Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonella Meloni | -- | 2510 | 2023-02-21 18:05:44 | | | |
| 2 | Rita Xu | Meta information modification | 2510 | 2023-02-22 04:25:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Meloni, A.; Frijia, F.; Panetta, D.; Degiorgi, G.; Gori, C.D.; Maffei, E.; Clemente, A.; Positano, V.; Cademartiri, F. Photon-Counting Computed Tomography. Encyclopedia. Available online: https://encyclopedia.pub/entry/41492 (accessed on 03 March 2026).
Meloni A, Frijia F, Panetta D, Degiorgi G, Gori CD, Maffei E, et al. Photon-Counting Computed Tomography. Encyclopedia. Available at: https://encyclopedia.pub/entry/41492. Accessed March 03, 2026.
Meloni, Antonella, Francesca Frijia, Daniele Panetta, Giulia Degiorgi, Carmelo De Gori, Erica Maffei, Alberto Clemente, Vincenzo Positano, Filippo Cademartiri. "Photon-Counting Computed Tomography" Encyclopedia, https://encyclopedia.pub/entry/41492 (accessed March 03, 2026).
Meloni, A., Frijia, F., Panetta, D., Degiorgi, G., Gori, C.D., Maffei, E., Clemente, A., Positano, V., & Cademartiri, F. (2023, February 21). Photon-Counting Computed Tomography. In Encyclopedia. https://encyclopedia.pub/entry/41492
Meloni, Antonella, et al. "Photon-Counting Computed Tomography." Encyclopedia. Web. 21 February, 2023.
Copy Citation
Photon-counting computed tomography (PCCT) is a new advanced imaging technique that is going to transform the standard clinical use of computed tomography (CT) imaging. Photon-counting detectors resolve the number of photons and the incident X-ray energy spectrum into multiple energy bins. Compared with conventional CT technology, PCCT offers the advantages of improved spatial and contrast resolution, reduction of image noise and artifacts, reduced radiation exposure, and multi-energy/multi-parametric imaging based on the atomic properties of tissues, with the consequent possibility to use different contrast agents and improve quantitative imaging.
photon-counting detectors
photon-counting CT
cardiac CT
1. Introduction
Vascular diseases refer to any abnormal condition that affects the blood vessels. The most important role among vascular diseases is played by atherosclerosis. The atherosclerotic plaques, composed by fatty substances, cholesterol, cellular waste products, calcium, and fibrin [1][2], can cause the narrowing or hardening of arteries, with consequent ischemia and functional impairment of the affected tissue or organ. Indeed, atherosclerosis is the leading cause of death and disability around the world [3]. The diagnosis of the ischemic disease at an early stage is the key to improve the effectiveness of patient treatment and to implement additional preventive measures, impeding its tragic sequelae.
Computed tomography (CT) has gained a pivotal role in the assessment of vascular disease processes and in the planning and follow-up of minimally invasive interventions. In particular, coronary CT angiography (CCTA) has progressively gained widespread adoption during the last two decades [4], thanks to increased axial coverage of multi-row detectors, increased rotation speed, and progress in prospective gating protocols based on electrocardiogram (ECG) phase-correlated triggering [5]. The main advantages of CT are its non-invasiveness, large availability, fast scanning speed, wide field of view, and excellent spatial and temporal resolution [6]. The main limitations of CT are the exposure to ionizing radiations and the necessity of administering iodinated contrast media, which can be problematic for patients with kidney disease [7].
Photon-counting computed tomography (PCCT) is a technology based on energy-resolving, direct-conversion X-ray detectors, which has been adopted just very recently in clinical CT equipment after 15 years of research and development. This is substantially different from conventional CT detectors, based on indirect X-ray conversion (with scintillators) and signal integration over the entire X-ray energy spectrum. PCCT conveys the potential for changing the clinical CT scenario, thanks to its many inherent advantages and the ability to overcome several of the shortcomings of current state-of-the-art CT systems.
2. Photon-Counting Detector Technology
2.1. Comparison between Conventional and Photon Counting Detectors
The X-ray detector is a core component of a CT scanner, determining both image quality and radiation dose. Conventional CT devices currently employ energy-integrating detectors (EIDs) equipped with scintillator elements and reflective layers (septa). The layer of ceramic scintillators converts the incident X-ray photons into low energy secondary photons in the visible spectrum. These latter photons are then absorbed by a photodiode array made of a semiconducting material, which generates an electrical signal proportional to the total deposited energy, summed to electronic thermal noise. Finally, the electrical signal is amplified and then converted to a digital signal, so that it can be processed for tomographic image reconstruction. Indeed, because the detector integrates the energy from all incident photons in a given time interval, any information regarding the energy of an individual X-ray photon is lost. Septa are incorporated between scintillating detector pixels to prevent light crosstalk between them. Such septa cause “dead space” on the detector surface and, since there is a physical limit to their thickness, they limit the geometric dose efficiency [8][9].
PC detectors (PCDs) are based on a direct conversion technique. They are made by coupling a semiconductor sensor with a high effective atomic number (typically, cadmium telluride or CdTe) and high thickness (1–2 mm) with a readout circuit (Figure 1). Upon absorption in the semiconductor, the incident X-ray photons are directly converted into electron-hole pairs. Charge collection efficiency is increased by applying an inverse bias voltage through either Schottky-type barrier contact or ohmic (metal semiconductor) contacts, thus improving the energy resolution, detection efficiency, and reducing the contribution of dark current [10]. When operated in counting mode, the height of the electric pulse is proportionate to the energy deposited by the interacting X-ray photon in the depleted semiconductor region. Pulse heights are then compared with a voltage that reflects a specified photon energy level (energy threshold) [11]. Multiple electronic comparators are used to count the number of pulses with an energy level equal to or greater than the preset thresholds [12], allowing researchers to sort the incoming photons into a number of energy bins (typically two to eight). In experimental settings, the energy thresholds (in kiloelectron volts, or keV) could be set up by the user before data acquisition; on the other hand, commercial systems are generally pre-set to factory values and do not allow the final user to create custom thresholds. The lower threshold is set at levels that are reasonably higher than the electronic noise level, so that the electronic noise is totally suppressed in the final signal. The other thresholds are either spaced uniformly or chosen to optimize a given imaging task [13].
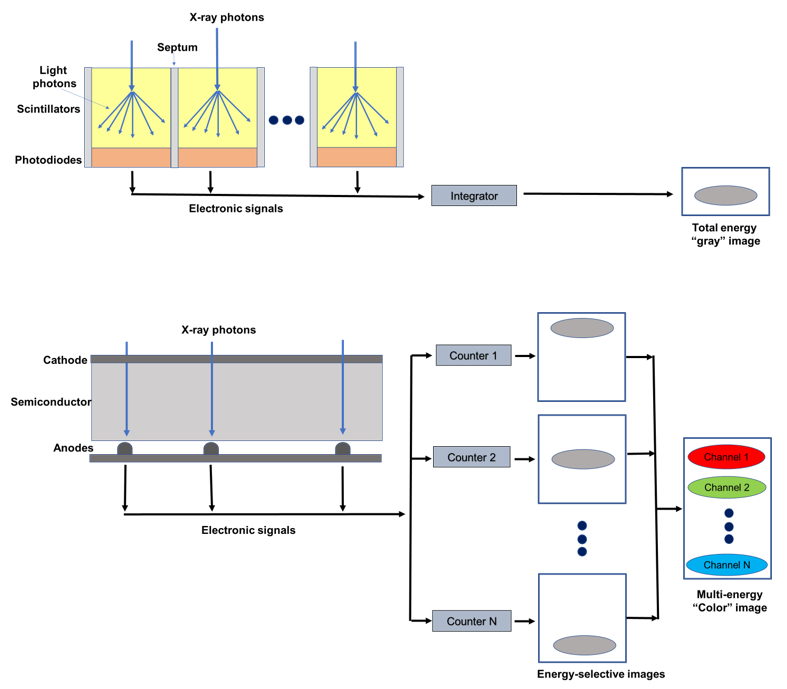
Figure 1. Schematic representation of an energy integrating detector (top) and of a photon-counting detector directly converting X-rays into an electrical signal (bottom). The photon-counting design allows the generation of energy-selective images, from which a set of material concentration maps can be obtained. Material concentration maps can then be combined in different ways to obtain monochromatic images, virtual non-contrast images, or material-specific color-overlay images.
2.2. Technical Challenges of PCDs
The adoption of PCDs in clinical CT has been made possible by the minimization or overcoming of several factors affecting the detector performance and, in turn, the final quality of reconstructed images. Count rate performance was one of the main obstacles to the introduction of counting mode CdTe detectors in clinical CT. Photon fluences in commercial scanners can be higher than 108 photons/(mm2 s) [14], which is several orders of magnitudes as compared to hit rates normally encountered in, for instance, nuclear medicine applications. In clinical PCDs, the requirement for high hit-rate-capable detectors (>106 counts per second) can be relaxed by using monolithic CdTe layers, bonded to pixelated application-specific integrated circuits (ASIC) with small pitch (<200 m). As an example, in 2021 Siemens Healthineers released the first commercial CT scanner based on PCD, with FDA clearance for clinical use. In this case, the pitch of the ASIC reading the CdTe detector was 0.150 × 0.176 mm2 at the isocenter, with standard reading after 2 × 2 binning. Reducing the active area for each pixel, and consequently also the count rate requirement, pulse pileup, and consequent count loss and spectral distortion, this configuration leads to a very high spatial resolution when operating the detector without rebinning (i.e., 1 × 1 reading) [15]. On the other hand, narrower detector pitch leads to secondary charge clouds being sensed by more than one neighboring pixels, which is known as charge sharing effect [16]. Charge sharing can be corrected by several schemes, such as dedicated circuits implementing a winner-takes-all strategy, where the pixel receiving the largest amount of charge is assigned to the total charge detected in a 2 × 2 neighborhood [17]. The Medipix3 ASIC, for instance, implements this strategy [18]. Besides secondary charge sharing, other types of pixel crosstalk are possible in PCDs, such as those related to the generation of fluorescence X-ray radiation in pixels involved in the first interaction, which is in turn detected in the nearby pixels. This effect is responsible for the lower limit of detector pixel size in practical applications [12].
3. Benefits of PCDs
3.1. Reduction of Electronic Noise
In CT the electronic noise is mainly caused by the analog electronic circuits in the detection system and is usually detected as a low-amplitude signal.
EIDs do not process the signals from individual photons but integrate the total energy deposited over a certain time period, including electronic noise. Conversely, in PCDs the noise affects the minimum detectable pulse-height (noise threshold) but not the number of pulses (photon count) above that threshold. Therefore, by setting up the low energy threshold of a PCD at levels exceeding the one associated with the noise floor (approximately 25 keV), the electronic noise can be effectively excluded from photon and/or pulse counts, although it is still present in the spectral information [12].
Therefore, for the same dose, the noise in the reconstructed image is lower with PCDs than with EIDs. The intrinsic advantage of PCDs is of particular benefit in CT scans performed at very low radiation dose or in obese patients, when the noise is not negligible. In these scenarios, images obtained with PCDs have demonstrated to be less affected by streak artifacts and signal uniformity and to produce more stable CT-numbers [19][20]. Importantly, the reduced noise benefit can be exploited to improve the dose efficiency, since with PCDs a noise level comparable to that of an EID can be obtained with a lower radiation dose.
3.2. Improvement in Spatial Resolution
In current clinical EIDs the pixel size is about 0.4–0.6 mm at the isocenter, limiting their resolution. In fact, the design of smaller detector pixels causes an increase in the relative area to the septum in comparison to the detector area, with a consequent reduction in the geometric dose efficiency. In PCDs, thanks to the absence of a mechanic separation, the pixel pitch does not have a technical limitation and can reach 0.15–0.225 mm at the isocenter [21][22][23][24].
Different solutions have been proposed to improve the spatial resolution of scintillator detectors. For example, a dedicated attenuating filter can be used to decrease the pixel aperture [25]. However, this approach reduces the radiation dose efficiency. An ultra-high-resolution (UHR) EID-CT, characterized by a thinner septa gap, has been introduced to the market [26]. This system has an effective detector pixel size of 0.25 × 0.25 mm, which reduces the gap with the PCDs. Anyway, a UHR imaging technique has been developed also for PCD-based CT systems [27]. A study conducted on anthropomorphic phantoms and cadaveric specimens has shown that UHR PCD images have 29% lower noise compared to the UHR EID images, which can be translated into a potential dose savings of 50% for equivalent image noise [27].
3.3. Contrast Improvement
Conventional EIDs weigh the photons based on the energy, so that the contribution to the signal is higher for high-energy than for low-energy photons. Since low-energy photons carry more contrast information than high-energy photons, their underweighting reduces the contrast-to-noise ratio (CNR). Moreover, the non-uniform weighting of photons increases the variance relative to the mean value, resulting in a reduced signal-to-noise ratio (Swank factor) [28].
Conversely, in PCDs all photons are equally weighted, independent from the energy (one photon, one count). The possibility to give relatively more weight to low-energy photons translates into a higher contrast in comparison with EIDs, in particular for low absorbing materials [29][30][31][32]. Importantly, the CNR for a given material can be maximized by tailoring the weighting scheme, that is by giving different weights to photons of different energy [15][33][34]. PCDs also avoid the Swank factor.
3.4. Reduction of Beam-Hardening
CT uses polyenergetic beams and, due to the energy dependence of mass attenuation coefficients, low-energy photons are more attenuated than high-energy photons [35]. This causes a shift of the mean energy of the X-ray beam toward the higher end of the spectrum, a phenomenon known as beam hardening. Beam hardening from a very dense target (i.e., cortical bone and metal implants) may result in characteristic artifacts: cupping artifacts and streaking (dark bands) artifacts [36]. These artifacts affect the image appearance and CT number accuracy for nearby soft tissues.
In PCDs the constant weighting reduces the beam-hardening artifacts [37][38]. In particular, in PCDs the best advantages in terms of immunity to beam-hardening effects are obtained with the use of high-energy thresholds [19][39].
3.5. Multienergy Acquisitions and K-Edge Imaging
Spectral CT refers to the use of energy-dependent attenuating characteristics of materials for achieving differentiation between tissue compositions (material decomposition) and improved lesion detection in contrast-enhanced scans and for reducing imaging artifacts [40].
Currently, material decomposition is performed using EID-based CT scanners capable of acquiring (with different approaches) dual-energy data, that are then combined through automated or semi-automated post-processing [41][42]. However, the conventional scanners do not allow for the separation of more than two materials (or three, by using some prior information and/or additional conditions) [43], require temporal image registration, and suffer from spectral overlap, which reduces the accuracy of material decomposition [44][45].
Since PCD can discriminate photons of different energies through pulse-height analysis, they inherently allow simultaneous multi-energy (n ≥ 2) acquisitions, with perfect spatial and temporal registrations and without spectral overlap [46]. Importantly, PCDs with more than three bin counters can allow to characterize the composition of each voxel as a combination of three or more basis materials [47].
The implementation of material decomposition algorithms from a number of energy-selective images results in a set of basis image maps that show the distribution within the imaged object of a certain material. These basic images can be processed to obtain virtual monochromatic images (VMI) [48][49][50], virtual non-contrast images [51], or material-specific color-overlay images [52]. PCCT provides several benefits over conventional EID-based CT scanners for these purposes. In addition to the comparable accuracy in iodine quantification and VMI CT number, PCCT offers the advantages of temporal and spatial alignment to avoid motion artifact, high spatial resolution, and improved contrast-to-noise ratio [49]. Due to its improved spectral separation, PCCT can foster more realistic virtual non-contrast images [49]. Moreover, with PCDs the higher number of energy measurements results in a more accurate measurement of each photon energy and, of consequence, in lower image noise in the material-specific images [53]. Importantly, PCDs permit to improve the accuracy for measurement of the concentration of a contrast agent. In fact, the techniques used to measure water, calcium, and contrast agent (i.e., iodine) with dual-energy-CT (DECT) require assumptions about the tissue composition [44] and if these assumptions are not correct, the measurement becomes inaccurate [54].
A key application of spectral/multi-energy CT is K-edge imaging, that is, the imaging of materials with a detectable K-edge in the diagnostic X-ray energy range. K-edge imaging is not feasible with DECT, while PCCT enables it, in virtue of the possibility of selecting energy thresholds lower and higher than the K-edge of a specific material. The detectability of a K-edge depends on the finite widths of the energy bins and then images can be reconstructed from the corresponding projections in the two energy bins. Different contrast agents can be distinguished according to their unique K-edges, besides the similar Hounsfield numbers in conventional CT images [55][56][57][58]. Therefore, PCTT provides the unique chance to use different contrast agents from iodine, including gold, platinum, silver, ytterbium, and bismuth, that are within the clinical X-ray tube spectrum [57][59][60][61], and to develop new types of contrast agents including nanoparticles targeted to specific cells or enzymes [58][62][63][64]. This approach opens the doors to molecular and functional CT imaging and to the simultaneous administration and detection of specific distribution of different contrast agents [52][65][66][67], conveying additional information. However, the promising results from animal or proof-of-concept (in silico) studies have not yet been translated into clinical practice.
3.6. Dose Efficiency
Thanks to the reduction of the electronic noise and the improvements in CNR and visualization of small objects, PCDs lead to better dose efficiency than EIDs [20][68]. This makes PCCT a promising technique for new low-dose imaging protocols, protecting patients from high radiation dose exposure while maintaining good image quality.
References
- Libby, P.; Ridker, P.M. Inflammation and atherosclerosis: Role of C-reactive protein in risk assessment. Am. J. Med. 2004, 116, 9–16.
- Aziz, M.; Yadav, K. Pathogenesis of atherosclerosis a review. Med. Clin. Rev. 2016, 2, 1–6.
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743.
- Andreini, D.; Martuscelli, E.; Guaricci, A.I.; Carrabba, N.; Magnoni, M.; Tedeschi, C.; Pelliccia, A.; Pontone, G. Clinical recommendations on Cardiac-CT in 2015: A position paper of the Working Group on Cardiac-CT and Nuclear Cardiology of the Italian Society of Cardiology. J. Cardiovasc. Med. 2016, 17, 73–84.
- Heseltine, T.D.; Murray, S.W.; Ruzsics, B.; Fisher, M. Latest Advances in Cardiac CT. Eur. Cardiol. 2020, 15, 1–7.
- Met, R.; Bipat, S.; Legemate, D.A.; Reekers, J.A.; Koelemay, M.J. Diagnostic performance of computed tomography angiography in peripheral arterial disease: A systematic review and meta-analysis. JAMA 2009, 301, 415–424.
- Rajiah, P. Updates in Vascular Computed Tomography. Radiol. Clin. N. Am. 2020, 58, 671–691.
- Kreisler, B. Photon counting Detectors: Concept, technical Challenges, and clinical outlook. Eur. J. Radiol. 2022, 149, 110229.
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312.
- Gnatyuk, V.; Maslyanchuk, O.; Solovan, M.; Brus, V.; Aoki, T. CdTe X/γ-ray Detectors with Different Contact Materials. Sensors 2021, 21, 3518.
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112.
- Taguchi, K.; Iwanczyk, J.S. Vision 20/20: Single photon counting x-ray detectors in medical imaging. Med. Phys. 2013, 40, 100901.
- Zheng, Y.; Yveborg, M.; Grönberg, F.; Xu, C.; Su, Q.; Danielsson, M.; Persson, M. Robustness of optimal energy thresholds in photon-counting spectral CT. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 953, 163132.
- Persson, M.; Bujila, R.; Nowik, P.; Andersson, H.; Kull, L.; Andersson, J.; Bornefalk, H.; Danielsson, M. Upper limits of the photon fluence rate on CT detectors: Case study on a commercial scanner. Med. Phys. 2016, 43, 4398–4411.
- Danielsson, M.; Persson, M.; Sjölin, M. Photon-counting x-ray detectors for CT. Phys. Med. Biol. 2021, 66, 03TR01.
- Hsieh, S.S.; Sjolin, M. Digital count summing vs analog charge summing for photon counting detectors: A performance simulation study. Med. Phys. 2018, 45, 4085–4093.
- Tanguay, J.; Cunningham, I.A. Cascaded systems analysis of charge sharing in cadmium telluride photon-counting x-ray detectors. Med. Phys. 2018, 45, 1926–1941.
- Procz, S.; Wartig, K.A.; Fauler, A.; Zwerger, A.; Luebke, J.; Ballabriga, R.; Blaj, G.; Campbell, M.; Mix, M.; Fiederle, M. Medipix3 CT for material sciences. J. Instrum. 2013, 8, C01025.
- Yu, Z.; Leng, S.; Kappler, S.; Hahn, K.; Li, Z.; Halaweish, A.F.; Henning, A.; McCollough, C.H. Noise performance of low-dose CT: Comparison between an energy integrating detector and a photon counting detector using a whole-body research photon counting CT scanner. J. Med. Imaging 2016, 3, 043503.
- Symons, R.; Cork, T.E.; Sahbaee, P.; Fuld, M.K.; Kappler, S.; Folio, L.R.; Bluemke, D.A.; Pourmorteza, A. Low-dose lung cancer screening with photon-counting CT: A feasibility study. Phys. Med. Biol. 2017, 62, 202–213.
- Si-Mohamed, S.A.; Sigovan, M.; Hsu, J.C.; Tatard-Leitman, V.; Chalabreysse, L.; Naha, P.C.; Garrivier, T.; Dessouky, R.; Carnaru, M.; Boussel, L.; et al. In Vivo Molecular K-Edge Imaging of Atherosclerotic Plaque Using Photon-counting CT. Radiology 2021, 300, 98–107.
- Leng, S.; Rajendran, K.; Gong, H.; Zhou, W.; Halaweish, A.F.; Henning, A.; Kappler, S.; Baer, M.; Fletcher, J.G.; McCollough, C.H. 150-μm Spatial Resolution Using Photon-Counting Detector Computed Tomography Technology: Technical Performance and First Patient Images. Invest. Radiol. 2018, 53, 655–662.
- Ferda, J.; Vendiš, T.; Flohr, T.; Schmidt, B.; Henning, A.; Ulzheimer, S.; Pecen, L.; Ferdová, E.; Baxa, J.; Mírka, H. Computed tomography with a full FOV photon-counting detector in a clinical setting, the first experience. Eur. J. Radiol. 2021, 137, 109614.
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-counting Detector CT System: Technical Evaluation. Radiology 2022, 303, 130–138.
- Flohr, T.G.; Stierstorfer, K.; Süss, C.; Schmidt, B.; Primak, A.N.; McCollough, C.H. Novel ultrahigh resolution data acquisition and image reconstruction for multi-detector row CT. Med. Phys. 2007, 34, 1712–1723.
- Oostveen, L.J.; Boedeker, K.L.; Brink, M.; Prokop, M.; de Lange, F.; Sechopoulos, I. Physical evaluation of an ultra-high-resolution CT scanner. Eur. Radiol. 2020, 30, 2552–2560.
- Leng, S.; Yu, Z.; Halaweish, A.; Kappler, S.; Hahn, K.; Henning, A.; Li, Z.; Lane, J.; Levin, D.L.; Jorgensen, S.; et al. Dose-efficient ultrahigh-resolution scan mode using a photon counting detector computed tomography system. J. Med. Imaging 2016, 3, 043504.
- Swank, R.K. Absorption and noise in x-ray phosphors. J. Appl. Phys. 1973, 44, 4199–4203.
- Iwanczyk, J.S.; Nygård, E.; Meirav, O.; Arenson, J.; Barber, W.C.; Hartsough, N.E.; Malakhov, N.; Wessel, J.C. Photon Counting Energy Dispersive Detector Arrays for X-ray Imaging. IEEE Trans. Nucl. Sci. 2009, 56, 535–542.
- Shikhaliev, P.M. Energy-resolved computed tomography: First experimental results. Phys. Med. Biol. 2008, 53, 5595–5613.
- Shikhaliev, P.M.; Fritz, S.G. Photon counting spectral CT versus conventional CT: Comparative evaluation for breast imaging application. Phys. Med. Biol. 2011, 56, 1905–1930.
- Silkwood, J.D.; Matthews, K.L.; Shikhaliev, P.M. Photon counting spectral breast CT: Effect of adaptive filtration on CT numbers, noise, and contrast to noise ratio. Med. Phys. 2013, 40, 051905.
- Giersch, J.; Niederlöhner, D.; Anton, G. The influence of energy weighting on X-ray imaging quality. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2004, 531, 68–74.
- Schmidt, T.G. Optimal “image-based” weighting for energy-resolved CT. Med. Phys. 2009, 36, 3018–3027.
- Brooks, R.A.; Chiro, G.D. Beam hardening in X-ray reconstructive tomography. Phys. Med. Biol. 1976, 21, 390–398.
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and avoidance. Radiographics 2004, 24, 1679–1691.
- Shikhaliev, P.M. Beam hardening artefacts in computed tomography with photon counting, charge integrating and energy weighting detectors: A simulation study. Phys. Med. Biol. 2005, 50, 5813–5827.
- Lee, C.-L.; Park, J.; Nam, S.; Choi, J.; Choi, Y.; Lee, S.; Lee, K.-Y.; Cho, M. Metal artifact reduction and tumor detection using photon-counting multi-energy computed tomography. PLoS ONE 2021, 16, e0247355.
- Gutjahr, R.; Halaweish, A.F.; Yu, Z.; Leng, S.; Yu, L.; Li, Z.; Jorgensen, S.M.; Ritman, E.L.; Kappler, S.; McCollough, C.H. Human Imaging With Photon Counting-Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Invest. Radiol. 2016, 51, 421–429.
- Yeh, B.M.; FitzGerald, P.F.; Edic, P.M.; Lambert, J.W.; Colborn, R.E.; Marino, M.E.; Evans, P.M.; Roberts, J.C.; Wang, Z.J.; Wong, M.J.; et al. Opportunities for new CT contrast agents to maximize the diagnostic potential of emerging spectral CT technologies. Adv. Drug Deliv. Rev. 2017, 113, 201–222.
- Hounsfield, G.N. Computerized transverse axial scanning (tomography): Part 1. Description of system. Br. J. Radiol. 1973, 46, 1016–1022.
- Coursey, C.A.; Nelson, R.C.; Boll, D.T.; Paulson, E.K.; Ho, L.M.; Neville, A.M.; Marin, D.; Gupta, R.T.; Schindera, S.T. Dual-Energy Multidetector CT: How Does It Work, What Can It Tell Us, and When Can We Use It in Abdominopelvic Imaging? RadioGraphics 2010, 30, 1037–1055.
- Xue, Y.; Jiang, Y.; Yang, C.; Lyu, Q.; Wang, J.; Luo, C.; Zhang, L.; Desrosiers, C.; Feng, K.; Sun, X.; et al. Accurate Multi-Material Decomposition in Dual-Energy CT: A Phantom Study. IEEE Trans. Comput. Imaging 2019, 5, 515–529.
- Liu, X.; Yu, L.; Primak, A.N.; McCollough, C.H. Quantitative imaging of element composition and mass fraction using dual-energy CT: Three-material decomposition. Med. Phys. 2009, 36, 1602–1609.
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653.
- Kappler, S.; Henning, A.; Kreisler, B.; Schoeck, F.; Stierstorfer, K.; Flohr, T. Photon Counting CT at Elevated X-ray Tube Currents: Contrast Stability, Image Noise and Multi-Energy Performance. In Proceedings of the Medical Imaging 2014: Physics of Medical Imaging, San Diego, CA, USA, 15–20 February 2014; SPIE: Bellingham, WA, USA, 2014; Volume 9033.
- Le, H.Q.; Molloi, S. Segmentation and quantification of materials with energy discriminating computed tomography: A phantom study. Med. Phys. 2011, 38, 228–237.
- Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Krauss, B.; Ulzheimer, S.; Kappler, S.; Bluemke, D.A.; Pourmorteza, A. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck: First In Vivo Human Results. Invest. Radiol. 2018, 53, 135–142.
- Leng, S.; Zhou, W.; Yu, Z.; Halaweish, A.; Krauss, B.; Schmidt, B.; Yu, L.; Kappler, S.; McCollough, C. Spectral performance of a whole-body research photon counting detector CT: Quantitative accuracy in derived image sets. Phys. Med. Biol. 2017, 62, 7216–7232.
- Laukamp, K.R.; Lennartz, S.; Neuhaus, V.F.; Große Hokamp, N.; Rau, R.; Le Blanc, M.; Abdullayev, N.; Mpotsaris, A.; Maintz, D.; Borggrefe, J. CT metal artifacts in patients with total hip replacements: For artifact reduction monoenergetic reconstructions and post-processing algorithms are both efficient but not similar. Eur. Radiol. 2018, 28, 4524–4533.
- Mergen, V.; Racine, D.; Jungblut, L.; Sartoretti, T.; Bickel, S.; Monnin, P.; Higashigaito, K.; Martini, K.; Alkadhi, H.; Euler, A. Virtual Noncontrast Abdominal Imaging with Photon-counting Detector CT. Radiology 2022, 305, 107–115.
- Symons, R.; Krauss, B.; Sahbaee, P.; Cork, T.E.; Lakshmanan, M.N.; Bluemke, D.A.; Pourmorteza, A. Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: An in vivo study. Med. Phys. 2017, 44, 5120–5127.
- Faby, S.; Kuchenbecker, S.; Sawall, S.; Simons, D.; Schlemmer, H.P.; Lell, M.; Kachelrieß, M. Performance of today’s dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: A simulation study. Med. Phys. 2015, 42, 4349–4366.
- Yveborg, M.; Danielsson, M.; Bornefalk, H. Theoretical comparison of a dual energy system and photon counting silicon detector used for material quantification in spectral CT. IEEE Trans. Med. Imaging 2015, 34, 796–806.
- Roessl, E.; Proksa, R. K-edge imaging in x-ray computed tomography using multi-bin photon counting detectors. Phys. Med. Biol. 2007, 52, 4679–4696.
- Schlomka, J.P.; Roessl, E.; Dorscheid, R.; Dill, S.; Martens, G.; Istel, T.; Bäumer, C.; Herrmann, C.; Steadman, R.; Zeitler, G.; et al. Experimental feasibility of multi-energy photon-counting K-edge imaging in pre-clinical computed tomography. Phys. Med. Biol. 2008, 53, 4031–4047.
- Pan, D.; Schirra, C.O.; Senpan, A.; Schmieder, A.H.; Stacy, A.J.; Roessl, E.; Thran, A.; Wickline, S.A.; Proska, R.; Lanza, G.M. An early investigation of ytterbium nanocolloids for selective and quantitative “multicolor” spectral CT imaging. ACS Nano. 2012, 6, 3364–3370.
- Si-Mohamed, S.; Cormode, D.P.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Langlois, J.-B.; Chalabreysse, L.; Coulon, P.; Blevis, I.; Roessl, E.; et al. Evaluation of spectral photon counting computed tomography K-edge imaging for determination of gold nanoparticle biodistribution in vivo. Nanoscale 2017, 9, 18246–18257.
- Schirra, C.O.; Brendel, B.; Anastasio, M.A.; Roessl, E. Spectral CT: A technology primer for contrast agent development. Contrast. Media. Mol. Imaging 2014, 9, 62–70.
- Müllner, M.; Schlattl, H.; Hoeschen, C.; Dietrich, O. Feasibility of spectral CT imaging for the detection of liver lesions with gold-based contrast agents—A simulation study. Phys. Med. 2015, 31, 875–881.
- Kim, J.; Bar-Ness, D.; Si-Mohamed, S.; Coulon, P.; Blevis, I.; Douek, P.; Cormode, D.P. Assessment of candidate elements for development of spectral photon-counting CT specific contrast agents. Sci. Rep. 2018, 8, 12119.
- Cormode, D.P.; Roessl, E.; Thran, A.; Skajaa, T.; Gordon, R.E.; Schlomka, J.P.; Fuster, V.; Fisher, E.A.; Mulder, W.J.; Proksa, R.; et al. Atherosclerotic plaque composition: Analysis with multicolor CT and targeted gold nanoparticles. Radiology 2010, 256, 774–782.
- Balegamire, J.; Vandamme, M.; Chereul, E.; Si-Mohamed, S.; Azzouz Maache, S.; Almouazen, E.; Ettouati, L.; Fessi, H.; Boussel, L.; Douek, P.; et al. Iodinated polymer nanoparticles as contrast agent for spectral photon counting computed tomography. Biomater. Sci. 2020, 8, 5715–5728.
- Dong, Y.C.; Kumar, A.; Rosario-Berríos, D.N.; Si-Mohamed, S.; Hsu, J.C.; Nieves, L.M.; Douek, P.; Noël, P.B.; Cormode, D.P. Ytterbium Nanoparticle Contrast Agents for Conventional and Spectral Photon-Counting CT and Their Applications for Hydrogel Imaging. ACS Appl. Mater. Interfaces 2022, 14, 39274–39284.
- Muenzel, D.; Daerr, H.; Proksa, R.; Fingerle, A.A.; Kopp, F.K.; Douek, P.; Herzen, J.; Pfeiffer, F.; Rummeny, E.J.; Noël, P.B. Simultaneous dual-contrast multi-phase liver imaging using spectral photon-counting computed tomography: A proof-of-concept study. Eur. Radiol. Exp. 2017, 1, 25.
- Symons, R.; Cork, T.E.; Lakshmanan, M.N.; Evers, R.; Davies-Venn, C.; Rice, K.A.; Thomas, M.L.; Liu, C.Y.; Kappler, S.; Ulzheimer, S.; et al. Dual-contrast agent photon-counting computed tomography of the heart: Initial experience. Int. J. Cardiovasc. Imaging 2017, 33, 1253–1261.
- Cormode, D.P.; Si-Mohamed, S.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Balegamire, J.; Lavenne, F.; Coulon, P.; Roessl, E.; Bartels, M.; et al. Multicolor spectral photon-counting computed tomography: In vivo dual contrast imaging with a high count rate scanner. Sci. Rep. 2017, 7, 4784.
- Rajagopal, J.R.; Farhadi, F.; Solomon, J.; Sahbaee, P.; Saboury, B.; Pritchard, W.F.; Jones, E.C.; Samei, E. Comparison of Low Dose Performance of Photon-Counting and Energy Integrating CT. Acad. Radiol. 2021, 28, 1754–1760.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
22 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





