Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucia La Mura | -- | 2137 | 2023-02-21 02:21:41 | | | |
| 2 | Catherine Yang | Meta information modification | 2137 | 2023-02-21 02:26:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Perone, F.; Guglielmo, M.; Coceani, M.; Mura, L.L.; Dentamaro, I.; Sabatino, J.; Gimelli, A. Aortic Dissection. Encyclopedia. Available online: https://encyclopedia.pub/entry/41449 (accessed on 08 March 2026).
Perone F, Guglielmo M, Coceani M, Mura LL, Dentamaro I, Sabatino J, et al. Aortic Dissection. Encyclopedia. Available at: https://encyclopedia.pub/entry/41449. Accessed March 08, 2026.
Perone, Francesco, Marco Guglielmo, Michele Coceani, Lucia La Mura, Ilaria Dentamaro, Jolanda Sabatino, Alessia Gimelli. "Aortic Dissection" Encyclopedia, https://encyclopedia.pub/entry/41449 (accessed March 08, 2026).
Perone, F., Guglielmo, M., Coceani, M., Mura, L.L., Dentamaro, I., Sabatino, J., & Gimelli, A. (2023, February 21). Aortic Dissection. In Encyclopedia. https://encyclopedia.pub/entry/41449
Perone, Francesco, et al. "Aortic Dissection." Encyclopedia. Web. 21 February, 2023.
Copy Citation
Acute aortic syndromes (AAS) represent a spectrum of interrelated disorders characterized by the disruption of the aortic integrity, and are associated with high morbidity and mortality. These conditions include aortic dissection (AD), accounting for the majority of AAS (80%), intramural hematoma (IMH, ~15%), and penetrating aortic ulcer (PAU, ~5%).
acute aortic syndromes
multimodality imaging
echocardiography
1. Transthoracic and Transesophageal Echocardiography
Echocardiography is renowned for being a non-invasive, safe, and accurate imaging modality used in the clinical evaluation of cardiovascular disease and plays an important role in the diagnosis of aortic diseases. Transthoracic echocardiography (TTE) can be performed at the patient’s bedside, as well as in the emergency and critical care units, and for this reason the European Society of Cardiology guidelines [1] recommend it in patients who are clinically suspected of having an AD (class I, level of evidence C).
The diagnosis of AD by TTE is based on detecting intimal flaps in the aorta. The sensitivity and specificity of TTE range from 77–80% and 93–96%, respectively, for ascending AD, and varies according to the use of contrast agents [2]. Nevertheless, TTE is successful in detecting the distal dissection of the thoracic aorta in only 70% of patients [3]. On transthoracic M-mode echocardiography, floating intimal flaps, the enlargement of the aortic root, and an increase in the aortic wall thickness were considered signs of AD. With the introduction of two-dimensional (2D) echocardiography and the feasibility of taking suprasternal, subcostal, and substernal views, it has become possible to directly visualize the presence of floating intimal membranes, intimal tears, and false lumens on the ascending aorta and aortic arch [4]. Thanks to the superior axial image resolution offered by parasternal long-axis views, mobile dissection membranes within the ascending aorta can be seen in AD patients with optimal windows (Figure 1A). The parasternal short-axis view is useful to evaluate the morphology of the aortic valve and location of any aortic regurgitation. Aortic regurgitation is a frequent finding in patients with type A AD, occurring in 40–76% of patients [5]. TTE can also offer immediate evidence of other complications which can accompany an AD, such as cardiac tamponade, severe aortic dilation, regional wall motion abnormalities, and severe left ventricular systolic dysfunction, all of which may influence surgical management [6]. Since sub-optimal image quality can be a limitation in some patients, an AD cannot always be completely ruled out by TTE alone, and further imaging such as transesophageal echocardiography (TOE) or computed tomography (CT) of the aorta should be considered if clinical suspicion remains high [7].
TOE is currently considered as one of the reference techniques in AD diagnosis, with a very high diagnostic accuracy for the detection of both ascending and descending aortic diseases (Figure 1B) [8]. Indeed, the proximity of the oesophagus and the thoracic aorta permits a high-resolution image. Furthermore, the availability of multiplane imaging allows incremental assessment of the aorta from its root to the descending aorta. Several studies have demonstrated the accuracy of TOE in the diagnosis of AD with a sensitivity of 86–100%, a specificity of 90–100%, and a negative predictive value of 86–100% [9]. Owing to the interposition of the right bronchus and trachea, a short segment of the distal ascending aorta, just before the innominate artery, remains a ‘blind spot’ [1]; Evangelista et al. demonstrated that contrast-enhanced echocardiography can solve this problem [8]. One of the main limitations of TOE is the presence of ultrasound artefacts in the ascending aorta which are common, particularly when dilated [10]; in this case, M-mode tracings differentiate between intimal flap and imaging reverberations [11]. TOE may also identify the presence of leaks and/or small re-entry tears with much higher sensitivity than angiography. This is also important for the prognosis of patients with residual patent false lumen in the descending aorta and the presence of a large proximal entry tear (>10 mm) defined by TOE, which indicates a high risk of mortality and the need for surgical or endovascular treatment during the follow-up [12]. Other limitations of this technique could concern the discomfort for patients, with the possible need for sedation, and the intra- and inter-operator variability.
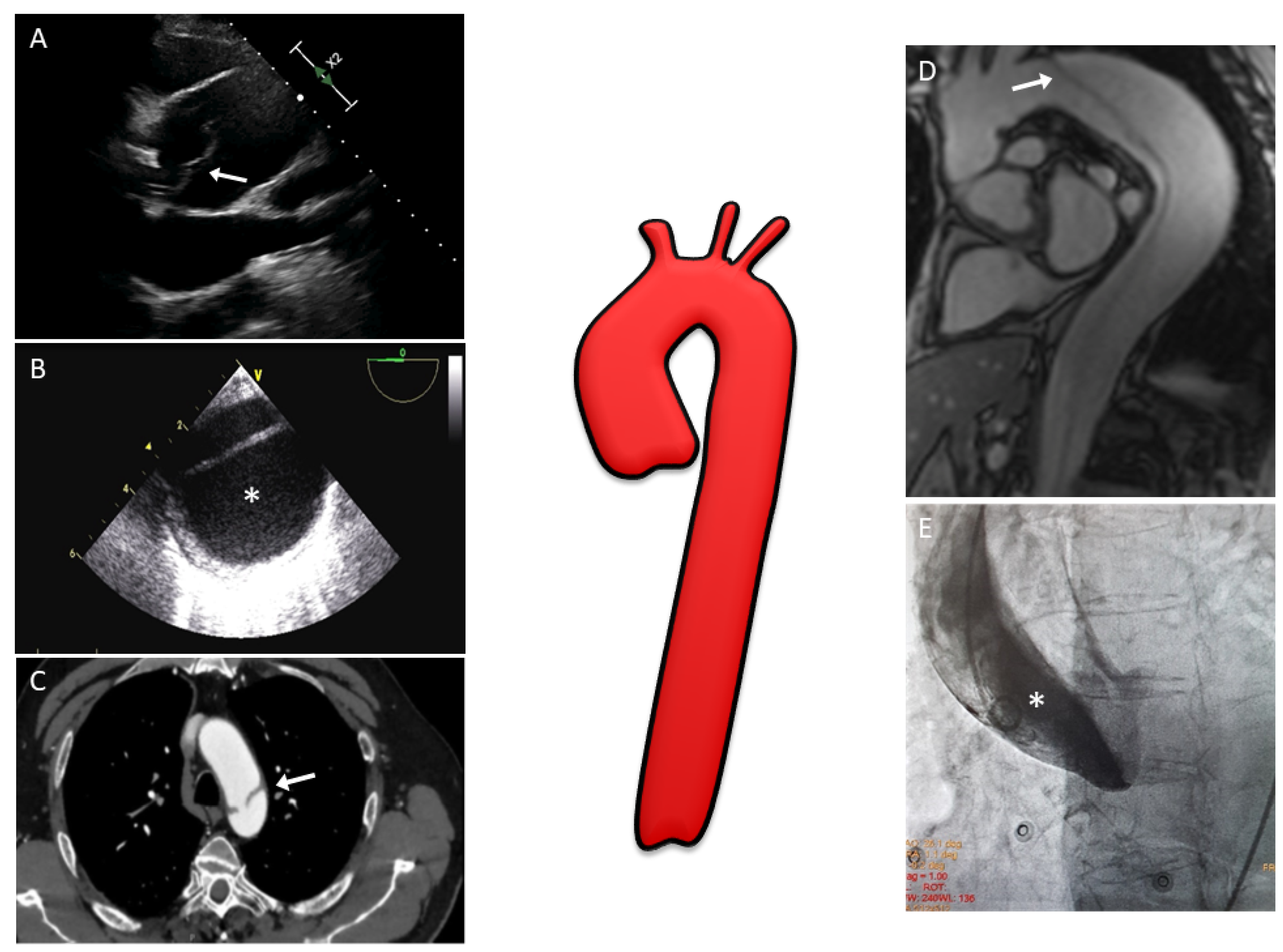
Figure 1. Multimodality imaging assessment of aortic dissection: (A) Two-dimensional transthoracic echocardiography showing a linear echo of an intimal flap (arrow) in a dilated aortic root above aortic valve level; (B) Two-dimensional transesophageal echocardiography in patients with aortic dissection involving the entire aorta; the false lumen (*) is typically larger and often compresses the true lumen, potentially affecting distal aortic flow; (C) CT image with evidence of the intimal tear (arrow) at the level of the aortic arch; (D) MRI with SSFP imaging in the oblique sagittal plane showing an intimal flap (arrow) from the aortic arch to abdominal aorta; (E) Aortic angiography performed in a patient with suspected inferior ST-segment elevation myocardial infarction revealing a type A aortic dissection (one may note that the pigtail catheter is located in the false lumen (*) of the dissection). CT, computed tomography; MRI, magnetic resonance imaging; SSFP, steady-state free precession.
2. Computed Tomography
Thanks to its high spatial resolution, full assessment of thoracoabdominal aorta, short acquisition time, and wide availability, CT represents the ideal technique for diagnosing AAS.
The typical scan protocol first includes a non-contrast CT followed by an arterial acquisition [13]. A late thoracoabdominal scan can be added to improve the detection of visceral malperfusion in the acute setting of AD and distinguish slow flow from thrombus in the false lumen [14]. Indeed, this late (venous) phase is often useful to confirm the circulation of the false lumen, which may not be opacified in the arterial phase. Electrocardiogram (ECG) gating should always be used to avoid motion artifacts that can be misinterpreted as intimal flaps. The entire aorta must be assessed to determine the distal extent of the dissection and to identify the ischemia of the abdominal organs, which have important prognostic implications. In the case of a cerebrovascular accident, a CT head scan is mandatory. Dedicated injection protocols are used, considering the speed of scan acquisition and coverage to optimize image quality and reduce the volume of contrast agents [15]. The main limitations concern the use of iodinated contrast and ionizing radiations.
The sensitivity and specificity of CT for AD is approximately 98–100% [16], and it represents the modality of choice for dissection in the majority of patients [17]. CT can differentiate between type A and B AD, helping to localize intimal entry site, involvement, relationship with the false or true lumen of the branch vessels, and organ ischemia (Figure 1C) [15]. Moreover, coronary ostia involvement can be detected with CT [18].
The classical finding of AD in CT is an intimal flap with a partition between the true and false lumen. Supporting signs are the internal displacement of intimal calcifications, the delayed enhancement of the false lumen, the widening of the aorta, mediastinal, pleural, or pericardial hematoma [19], and signs of cardiac tamponade. The latter is suspected in the presence of a large pericardial effusion, dilatation of superior and inferior vena cava, reflux of contrast agents into the inferior vena cava and azygos vein, compression of the cardiac chambers, and the bowing of the interventricular septum [20]. A thorough clinical evaluation does not always provide a clear understanding of the cause of acute chest pain. For these cases, a triple rule out protocol has been proposed in which coronary arteries, the thoracic aorta, and pulmonary arteries are simultaneously examined with CT. However, triple rule out scans provide greater anatomic coverage than dedicated protocols, which leads to a higher radiation dose. In addition, they require a higher contrast load to opacify both the right and left circulations, as well as more time to report the scan. Multiple studies have failed to demonstrate superior clinical outcomes with triple rule out to justify the increased radiation, contrast dose, and readout time [21]. Apart from its essential crucial diagnostic role, CT is used in patients with AD for planning interventional surgical or percutaneous interventions. Specifically, measurements of vessel diameter, angles, aneurysm neck size, and proximal and distal landing zones for stent grafts are essential in determining thoracic endovascular aortic repair (TEVAR) eligibility [22].
3. Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a highly accurate, noninvasive imaging modality, with a sensitivity and specificity of almost 98% for detecting AD [23]. Additionally, it does not require ionizing radiation or iodinated contrast, making it the most suitable test for patients with impaired renal function, known severe allergies to iodated contrast material, and who are pregnant. The limitations of MRI in acute AD apparently include long imaging time, the need for patient cooperation to avoid motion-related image degradation, and difficulty in monitoring acutely ill patients. Nevertheless, Wang et al. demonstrated that the use of MRI for evaluation of thoracic aortic dissection is well-tolerated by emergency department patients [24], despite the fact that MRI is currently not used in the vast majority of centres to search for acute dissection, it being more useful in the follow-up of chronic type B dissection. Other limitations include the risk of nephrogenic systemic fibrosis with GFR < 30 mL/min/1.73 m2 when gadolinium is used, and the poor assessment of arterial wall calcification.
In a suspected case of AD, the standard MRI examination should begin with rapid spin-echo black blood acquisitions covering the aorta to outline aortic shape and diameter and to rule out alterations in wall structure [25]. In the axial plane, the intimal flap is detected as a line inside of the aortic lumen. In stable patients, adjunctive gradient-echo sequences or phase contrast images can be instrumental in identifying aortic regurgitation and entry or re-entry sites as well as in differentiating slow flow from the thrombus in the false lumen [26][27][28][29]. With balanced steady-state free precession (SSFP) techniques, image contrast is determined by T2/T1 ratios, allowing the detection of the intimal media flap, with no gadolinium contrast media [30][31]. These images are typically acquired initially in axial and oblique sagittal projections and gated to the cardiac diastolic phase (Figure 1D). Cine SSFP MRI can be used to further delineate the entry and exit zones of the intimal media flap and the presence of aortic regurgitation thanks to the detection of flow turbulence. Quantitative data on flow velocity and volume, in the true and false lumen, can be obtained from the velocity maps of phase contrast sequences. In addition, contrast-enhanced MRI angiography may be performed quickly (in a few seconds without any need of ECG triggering) and will provide information regarding branch vessel involvement, the presence of intraluminal abnormalities, PAU, and will demonstrate the intimo-medial flap location and the entry and exit tears in the dissection. Despite the advantages, the image quality of dynamic MRI angiography proved to be inferior to high-resolution three-dimensional (3D) MRI [32]. Four-dimensional (4D) flow MRI could be a potential tool in AD patients thanks to flow quantification and hemodynamic information. Unfortunately, so far it has currently been used only in ex vivo studies and in patients with chronic dissection [33][34].
4. Aortography
In the past, aortography represented the gold standard for the diagnosis of AD [35]. In more recent years, aortography has been almost completely replaced by equally accurate, but less invasive, techniques. Nevertheless, aortography remains the benchmark against which all other imaging modalities should be measured [36]. Furthermore, aortography still maintains an important role in patients who undergo coronary angiography (for example, in stable patients in which concomitant coronary artery disease is suspected). It should also be kept in mind that AAS may mimic an acute coronary syndrome and, as a result, the correct differential diagnosis will be made only at the time of invasive coronary angiography. Finally, aortography is an indispensable guide for endovascular procedures, which are more frequently carried out in patients with type B dissection [1].
Aortography visualizes all features of AD: the intimal flap, true lumen (which is usually compressed), false lumen, craniocaudal extension, indirect visualization of the coronary arteries, possible aortic regurgitation, and aortic rupture (Figure 1E). Various projections may be necessary to evaluate every aspect of aortic anatomy and the potential involvement of aortic side branches [18]. In the case of a type A dissection, aortography permits the assessment of the blood vessels which originate from the aortic arch, thus facilitating surgical planning. However, because aortography is a “lumenogram”, it does not provide any information on aortic wall thickness. Caution must be employed in performing aortography because, if the false lumen is cannulated with the diagnostic catheter, a sudden increase in pressure during contrast injection may lead to aortic rupture. To this end, manual injection may be safer, although less effective in the visualization of the entire dissection, especially in the case of low blood flow. Moreover, in patients with chronic kidney disease, aortography may lead to contrast induced nephropathy. In addition to the use of contrast medium, the invasive nature of the procedure and radiation exposure are other limitations to this technique. The specificity of aortography is high at 95%, but sensitivity may be lower than other techniques due to the inability in certain cases to differentiate the two lumens of the aorta (for example, because of a completely thrombosed false lumen) [37].
References
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926.
- Khandheria, B.K.; Tajik, A.J.; Taylor, C.L.; Safford, R.E.; Miller, F.A., Jr.; Stanson, A.W.; Sinak, L.J.; Oh, J.K.; Seward, J.B. Aortic dissection: Review of value and limitations of two-dimensional echocardiography in a six-year experience. J. Am. Soc. Echocardiogr. 1989, 2, 17–24.
- Iliceto, S.; Ettorre, G.; Francioso, G.; Antonelli, G.; Biasco, G.; Rizzon, P. Diagnosis of aneurysm of the thoracic aorta. Comparison between two non invasive techniques: Two-dimensional echocardiography and computed tomography. Eur. Heart J. 1984, 5, 545–555.
- Czerny, M.; Schmidli, J.; Adler, S.; van den Berg, J.C.; Bertoglio, L.; Carrel, T.; Chiesa, R.; Clough, R.E.; Eberle, B.; Etz, C.; et al. EACTS/ESVS scientific document group. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: An expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur. J. Cardiothorac. Surg. 2019, 55, 133–162.
- Evangelista, A.; Flachskampf, F.A.; Erbel, R.; Antonini-Canterin, F.; Vlachopoulos, C.; Rocchi, G.; European Association of Echocardiography. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur. J. Echocardiogr. 2010, 11, 645–658.
- Sobczyk, D.; Nycz, K. Feasibility and accuracy of bedside transthoracic echocardiography in diagnosis of acute proximal aortic dissection. Cardiovasc. Ultrasound 2015, 13, 15.
- Baliga, R.R.; Nienaber, C.A.; Bossone, E.; Oh, J.K.; Isselbacher, E.M.; Sechtem, U.; Fattori, R.; Raman, S.V.; Eagle, K.A. The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc. Imaging 2014, 7, 406–424.
- Evangelista, A.; Avegliano, G.; Aguilar, R.; Cuellar, H.; Igual, A.; González-Alujas, T.; Rodríguez-Palomares, J.; Mahia, P.; García-Dorado, D. Impact of contrast-enhanced echocardiography on the diagnostic algorithm of acute aortic dissection. Eur. Heart J. 2010, 31, 472–479.
- Flachskampf, F.A.; Wouters, P.F.; Edvardsen, T.; Evangelista, A.; Habib, G.; Hoffman, P.; Hoffmann, R.; Lancellotti, P.; Pepi, M. Recommendations for transoesophageal echocardiography: EACVI update 2014. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 353–365.
- Pepi, M.; Campodonico, J.; Galli, C.; Tamborini, G.; Barbier, P.; Doria, E.; Maltagliati, A.; Alimento, M.; Spirito, R. Rapid diagnosis and management of thoracic aortic dissection and intramural haematoma: A prospective study of advantages of multiplane vs. biplane transoesophageal echocardiography. Eur. J. Echocardiogr. 2000, 1, 72–79.
- Evangelista, A.; Garcia-del-Castillo, H.; Gonzalez-Alujas, T.; Dominguez-Oronoz, R.; Salas, A.; Permanyer-Miralda, G.; Soler-Soler, J. Diagnosis of ascending aortic dissection by transesophageal echocardiography: Utility of M-mode in recognizing artifacts. J. Am. Coll. Cardiol. 1996, 27, 102–107.
- Evangelista, A.; Salas, A.; Ribera, A.; Ferreira-González, I.; Cuellar, H.; Pineda, V.; González-Alujas, T.; Bijnens, B.; Permanyer-Miralda, G.; Garcia-Dorado, D. Long-term outcome of aortic dissection with patent false lumen: Predictive role of entry tear size and location. Circulation 2012, 125, 3133–3141.
- Ueda, T.; Chin, A.; Petrovitch, I.; Fleischmann, D. A pictorial review of acute aortic syndrome: Discriminating and overlapping features as revealed by ECG-gated multidetector-row CT angiography. Insights Imaging 2012, 3, 561–571.
- Grewal, S.; Contrella, B.N.; Sherk, W.M.; Khaja, M.S.; Williams, D.M. Endovascular Management of Malperfusion Syndromes in Aortic Dissection. Tech. Vasc. Interv. Radiol. 2021, 24, 100751.
- McMahon, M.A.; Squirrell, C.A. Multidetector CT of Aortic Dissection: A Pictorial Review. Radiographics 2010, 30, 445–460.
- Carroll, B.J.; Schermerhorn, M.L.; Manning, W.J. Imaging for acute aortic syndromes. Heart 2020, 106, 182–189.
- Moore, A.G.; Eagle, K.A.; Bruckman, D.; Moon, B.S.; Malouf, J.F.; Fattori, R.; Evangelista, A.; Isselbacher, E.M.; Suzuki, T.; Nienaber, C.A.; et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am. J. Cardiol. 2002, 89, 1235–1238.
- Nienaber, C.A. The role of imaging in acute aortic syndromes. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 15–23.
- Sebastià, C.; Pallisa, E.; Quiroga, S.; Alvarez-Castells, A.; Dominguez, R.; Evangelista, A. Aortic dissection: Diagnosis and follow-up with helical CT. Radiographics 1999, 19, 45–60, quiz 149–150.
- Restrepo, C.S.; Lemos, D.F.; Lemos, J.A.; Velasquez, E.; Diethelm, L.; Ovella, T.A.; Martinez, S.; Carrillo, J.; Moncada, R.; Klein, J.S. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics 2007, 27, 1595–1610.
- Goldstein, S.A.; Evangelista, A.; Abbara, S.; Arai, A.; Asch, F.M.; Badano, L.P.; Bolen, M.A.; Connolly, H.M.; Cuéllar-Calàbria, H.; Czerny, M.; et al. Multimodality imaging of diseases of the thoracic aorta in adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging: Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2015, 28, 119–182.
- Murillo, H.; Molvin, L.; Chin, A.S.; Fleischmann, D. Aortic Dissection and Other Acute Aortic Syndromes: Diagnostic Imaging Findings from Acute to Chronic Longitudinal Progression. Radiographics 2021, 41, 425–446.
- Shiga, T.; Wajima, Z.; Apfel, C.C.; Inoue, T.; Ohe, Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: Systematic review and meta-analysis. Arch. Intern. Med. 2006, 166, 1350–1356.
- Wang, G.X.; Hedgire, S.S.; Le, T.Q.; Sonis, J.D.; Yun, B.J.; Lev, M.H.; Raja, A.S.; Prabhakar, A.M. MR angiography can guide ED management of suspected acute aortic dissection. Am. J. Emerg. Med. 2017, 35, 527–530.
- Evangelista, A.; Carro, A.; Moral, S.; Teixido-Tura, G.; Rodríguez-Palomares, J.F.; Cuéllar, H.; García-Dorado, D. Imaging modalities for the early diagnosis of acute aortic syndrome. Nat. Rev. Cardiol. 2013, 10, 477–486.
- Sakuma, H.; Bourne, M.W.; O’Sullivan, M.; Merrick, S.H.; Ullyot, D.J.; Chatterjee, K.; Shimakawa, A.; Foo, T.K.; Higgins, C.B. Evaluation of thoracic aortic dissection using breath-holding cine MRI. J. Comput. Assist. Tomogr. 1996, 20, 45–50.
- Powell, A.J.; Maier, S.E.; Chung, T.; Geva, T. Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: In vitro and in vivo validation. Pediatr. Cardiol. 2000, 21, 104–110.
- Chang, J.M.; Friese, K.; Caputo, G.R.; Kondo, C.; Higgins, C.B. MR measurement of blood flow in the true and false channel in chronic aortic dissection. J. Comput. Assist. Tomogr. 1991, 15, 418–423.
- Silverman, J.M.; Raissi, S.; Tyszka, J.M.; Trento, A.; Herfkens, R.J. Phase-contrast cine MR angiography detection of thoracic aortic dissection. Int. J. Card. Imaging 2000, 16, 461–470.
- Pereles, F.S.; McCarthy, R.M.; Baskaran, V.; Carr, J.C.; Kapoor, V.; Krupinski, E.A.; Finn, J.P. Thoracic aortic dissection and aneurysm: Evaluation with nonenhanced true FISP MR angiography in less than 4 minutes. Radiology 2002, 223, 270–274.
- Gebker, R.; Gomaa, O.; Schnackenburg, B.; Rebakowski, J.; Fleck, E.; Nagel, E. Comparison of different MRI techniques for the assessment of thoracic aortic pathology: 3D contrast enhanced MR angiography, turbo spin echo and balanced steady state free precession. Int. J. Cardiovasc. Imaging 2007, 23, 747–756.
- Kinner, S.; Eggebrecht, H.; Maderwald, S.; Barkhausen, J.; Ladd, S.C.; Quick, H.H.; Hunold, P.; Vogt, F.M. Dynamic MR angiography in acute aortic dissection. J. Magn. Reson. Imaging 2015, 42, 505–514.
- Veger, H.T.C.; Pasveer, E.H.; Westenberg, J.J.M.; Wever, J.J.; van Eps, R.G.S. Wall Shear Stress Assessment of the False Lumen in Acute Type B Aortic Dissection Visualized by 4-Dimensional Flow Magnetic Resonance Imaging: An Ex-Vivo Study. Vasc. Endovasc. Surg. 2021, 55, 696–701.
- Chen, C.W.; Tseng, Y.H.; Lin, C.C.; Kao, C.C.; Wong, M.Y.; Ting, H.; Huang, Y.K. Aortic dissection assessment by 4D phase-contrast MRI with hemodynamic parameters: The impact of stent type. Quant. Imaging Med. Surg. 2021, 11, 490–501.
- Khandheria, B.K. Aortic dissection. The last frontier. Circulation 1993, 87, 1765–1768.
- Bansal, R.C.; Chandrasekaran, K.; Ayala, K.; Smith, D.C. Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography. J. Am. Coll. Cardiol. 1995, 25, 1393–1401.
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute aortic syndromes: Diagnosis and management, an update. Eur. Heart J. 2018, 39, 739–749d.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
21 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





