Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miriam Cerrillo | -- | 1435 | 2023-02-20 15:13:45 | | | |
| 2 | Conner Chen | Meta information modification | 1435 | 2023-02-21 04:39:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cerrillo, M.; Riau, V.; Bonmatí, A. Principles of Nutrient Recovery in BESs Using Membranes. Encyclopedia. Available online: https://encyclopedia.pub/entry/41435 (accessed on 07 February 2026).
Cerrillo M, Riau V, Bonmatí A. Principles of Nutrient Recovery in BESs Using Membranes. Encyclopedia. Available at: https://encyclopedia.pub/entry/41435. Accessed February 07, 2026.
Cerrillo, Míriam, Victor Riau, August Bonmatí. "Principles of Nutrient Recovery in BESs Using Membranes" Encyclopedia, https://encyclopedia.pub/entry/41435 (accessed February 07, 2026).
Cerrillo, M., Riau, V., & Bonmatí, A. (2023, February 20). Principles of Nutrient Recovery in BESs Using Membranes. In Encyclopedia. https://encyclopedia.pub/entry/41435
Cerrillo, Míriam, et al. "Principles of Nutrient Recovery in BESs Using Membranes." Encyclopedia. Web. 20 February, 2023.
Copy Citation
Bioelectrochemical systems (BESs) have emerged as a technology that is able to recover resources from different kinds of substrates, especially wastewater. Nutrient recovery, mostly based on membrane reactor configuration, is a clear niche for BES application. The recovery of nitrogen or phosphorus allows for treatment of wastewater while simultaneously collecting a concentrated stream with nutrients that can be reintroduced into the system, becoming a circular economy solution.
ammonium
phosphorus
bioelectrochemical systems
1. Introduction
Bioelectrochemical systems (BESs) are bioreactors that are equipped with two electrodes, an anode, and a cathode and use exoelectrogenic microorganisms to catalyse oxidation and/or reduction reactions. BESs have become a highly versatile technology that allows wastewater treatment to be combined with the recovery or production of compounds such as nutrients and energy carriers. BESs can be operated as a stand-alone technology or in combination with other technologies, such as anaerobic digestion, in order to improve the recovery of resources and energy within a circular economy approach [1][2][3][4].
BESs can be used to treat a wide range of organic substrates and wastewaters, such as digested sludge and agroindustry, food industry, and urban wastewater [5]. In a changing paradigm in waste and wastewater management technologies from the purpose of waste disposal to the biorefinery concept, the recovery and reuse of nutrients from wastewater is a priority over their disposal through technologies such as the nitrification–denitrification process [6]. For example, fertilisation activities are highly dependent on ammonia and phosphate, and their recovery from waste streams will reduce the demand for phosphate mined from rock or ammonium produced by industrial processes. Ammonium is industrially produced by nitrogen conversion from atmospheric N2 gas through the Haber–Bosch process, which is energy intensive due to the need to supply high temperatures (400–500 °C) and high pressure (100–200 atm) to the reactors. Furthermore, 1.87 tonnes of carbon dioxide (CO2) is generated with every tonne of NH3 produced by the Haber–Bosch process, contributing 1.2% of global anthropogenic CO2 emissions [7]. With regard to phosphate, most of it currently comes from a non-renewable resource, phosphate rock, which has no feasible substitute and can be contaminated by heavy metals. Mining costs increase over time as P rock becomes less available, constraining further mining action. Without proper management, phosphate rock is projected to be depleted in the next 70–140 years [8].
Although BESs can be configured as a single-chambered cell, the most typical setting is a dual-chambered device consisting of an anodic compartment and a cathodic compartment separated by a membrane. This configuration has been widely investigated for nutrient recovery, with different kinds of membranes tested to increase the selectivity of the recovered compound. The most commonly used membranes in the construction of BESs are ion-exchange membranes (IEM), typically to recover ammonium and phosphates for reuse as fertilisers.
BESs have been the subject of various literature reviews focused on materials and their many applications [9][10] along with nutrient removal and recovery [11][12][13], including membrane and membraneless reactors. The most recent reviews focused specifically on the recovery of nitrogen from a sustainability perspective [14][15]. To the authors’ knowledge, no reviews concerning ammonia and phosphate recovery using membrane-based BESs have to date been published. Furthermore, in the last few years, several papers have dealt with nitrogen and phosphate recovery including new advances and focusing both on achieving high efficiency in nutrient recovery and reducing the associated chemical requirements and energy consumption. Thus, an update on new advances in this field is needed.
2. Principles of Nutrient Recovery in BESs Using Membranes
BESs for nutrient recovery generally consist of an anodic compartment and a cathodic compartment divided by a membrane. Exoelectrogenic bacteria grow on the anode, forming a biofilm, and are capable of oxidising organic matter that is introduced to the anode compartment. The electrons and protons produced by the organic matter oxidation at the anode are directed to the cathode compartment by an external electrical circuit and through the internal membrane, respectively, and are combined by a reduction reaction. The circulation of electrons through the external electrical circuit generates electricity (microbial fuel cell, MFC). A charge imbalance is produced in the cell due to electron transfer from the anode to the cathode, which leads to positive charge migration, such as of protons, through the cation-exchange membrane (CEM, the most common IEM for ammonia recovery). However, when complex substrates are introduced to the anode compartment, other cations such as NH4+ will be in higher concentrations than the protons and preferably transfer (migrate) to the cathode compartment against the protons. In a similar way to ammonium, when an anion-exchange membrane (AEM) is used as a separator, phosphate ions can migrate from the cathode to the anode compartment.
Since cation/anion migration through the CEM or AEM is linked to the amount of charge circulating through the external electrical circuit, the transfer rate can be improved by an external power source connection in addition to producing hydrogen gas or other nonspontaneous reactions in the cathode (microbial electrolysis cell, MEC). Ammonia recovery improves with higher current densities; therefore, externally powered MECs perform better than MFCs for the recovery of ions. On the other hand, in the cathode chamber of CEM-equipped MFCs and MECs, a local high pH is created around the cathode surface through proton consumption or release of hydroxyls, which may improve nutrient recovery, i.e., by inducing ammonium conversion to ammonia or struvite crystallisation [16].
Membranes are a key element in nutrient recovery in BESs since they allow for the separation of nitrogen and phosphorus from the wastewater matrix and subsequent recovery in a clean solution, in contrast to BESs without a membrane. Several types of IEMs have been applied in the construction of BESs, such as proton-exchange membranes (PEM), CEMs, AEMs, ultrafiltration, microfiltration, and bipolar membranes. Comprehensive reviews of membrane separators in BESs have been published in recent years [17][18]. Membrane-BESs can be configured in different setups using a unique membrane or a combination of different kinds of membranes, achieving different ways of recovering nutrients, as shown in Figure 1.
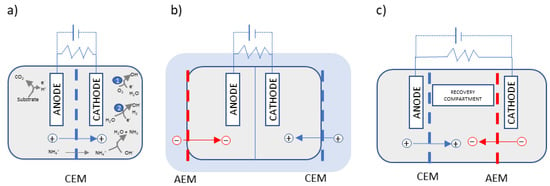
Figure 1. Examples of three typical configurations of membrane-based BESs. + and − stand for ions charged positively (i.e., NH4+) and negatively (i.e., PO43−), respectively. (a) Dual-chambered BESs, (b) submersed BESs [19], and (c) three-chambered cell with recovery compartment. The main reactions taking place in BESs are shown in (a) by way of example, where ❶ and ❷ stand for MFC and MEC cathodic reactions, respectively.
Although membranes are of great importance in the recovery of nutrients in BESs, reactor performance has been reported to be significantly affected by IEMs since they generally increase the internal electrical resistance and coulombic efficiencies, but also in relation to capital cost, as they represent up to 40% of the total cost of BESs [20]. Nevertheless, membrane-based BESs for nutrient recovery are a subject of great interest, as demonstrated by the 49 papers published since 2016 included in this review related to ammonia recovery (63%) and combined ammonia and phosphate recovery (37%). Although phosphate recovery or remobilisation from iron phosphate in BESs has been reported [21][22], combined nitrogen and phosphorus recovery systems are usually developed.
2.1. Ammonia Recovery by Stripping and Absorption
In CEM-equipped BESs, NH4+ migrates to the cathode chamber through the membrane combined with other cations (Figure 1a). In order to obtain a pure ammonia solution, a subsequent step of stripping and absorption is usually applied. The stripping and absorption process consists of injecting an air flux through the catholyte to drag dissolved gases, in this case ammonia gas. The gaseous stream then comes into contact with an acidic solution, where the ammonia is absorbed, transforming it again into ammonium. In conventional ammonia stripping, the temperature or pH of the substrate needs to be externally increased to favour a shift in the ammonium/ammonia equilibrium towards the second gaseous species. While in BESs, this step is greatly enhanced with no need for an alkali addition or temperature increase, thus reducing reactant costs and energy consumption due to the catholyte spontaneous increase in pH value [14]. Using a NaCl solution in the cathode compartment, the catholyte pH value has been reported to increase from 9.1 to around 10.8–12.1 at the end of the batch [23].
2.2. Nutrient Recovery by Precipitation
Combined nitrogen and phosphorus recovery can generally be achieved by obtaining a concentrate solution or by struvite precipitation. Struvite is a white salt that crystallises with an orthorhombic structure under alkaline conditions when magnesium, ammonium, and phosphate are present in equimolar concentrations, as shown by reaction (1). Struvite is considered to be a slow-release fertiliser.
Mg2+ + NH4+ + HnPO43−n + 6H2O → MgNH4PO4 + 6H2O + nH+
The cost of struvite crystallisation, which in conventional reactors can be high due to pH adjustment using basic chemicals [24] such as Mg(OH)2, NaOH, and Ca(OH)2, can be reduced thanks to catholyte basification in BESs.
References
- Barbosa, S.G.; Rodrigues, T.; Peixoto, L.; Kuntke, P.; Alves, M.M.; Pereira, M.A.; Ter Heijne, A. Anaerobic biological fermentation of urine as a strategy to enhance the performance of a microbial electrolysis cell (MEC). Renew. Energy 2019, 139, 936–943.
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Overcoming organic and nitrogen overload in thermophilic anaerobic digestion of pig slurry by coupling a microbial electrolysis cell. Bioresour. Technol. 2016, 216, 362–372.
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Unravelling the active microbial community in a thermophilic anaerobic digester-microbial electrolysis cell coupled system under different conditions. Water Res. 2017, 110, 192–201.
- Hassanein, A.; Witarsa, F.; Guo, X.; Yong, L.; Lansing, S.; Qiu, L. Next generation digestion: Complementing anaerobic digestion (AD) with a novel microbial electrolysis cell (MEC) design. Int. J. Hydrogen Energy 2017, 42, 28681–28689.
- Kumar, S.S.; Kumar, V.; Malyan, S.K.; Sharma, J.; Mathimani, T.; Maskarenj, M.S.; Ghosh, P.C.; Pugazhendhi, A. Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel 2019, 254, 115526.
- Vanotti, M.B.; Szogi, A.A.; Millner, P.D.; Loughrin, J.H. Development of a second-generation environmentally superior technology for treatment of swine manure in the USA. Bioresour. Technol. 2009, 100, 5406–5416.
- Wang, Y.; Meyer, T.J. A Route to Renewable Energy Triggered by the Haber-Bosch Process. Chem 2019, 5, 496–497.
- Li, B.; Boiarkina, I.; Young, B.; Yu, W.; Singhal, N. Prediction of Future Phosphate Rock: A Demand Based Model. J. Environ. Inform. 2018, 31, 41–53.
- Al-Sahari, M.; Al-Gheethi, A.; Radin Mohamed, R.M.S.; Noman, E.; Naushad, M.; Rizuan, M.B.; Vo, D.-V.N.; Ismail, N. Green approach and strategies for wastewater treatment using bioelectrochemical systems: A critical review of fundamental concepts, applications, mechanism, and future trends. Chemosphere 2021, 285, 131373.
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Dominguez Benneton, X.; Strik, D.P.B.T.B.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170.
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360.
- Li, N.; Wan, Y.; Wang, X. Nutrient conversion and recovery from wastewater using electroactive bacteria. Sci. Total Environ. 2020, 706, 135690.
- Rodríguez Arredondo, M.; Kuntke, P.; Jeremiasse, A.W.; Sleutels, T.H.J.A.; Buisman, C.J.N.; ter Heijne, A. Bioelectrochemical systems for nitrogen removal and recovery from wastewater. Environ. Sci. Water Res. Technol. 2015, 1, 22–33.
- Kuntke, P.; Sleutels, T.H.J.A.; Rodríguez Arredondo, M.; Georg, S.; Barbosa, S.G.; ter Heijne, A.; Hamelers, H.V.M.; Buisman, C.J.N. (Bio)electrochemical ammonia recovery: Progress and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 3865–3878.
- Yang, K.; Qin, M. The Application of Cation Exchange Membranes in Electrochemical Systems for Ammonia Recovery from Wastewater. Membranes 2021, 11, 494.
- Zeppilli, M.; Paiano, P.; Torres, C.; Pant, D. A critical evaluation of the pH split and associated effects in bioelectrochemical processes. Chem. Eng. J. 2021, 422, 130155.
- Bakonyi, P.; Koók, L.; Kumar, G.; Tóth, G.; Rózsenberszki, T.; Nguyen, D.D.; Chang, S.W.; Zhen, G.; Bélafi-Bakó, K.; Nemestóthy, N. Architectural engineering of bioelectrochemical systems from the perspective of polymeric membrane separators: A comprehensive update on recent progress and future prospects. J. Memb. Sci. 2018, 564, 508–522.
- Daud, S.M.; Kim, B.H.; Ghasemi, M.; Daud, W.R.W. Separators used in microbial electrochemical technologies: Current status and future prospects. Bioresour. Technol. 2015, 195, 170–179.
- Zhang, Y.; Angelidaki, I. Counteracting ammonia inhibition during anaerobic digestion by recovery using submersible microbial desalination cell. Biotechnol. Bioeng. 2015, 112, 1478–1482.
- San-Martín, M.I.; Carmona, F.J.; Alonso, R.M.; Prádanos, P.; Morán, A.; Escapa, A. Assessing the ageing process of cation exchange membranes in bioelectrochemical systems. Int. J. Hydrogen Energy 2019, 44, 25287–25296.
- Happe, M.; Sugnaux, M.; Cachelin, C.P.; Stauffer, M.; Zufferey, G.; Kahoun, T.; Salamin, P.-A.; Egli, T.; Comninellis, C.; Grogg, A.-F.; et al. Scale-up of phosphate remobilization from sewage sludge in a microbial fuel cell. Bioresour. Technol. 2016, 200, 435–443.
- Blatter, M.; Vermeille, M.; Furrer, C.; Pouget, G.; Fischer, F. Mechanisms and Model Process Parameters in Bioelectrochemical Wet Phosphate Recovery from Iron Phosphate Sewage Sludge. ACS Sustain. Chem. Eng. 2019, 7, 5856–5866.
- Cerrillo, M.; Oliveras, J.; Viñas, M.; Bonmatí, A. Comparative assessment of raw and digested pig slurry treatment in bioelectrochemical systems. Bioelectrochemistry 2016, 110, 69–78.
- Jing, H.-P.; Li, Y.; Wang, X.; Zhao, J.; Xia, S. Simultaneous recovery of phosphate, ammonium and humic acid from wastewater using a biochar supported Mg(OH) 2 /bentonite composite. Environ. Sci. Water Res. Technol. 2019, 5, 931–943.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
527
Revisions:
2 times
(View History)
Update Date:
21 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




