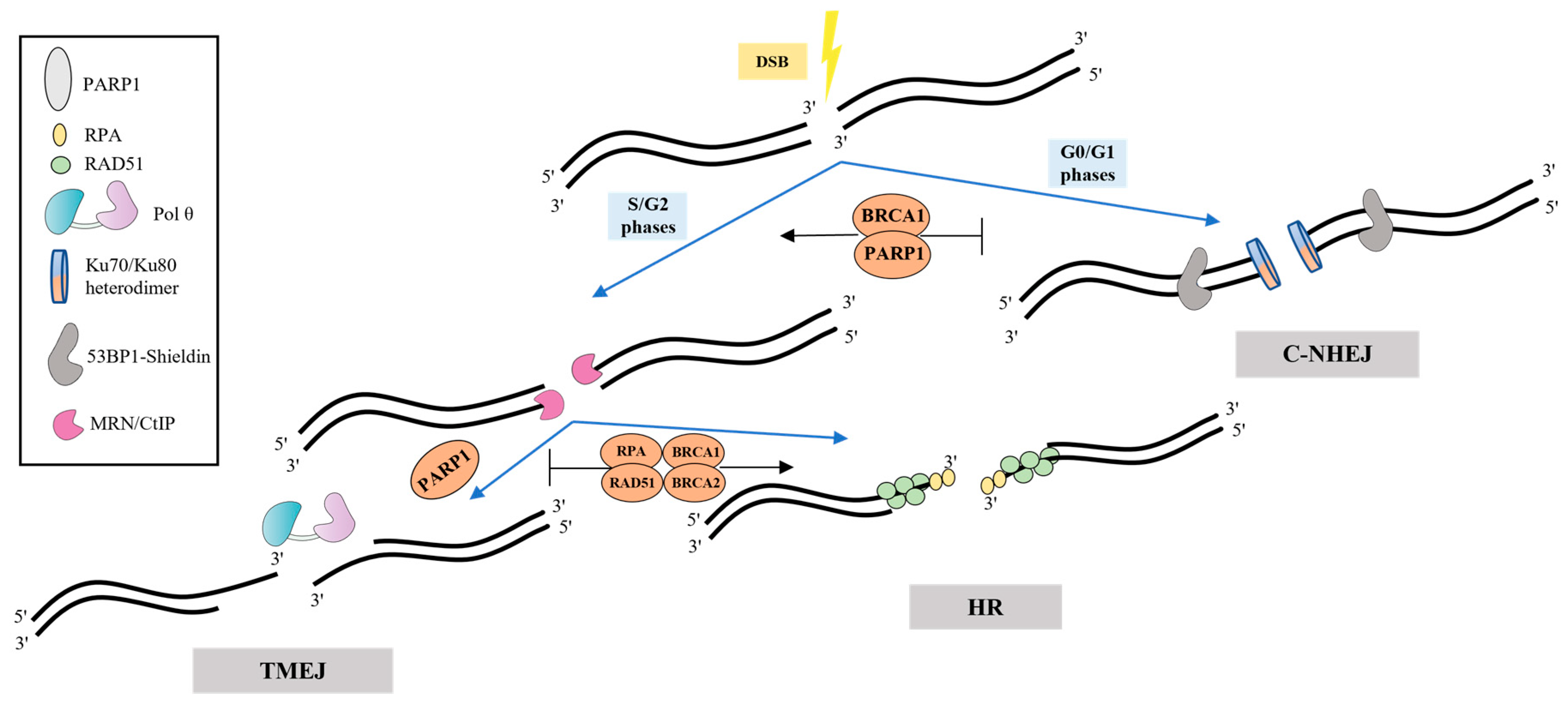
Figure 1. Regulation of TMEJ. Loading of Ku heterodimer, which prevents resection and promotes c-NHEJ, is very effective at blunt DNA ends and extremely short overhangs. Another factor, 53BP1 recruits the shieldin complex inhibiting 5′-3′ resection of DNA ends, thereby promoting c-NHEJ and antagonizing HR and TMEJ. The MRN/CtIP complex performs end resection accompanied by formation of intermediates that are common for both HR and TMEJ. HR factors (RPA, RAD51, BRCA1 and BRCA2) contribute to more accurate repair, inhibiting error-prone TMEJ. PARP1 appears to mediate the recruitment of Pol θ.
TMEJ is also regulated by poly(ADP) ribose polymerase 1 (PARP1). PARP1 is activated upon binding DNA strand breaks and performs post-translational modification (PARylation) of many DNA repair proteins. Competing with Ku proteins for DNA ends binding, PARP1 promotes a-EJ, and its inhibition impairs Pol θ recruitment to DNA break sites [13][14]. Chromosomal TMEJ assays revealed that PARP1/PARP2 deficiency reduces TMEJ activity only 2–4-fold through a decrease in the 5′-3′ resection of DSB, and, thus, re-channels repair to NHEJ [15]. However, PARP1-dependent PARylation of the Pol θ HD in vitro decreases the ssDNA-binding affinity of Pol θ. It was suggested that PARylation can promote the dissociation of Pol θ from DNA strands and termination of DNA synthesis in the end of TMEJ [16]. The alternative clamp RAD9-RAD1-HUS1 complex (9-1-1), Fanconi anaemia group D2 protein (FANCD2) and 5-hydroxymethylcytosine-binding protein (NMCES) possibly also play a role in the recruitment of Pol θ to DNA [reviewed in [5].
Before binding to DNA, it is necessary to displace replication protein A (RPA), which coats ssDNA regions limiting TMEJ and contributing to HR. Pol θ promotes the removal of RPA from single-stranded overhangs via ATP hydrolysis [17]. Moreover, the assembly of RAD51 filaments on ssDNA sites in order to promote HR can be suppressed by Pol θ. Thus, POLQ-knockdown in HR-proficient cells contributes to the RAD51 filaments assembly and accumulation [14][18] Disassembly of RAD51 nucleofilament by Pol θ is carried out in ATPase-dependent manner but with lower efficiency compared to RPA [16].
The 3′-overhangs lacking RPA should be paired and aligned, followed by the search for microhomologies and annealing (Figure 2). There are a number of different conditions for completing these steps [9][11][19][20]. In vitro assays indicated that the 3′-ssDNA overhangs shorter than 15 nt are favorable for TMEJ [19]. However, the presence of 3′-tails of >45 nt is an important requirement for promoting TMEJ in cells [11].
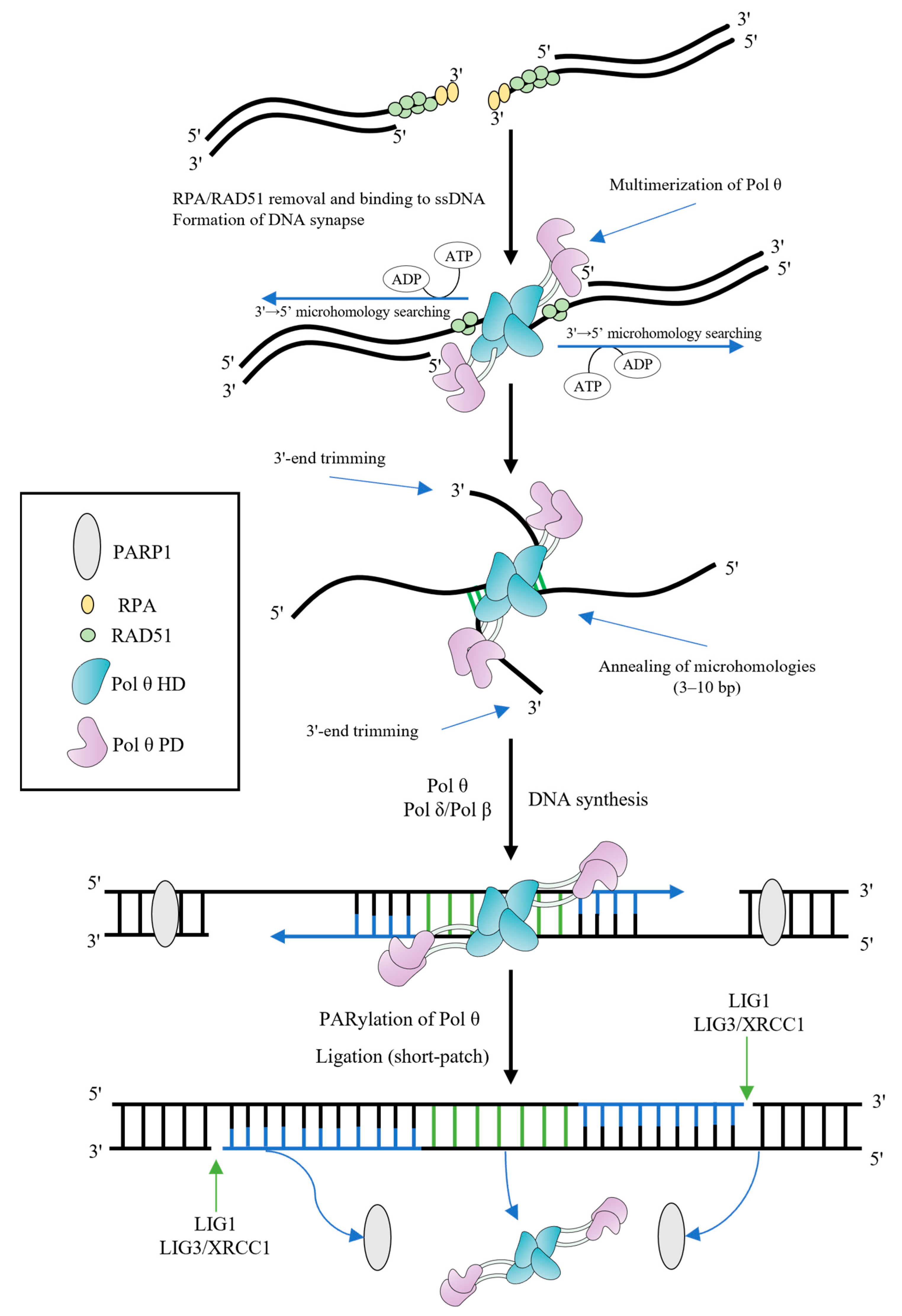
Figure 2. Model of TMEJ. The resected 3′-overhangs are coated by RPA and RAD51 filaments. The HD of Pol θ removes both of them in ATP-dependent manner, contributing to TMEJ. Multimerization of Pol θ promotes the DNA synapse formation and initiates the 3′→5′ bidirectional searching for microhomologies, using the energy of ATP hydrolysis. Aligned 3′-ends are annealed in microhomology-rich regions. Unannealed 3′-overhangs are trimmed before DNA extension. Pol θ (or Pol β) fills gaps from annealed microhomologies, using them as primers. PARP1 PARylates Pol θ in order to remove the polymerase from DNA and complete the synthesis step. LIG1 or LIG3/XRCC1 complex finish the end joining (the short-patch resolution is shown).
Some structural studies demonstrated that the HD and PD form multimers regulated by the CD and Insert 2 [19][21][22][23]. Multimerization of Pol θ might contribute to both bridging non-complementary DNA ends to form a DNA synapse, and searching for microhomologies [16][21]. Identification of the site of microhomology relies on a mechanism associated with the HD and involves initiation of a bidirectional search from the 3′-end [20]. As a rule, Pol θ successfully recognizes the microhomology when it is within the 15-nt region on either side of the break site [20], though some works indicated that microhomologies may be at a farther distance from the 3′-overhangs. Microhomologies need to comprise 3–10 bp for effective DSB repair by TMEJ [20].
Aligned 3′-ends are expected to promote the annealing accompanied by predominantly intramolecular hairpin formation rather than intermolecular annealing and DNA synthesis. Nevertheless, the unknown endonuclease activity can cleave hairpin structures that form during intramolecular annealing, facilitating intermolecular annealing as well as multimerization of Pol θ. The Pol θ HD suppresses snap-back replication, favoring intermolecular annealing and synthesis [21][23][24].
The presence of nonhomologous segments flanking the microhomologies at the 3′-ends complicates TMEJ. Nonhomologous regions should be removed and the 3′-tails should be shortened before initiation of DNA synthesis from annealed microhomologies. Initially, it was suggested that Pol θ performs end trimming using the intrinsic endonuclease activity associated with the PD (repurposing of metal ions in the polymerase active site for endonucleolytic cleavage) [24]. However, in a further paper, an alternative explanation of the results was provided which made the authors doubt the existence of trimming endonuclease activity [25]. The PD structure lacks a potential endonuclease domain [22]. Thus, the mechanism of the 3′-end processing is yet to be established. The 3′-flap endonuclease complex excision repair cross complementation group 1-xeroderma pigmentosum group F (ERCC1-XPF) is a possible candidate since the XPF deficiency is associated with a reduced DSB repair efficiency (independently from the NHEJ repair pathway) [26].
After the 3′-end-trimming step, Pol θ initiates template-dependent fill-in DNA synthesis [24]. Annealed microhomologies represent themselves as DNA primers and usually contain fewer than 10 nucleotides. The solved crystal structure of the PD reveals how Pol θ grasps the primer by making contacts with its phosphate backbone [22]. The processive DNA synthesis is important for efficient TMEJ, since the length of the 3′-overhangs before trimming often varies. In fact, the processivity of Pol θ is generally higher as compared to Y-family DNA polymerases. Pol θ is able to incorporate up to 100 nucleotides in one round of template binding in vitro [27][28][29]. Yet, sometimes processivity may not be sufficient during TMEJ (e.g., in the case of an only 1–2 nt microhomology), and it was suggested that Pol θ can carry out DNA synthesis in several rounds of DNA binding [4][11][20][30]. It was shown that the thumb subdomain Insert 1 residues contacting the DNA minor grove are important for processive DNA synthesis by Pol θ [22][31].
The DNA synthesis step is apparently not restricted to Pol θ; the high-fidelity replicative DNA polymerase Pol δ may be involved both in yeast and mammals [32][33]. It has been also hypothesized that, due to insufficient processivity, Pol θ only initiates synthesis from annealed microhomologies, and, subsequently, Pol δ fills the gap. Pol β, which can operate on the 3′-overhangs during gap-filling synthesis in vitro, is considered to be a back-up DNA polymerase for Pol θ [34].
After filling the gap, DNA ligase 1 (LIG1) or (preferentially) the complex of DNA ligase 3 (LIG3)/X-ray repair cross-complementing protein 1 (XRCC1) seals the DNA ends during a short-patch resolution, restoring a phosphodiester bond [4][14]. Alternatively, a DNA polymerase can displace DNA strand and generate a 5′-flap, which is removed by flap endonuclease 1 (FEN1) or DNA2 followed by ligation (a long-patch resolution) [35][36].
References
- Zhao, B.; Rothenberg, E.; Ramsden, D.A.; Lieber, M.R. The Molecular Basis and Disease Relevance of Non-Homologous DNA End Joining. Nat. Rev. Mol. Cell Biol. 2020, 21, 765–781.
- Shima, N.; Munroe, R.J.; Schimenti, J.C. The Mouse Genomic Instability Mutation Chaos1 Is an Allele of Polq That Exhibits Genetic Interaction with Atm. Mol. Cell. Biol. 2004, 24, 10381–10389.
- Goff, J.P.; Shields, D.S.; Seki, M.; Choi, S.; Epperly, M.W.; Wang, H.; Bakkenist, C.J.; Dertinger, S.D.; Dorothea, K.; Wittschieben, J.; et al. Lack of DNA Polymerase θ (POLQ) Radiosensitizes Bone Marrow Stromal Cells In Vitro and Increases Reticulocyte Micronuclei after Total-Body Irradiation. Radiat. Res. 2009, 172, 165–174.
- Chan, S.H.; Yu, A.M.; McVey, M. Dual Roles for DNA Polymerase Theta in Alternative End-Joining Repair of Double-Strand Breaks in Drosophila. PLoS Genet. 2010, 6, e1001005.
- Ramsden, D.A.; Carvajal-Garcia, J.; Gupta, G.P. Mechanism, Cellular Functions and Cancer Roles of Polymerase-Theta-Mediated DNA End Joining. Nat. Rev. Mol. Cell Biol. 2022, 23, 125–140.
- Truong, L.N.; Li, Y.; Shi, L.Z.; Hwang, P.Y.H.; He, J.; Wang, H.; Razavian, N.; Berns, M.W.; Wu, X. Microhomology-Mediated End Joining and Homologous Recombination Share the Initial End Resection Step to Repair DNA Double-Strand Breaks in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7720–7725.
- Bothmer, A.; Robbiani, D.F.; Feldhahn, N.; Gazumyan, A.; Nussenzweig, A.; Nussenzweig, M.C. 53BP1 Regulates DNA Resection and the Choice between Classical and Alternative End Joining during Class Switch Recombination. J. Exp. Med. 2010, 207, 855–865.
- Foster, S.S.; Balestrini, A.; Petrini, J.H.J. Functional Interplay of the Mre11 Nuclease and Ku in the Response to Replication-Associated DNA Damage. Mol. Cell Biol. 2011, 31, 4379–4389.
- Deng, S.K.; Gibb, B.; De Almeida, M.J.; Greene, E.C.; Symington, L.S. RPA Antagonizes Microhomology-Mediated Repair of DNA Double-Strand Breaks. Nat. Struct. Mol. Biol. 2014, 21, 405–412.
- Ahrabi, S.; Sarkar, S.; Pfister, S.X.; Pirovano, G.; Higgins, G.S.; Porter, A.C.G.; Humphrey, T.C. A Role for Human Homologous Recombination Factors in Suppressing Microhomology-Mediated End Joining. Nucleic Acids Res. 2016, 44, 5743–5757.
- Wyatt, D.W.; Feng, W.; Conlin, M.P.; Yousefzadeh, M.J.; Roberts, S.A.; Mieczkowski, P.; Wood, R.D.; Gupta, G.P.; Ramsden, D.A. Essential Roles for Polymerase θ-Mediated End Joining in the Repair of Chromosome Breaks. Mol. Cell 2016, 63, 662–673.
- Yu, W.; Lescale, C.; Babin, L.; Bedora-Faure, M.; Lenden-Hasse, H.; Baron, L.; Demangel, C.; Yelamos, J.; Brunet, E.; Deriano, L. Repair of G1 Induced DNA Double-Strand Breaks in S-G2/M by Alternative NHEJ. Nat. Commun. 2020, 11, 5239.
- Audebert, M.; Salles, B.; Weinfeld, M.; Calsou, P. Involvement of Polynucleotide Kinase in a Poly(ADP-Ribose) Polymerase-1-Dependent DNA Double-Strand Breaks Rejoining Pathway. J. Mol. Biol. 2006, 356, 257–265.
- Mateos-Gomez, P.A.; Gong, F.; Nair, N.; Miller, K.M.; Lazzerini-Denchi, E.; Sfeir, A. Mammalian Polymerase θ Promotes Alternative NHEJ and Suppresses Recombination. Nature 2015, 518, 254–257.
- Luedeman, M.E.; Stroik, S.; Feng, W.; Luthman, A.J.; Gupta, G.P.; Ramsden, D.A. Poly(ADP) Ribose Polymerase Promotes DNA Polymerase Theta-Mediated End Joining by Activation of End Resection. Nat. Commun. 2022, 13, 4547.
- Schaub, J.M.; Soniat, M.M.; Finkelstein, I.J. Polymerase Theta-Helicase Promotes End Joining by Stripping Single-Stranded DNA-Binding Proteins and Bridging DNA Ends. Nucleic Acids Res. 2022, 50, 3911–3921.
- Mateos-Gomez, P.A.; Kent, T.; Deng, S.K.; Mcdevitt, S.; Kashkina, E.; Hoang, T.M.; Pomerantz, R.T.; Sfeir, A. The Helicase Domain of Polθ Counteracts RPA to Promote Alt-NHEJ. Nat. Struct. Mol. Biol. 2017, 24, 1116–1123.
- Ceccaldi, R.; Liu, J.C.; Amunugama, R.; Hajdu, I.; Primack, B.; Petalcorin, M.I.R.; O’Connor, K.W.; Konstantinopoulos, P.A.; Elledge, S.J.; Boulton, S.J.; et al. Homologous-Recombination-Deficient Tumours Are Dependent on Polθ-Mediated Repair. Nature 2015, 518, 258–262.
- Kent, T.; Chandramouly, G.; Mcdevitt, S.M.; Ozdemir, A.Y.; Pomerantz, R.T. Mechanism of Microhomology-Mediated End-Joining Promoted by Human DNA Polymerase θ. Nat. Struct. Mol. Biol. 2015, 22, 230–237.
- Carvajal-Garcia, J.; Cho, J.E.; Carvajal-Garcia, P.; Feng, W.; Wood, R.D.; Sekelsky, J.; Gupta, G.P.; Roberts, S.A.; Ramsden, D.A. Mechanistic Basis for Microhomology Identification and Genome Scarring by Polymerase Theta. Proc. Natl. Acad. Sci. USA 2020, 117, 8476–8485.
- Newman, J.A.; Cooper, C.D.O.; Aitkenhead, H.; Gileadi, O. Structure of the Helicase Domain of DNA Polymerase Theta Reveals a Possible Role in the Microhomology-Mediated End-Joining Pathway. Structure 2015, 23, 2319–2330.
- Zahn, K.E.; Averill, A.M.; Aller, P.; Wood, R.D.; Doublié, S. Human DNA Polymerase θ Grasps the Primer Terminus to Mediate DNA Repair. Nat. Struct. Mol. Biol. 2015, 22, 304–311.
- Black, S.J.; Ozdemir, A.Y.; Kashkina, E.; Kent, T.; Rusanov, T.; Ristic, D.; Shin, Y.; Suma, A.; Hoang, T.; Chandramouly, G.; et al. Molecular Basis of Microhomology-Mediated End-Joining by Purified Full-Length Polθ. Nat. Commun. 2019, 10, 4423.
- Zahn, K.E.; Jensen, R.B.; Wood, R.D.; Doublié, S. Human DNA Polymerase θ Harbors DNA End-Trimming Activity Critical for DNA Repair. Mol. Cell 2021, 81, 1534–1547.e4.
- Wood, R.D.; Doublié, S. Genome Protection by DNA Polymerase θ. Annu. Rev. Genet. 2022, 56, 207–228.
- Ahmad, A.; Robinson, A.R.; Duensing, A.; van Drunen, E.; Beverloo, H.B.; Weisberg, D.B.; Hasty, P.; Hoeijmakers, J.H.J.; Niedernhofer, L.J. ERCC1-XPF Endonuclease Facilitates DNA Double-Strand Break Repair. Mol. Cell. Biol. 2008, 28, 5082–5092.
- Arana, M.E.; Seki, M.; Wood, R.D.; Rogozin, I.B.; Kunkel, T.A. Low-Fidelity DNA Synthesis by Human DNA Polymerase Theta. Nucleic Acids Res. 2008, 36, 3847–3856.
- Masutani, C.; Rika Kusumoto, S.I. and F.H. Mechanisms of Accurate Translesion Synthesis by Human DNA Polymerase η. EMBO J. 2000, 19, 3100–3109.
- Haracska, L.; Johnson, R.E.; Unk, I.; Phillips, B.B.; Hurwitz, J.; Prakash, L.; Prakash, S. Targeting of Human DNA Polymerase ι to the Replication Machinery via Interaction with PCNA. Proc. Natl. Acad. Sci. USA 2001, 98, 14256–14261.
- van Schendel, R.; van Heteren, J.; Welten, R.; Tijsterman, M. Genomic Scars Generated by Polymerase Theta Reveal the Versatile Mechanism of Alternative End-Joining. PLoS Genet. 2016, 12, e1006368.
- Hogg, M.; Seki, M.; Wood, R.D.; Doublié, S.; Wallace, S.S. Lesion Bypass Activity of DNA Polymerase θ (POLQ) Is an Intrinsic Property of the Pol Domain and Depends on Unique Sequence Inserts. J. Mol. Biol. 2011, 405, 642–652.
- Meyer, D.; Fu, B.X.H.; Heyer, W.D. DNA Polymerases δ and λ Cooperate in Repairing Double-Strand Breaks by Microhomology-Mediated End-Joining in Saccharomyces Cerevisiae. Proc. Natl. Acad. Sci. USA 2015, 112, E6907–E6916.
- Layer, J.V.; Debaize, L.; van Scoyk, A.; House, N.C.; Brown, A.J.; Liu, Y.; Stevenson, K.E.; Hemann, M.; Roberts, S.A.; Price, B.D.; et al. Polymerase δ Promotes Chromosomal Rearrangements and Imprecise Double-Strand Break Repair. Proc. Natl. Acad. Sci. USA 2020, 117, 27566–27577.
- Ray, S.; Breuer, G.; DeVeaux, M.; Zelterman, D.; Bindra, R.; Sweasy, J.B. DNA Polymerase Beta Participates in DNA End-Joining. Nucleic Acids Res. 2018, 46, 242–255.
- Mengwasser, K.E.; Adeyemi, R.O.; Leng, Y.; Choi, M.Y.; Clairmont, C.; D’Andrea, A.D.; Elledge, S.J. Genetic Screens Reveal FEN1 and APEX2 as BRCA2 Synthetic Lethal Targets. Mol. Cell 2019, 73, 885–899.e6.
- Howard, S.M.; Yanez, D.A.; Stark, J.M. DNA Damage Response Factors from Diverse Pathways, Including DNA Crosslink Repair, Mediate Alternative End Joining. PLoS Genet. 2015, 11, e1004943.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
22 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




