Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed Kouhen | -- | 3787 | 2023-02-20 10:41:18 | | | |
| 2 | Jason Zhu | -1 word(s) | 3786 | 2023-02-21 03:07:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kouhen, M.; Dimitrova, A.; Scippa, G.S.; Trupiano, D. The Plant Response to Mechanical Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/41420 (accessed on 07 February 2026).
Kouhen M, Dimitrova A, Scippa GS, Trupiano D. The Plant Response to Mechanical Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/41420. Accessed February 07, 2026.
Kouhen, Mohamed, Anastazija Dimitrova, Gabriella Stefania Scippa, Dalila Trupiano. "The Plant Response to Mechanical Stress" Encyclopedia, https://encyclopedia.pub/entry/41420 (accessed February 07, 2026).
Kouhen, M., Dimitrova, A., Scippa, G.S., & Trupiano, D. (2023, February 20). The Plant Response to Mechanical Stress. In Encyclopedia. https://encyclopedia.pub/entry/41420
Kouhen, Mohamed, et al. "The Plant Response to Mechanical Stress." Encyclopedia. Web. 20 February, 2023.
Copy Citation
Mechanical stimuli, together with the corresponding plant perception mechanisms and the finely tuned thigmomorphogenetic response, has been of scientific and practical interest since the mid-17th century. As an emerging field, there are many challenges in the research of mechanical stress. Indeed, studies on different plant species (annual/perennial) and plant organs (stem/root) using different approaches (field, wet lab, and in silico/computational) have delivered insufficient findings that frequently impede the practical application of the acquired knowledge.
calcium signaling
gravitropism
mechanosensitive channels
reaction wood
1. Introduction
In response to various mechanical stressors, plants exhibit various morphological responses, preceded by a range of biochemical changes. These responses depend on the type of mechanical stress (MS) as well as the plant developmental stage and species' biological characteristics (e.g., see [1][2]). This outlines the common thigmomorphogenetic characteristics of the stem and root; researchers note that, to the best of current knowledge and as other authors have shown, e.g., [3], there is no universal response to MS in woody and annual plants (Figure 1).
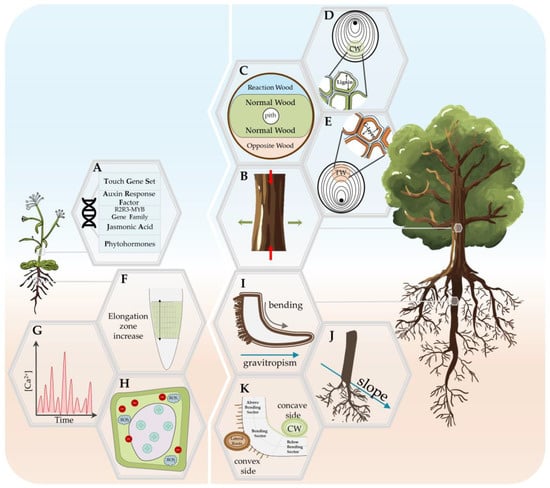
Figure 1. Summary of the mechanical stress response in annual (Arabidopsis; left panel) and woody plants (right panel). (A) Mechanical stress-related molecular responses in Arabidopsis stem. (B) Zones of differentiated growth in woody plant stems subjected to mechanical stress (reaction wood, RW; normal wood, NW; opposite wood, OW). (C) Location and characteristics of the tension wood (TW) formation, i.e., the RW in the stem of the Angiosperm specie. (D) Location and characteristics of the compression wood (CW) formation, i.e., the RW in the stem of the Gymnosperm specie. (E) Reduction in the elongation growth and increase in the radial thickness in the stem of woody plants. (F) Increase in the elongation zone with radially symmetric changes in cell expansion and elongation in the root of Arabidopsis. (G) Stimulus-specific rapid and transient increase in cytosolic calcium in Arabidopsis root. (H) Apoplastic alkalinization, cytoplasmic acidification, and the production of apoplastic reactive oxygen species (ROS). (I) Lateral root initiation as a response to either gravitropic curvature or manual woody root bending. (J) ‘Bilateral-fan shape’ lateral roots root distribution in slope conditions. (K) Asymmetric response of three root bending sectors on the concave and convex side of bent woody roots.
2. Thigmomorphogenesis in Stem
As Braam [4] reviewed, thigmomorphogenesis impacts plant species differently, and the touch stimuli can trigger different responses in the plant above-ground organs, i.e., leaves in carnivorous plants, modified leaves/stems in climbing plants, flowers in some species where self-pollination is possible, etc. The most iconic illustration of this is the thigmomorphogenetic response of Mimosa pudica, also referred to as ‘touch me not’. Mediating a motor organ named pulvinus, the plant leaflets rapidly fold in response to exogenous MS, using a long-distance rapid electrical signal [5] and calcium fluxes [6].
2.1. Mechanical Stress Response in Annual Plants: Arabidopsis Model
In response to mechanical stimuli such as wind or touch, stems undergo physiological and developmental changes that enhance resistance to subsequent MS (Figure 1A). In general, plants growing in windy environments are shorter, stockier, and often have altered flexibility [7][8].
In Arabidopsis, as a rosette plant, the ontogenetical function of the stem is different from the perennial counterpart, in which it contributes for long-term stability, structural and mechanical fitness, and where the aforementioned services are not prioritized [9][10][11]. However, the biological characteristics of Arabidopsis, i.e., the possibility to induce a stem in the secondary structure by decapitation, by reducing light exposure (‘short-day conditions’), or by increasing the weight load of the stem [4][12], along with the knowledge/data availability regarding the wide scope of the species’ physio-molecular process have been exploited in terms of studying the mechanisms of the stem response to MS. Wind stimulation has been shown to proportionally impact the degree of branching and basal fruit production in Arabidopsis plants [13].
The serendipitous discovery of the Arabidopsis touch (TCH) gene set has spiked an interest in the thigmomorphogenetic molecular mechanisms [3][14]. The roles played by the TCH gene family is not limited to MS as they were associated with upregulation via exogenous auxin and/or brassinosteroid and the fluctuation of free cytosolic calcium ion (Ca2+) as a secondary messenger in a variety of signal transduction pathways [14], opening new research channels. The generally observed stem thickness increase in response to MS does not always occur in Arabidopsis, but through the application of weight on the stem, a type of compression force can induce the formation of cambium-like tissues [3]. Auxin was found to support the secondary xylem formation, and three auxin response factor (ARF) genes (ARF2, ARF4, and ARF12) are assumed to play a particularly significant role during the wood formation [15]. From the previously mentioned R2R3-MYB gene family, four MYB transcription factors are considered as candidate regulatory genes for wood formation, and three of them (AtMYB77, AtMYB73, AtMYB44) seem to have similar functions in stem development [15]. Some of the TCH genes have additionally been linked to jasmonate signaling [16]. Mechanostimulation involves jasmonic acid (JA) signaling pathways as part of the cambium regulation, which induces the JA production and expression of JA biosynthesis genes [17] and is required for the wound-induced growth-regulation [18]. Katanin-dependent microtubule dynamics were found to increase the cell competence to respond to MS by enhancing the cells’ ability to adapt to their growth according to the neighbors [19]. The role of ethylene, auxin, cytokinins, and gibberellins in the vascular development of Arabidopsis has also been confirmed [20].
2.2. Woody Plants’ Stem Response—The Role of Reaction Wood
The perennial habit is associated with a wide range of morphological and physiological traits that are likely necessitated by the greater range of environmental and seasonal cues encountered by these plants compared with their annual counterparts [21]. In the stem of perennial woody plants, mechanical stress induced a reduction in elongation growth while increasing the radial thickness, i.e., reduced height and increased diameter, respectively [4][22], having a visible and direct impact on the yield/biomass production (Figure 1B). The objective of the woody plants thigmomorphogenetic response is non-vertical axis reorientation, which is achieved through the RW formation at points where the force (compression or tension) can push the stem towards its original position [23]. This response occurs due to the heterogeneity of the cambial region activity, and it mainly involves the wood, also called secondary xylem, which ensures the mechanical support and long-distance conductivity of water and nutrients [24][25][26]. Wood is naturally composed of cellulose microfibrils situated in the hemicelluloses and lignin matrix which, under load and over time, exhibit anatomical and chemical deformations [27]. These changes can reduce the wood value from an economical aspect, which has encouraged a significant body of literature to address the stem response to MS [28]. MS is also considered necessary for the differentiation of xylem cells, but the exact mechanisms of its impact are not clear [26][29]. Once a stem is bent, the asymmetrical response is exhibited as a formation of RW and opposite wood (OW) (Box 1; Figure 1C). The location and characteristics of RW differ between gymnosperms and angiosperms, respectively, and compression wood (CW) and tension wood (TW) (Box 1), and they further impact the hydraulic and mechanical wood properties through the changes in the wood properties [26][30]. Variations in both CW and TW appear due to the species characteristics and age, environmental conditions, stress type, and compression severity [23][24][25][26][27][28]. However, both employ similar basic mechanisms for sensing the stress stimulus and thigmomorphogenetic response, which differs in structural and mechanical context [23] and will be briefly summarized in the following paragraphs (Figure 1D,E).
The CW forms on the lower (concave) compressed side of the bent stem/branch in gymnosperm perennials (Figure 1D), and its main function is to push back the leaning stem to an upright position by compression stress [31]. The changes associated with CW are one of main contributors to reduced wood quality and fiber products [32]. The compression induces longitudinal shrinkage in comparison to normal wood (NW), which is related to a larger microfibril angle and increased lignification, both of which have been used to investigate the wood mechanical behavior and contribute to the lower stiffness of CW [27][28][33]. Another anatomical particularity of CW is shortened tracheids compared with NW and OW from the same tree, with changes in the shape and deformation of the tips [25][28]. In addition to higher lignin, the CW also contains higher amounts of (1-4)-β-galactan and lower amounts of cellulose, mannan, and xylan [28].
Box 1. Glossary of terms related to woody structure response to mechanical stress.
Reaction wood (RW)—natural response of woody plants to mechanical stress via asymmetrical formation of secondary xylem tissue, aimed to reinforce the structure and redirect the growth [30][34];
Normal wood (NW)—wood formed in the absence of stimulus [30];
Flexure wood (FW)—specific wood produced by the vascular cambium in trees growing in a windy environment, characterized with increased secondary xylem production and decreased elastic modulus in comparison to normal wood [30];
Compression wood (CW)—RW in Gymnosperm formed on the lower side of inclined stems or branches, characterized by a high lignin and low cellulose composition due to the generation of a compressive force to push the stem up [30][34]. Tissue with the same characteristics has been noted on the lower (concave) side in bent poplar root [30][35];
The TW forms on the upper (convex) stretched side of the bent stem/branch in angiosperm perennials (Figure 1E). Similar to CW, across species, TW exhibits a wide range of organizational variations (see comprehensive review in [36]), but its main anatomical characteristic are the G-fibers, which are xylem fibers with a smaller radial diameter that have an additional thick layer on the inner side of the secondary wall and which form an additional layer with a translucent gelatinous appearance, which is not very cohesive with the rest of the cell wall layers, named the G-layer [24][25]. The G-layer is mainly completely composed of cellulose, but the presence of lignin, xyloglucans and xyloglucan-synthesising proteins, pectins, and rhamnogalacturonan I, arabinogalactan, and arabinogalactan proteins has been confirmed [23][25]. However, as the detected lignin content has been minimal or non-existing, TW is generally considered to have increased cellulose and reduced lignin content [23][24] as the other side of the previously mentioned negative correlation. The G-layer is further characterized by a higher porosity, allowing for higher water content, which is assumed to be the reason for the gelatinous appearance and capacity of the G-layer for transversal swelling/shrinking [23]. The TW formation results from an increased cell division rate, i.e., cambial activity [25].
The role of phytohormones in the thigmomorphogenetic response and their involvement in CW and TW formation have been acknowledged and studied, but the results and conclusions are not consistent and are difficult to compare (for a detailed review, see [28] for CW and [23] for TW). This is not only due to the general involvement of the phytohormones in many aspects of the plant development, but also due to the combined effect that hormones have with each other and with other parts of the stress regulation mechanism. To briefly summarize, it appears that CW formation mainly involves auxin and ethylene along with reduced endogenous cytokinins and abscisic acid [28][37]. In TW formation, while the role of ethylene has been continuously confirmed, the role of auxin is not yet clearly defined but appears to be a crucial part for RW formation [23][24]. More recently, cytokinins and brassinosteroids were also associated with TW formation by [30] and [34], respectively. Gibberellin’s role in CW formation has been dismissed, but there has been some evidence for its role in TW formation, where it has been shown to be able to induce cambial growth and G-fibers differentiation [23][34][38].
Previous research has largely focused on the anatomical and morphological characteristics of RW. The same attention has not been given to the molecular mechanisms of secondary growth [12], and the current interest is indeed focused on the molecular and signaling aspects of RW formation. However, there has been no consensus whether a model species comparison is a suitable approach between Arabidopsis and perennials, as well as different perennial species. While key regulators have been observed when it comes to the secondary development for both herbaceous and woody plants [20], the issue remains that RW formation does not naturally occur in herbaceous model species such as Arabidopsis [39]. Using poplars as a model species does help overcome some difficulties, but the shortfall regarding molecular studies in perennial species and, in gymnosperms even more so, remains [39][40]. In CW, gene expression analysis uncovered the upregulation of genes involved in the gravitropic response of the stem, i.e., lignin biosynthesis, ethylene forming enzymes, and cell wall proteins (biosynthetic enzymes, carbohydrate metabolism and regulatory proteins, monolignol biosynthesis, arabinogalactan, and proline-rich proteins) [28][32]. A particular point of interest is the R2R3-MYB family, which regulates the lignin and phenylpropanoid metabolism during wood formation and whose involvement has been confirmed in conifers [40]. Pilate et al. [39] provided a comprehensive review regarding TW genomic studies, indicating the potential of TW to provide a better understanding of the molecular mechanisms of wood formation and their properties. More recently, studies focusing on the early changes in the poplar transcriptome have contributed to a better understanding of the thigmomorphogenesis. Pomiès et al. [41] have investigated the response due to single or repeated bending, concluding that while major gene expression changes take place in the first two hours post-bending, there are several mechanistic pathways involved in the response, starting from the genes involved in the general response to abiotic stress (ROS, Ca2+, and jasmonic acid signalling) to more specific genes involved in cell wall and wood development. Using an innovative isotropic device, Lopez et al. [34] have managed to isolate the early (30 min) molecular response to gravistimulation, confirming again the activity in the cell wall and wood formation and noting on about 200 xylem-regulated genes that have not yet been functionally characterized.
3. Thigmomorphogenesis in the Roots
The obstacles encountered by roots during soil penetration invariably cause thigmomorphogenesis [16]. Root ecology research faces numerous challenges, ranging from the biological characteristics of the organ to the design of relevant experimental studies [42]. These difficulties are even more accentuated in the case of root thigmomorphogenesis due to the previously mentioned factors of MS variability in experimental design and sampling difficulties.
3.1. Young Roots Response to Mechanical Stress
The impact of mechanical stimuli on primary roots induces changes in the growth direction that can alter the lateral root (LR) location, with the LRs emanating from the convex side of the arising curves rather than being in a preset distribution [43][44][45][46][47]. The cascade of events leading to LR formation from the xylem pericycle cells has been well-studied in Arabidopsis, which is shown to be strictly related to a transient spatio-temporal accumulation of auxins along the parental root axis [48][49]. In particular, Ditengou et al. [43] observed a delocalization of the auxin carrier PIN1 in a single protoxylem cell, followed by auxin accumulation at the site of lateral root induction. Another study confirmed the LR emission on the convex side of the bending in a timepoint as short as 20 s of transient bend [50]. LR emission has also been linked to calcium signaling, which translates the mechanical forces to a developmental response in the roots [50]. Because plants detect mechanical stimuli to identify neighboring barriers and alter their growth patterns to acclimate to their surroundings, another commonly used approach in MS studies is root barrier exposure. External and endogenously generated mechanical forces consistently cause stimulus-specific, rapid, and transient increases in cytosolic Ca2+ [51]. Barrier exposure was shown to trigger an apoplastic alkalinization, cytoplasmic acidification, and the production of apoplastic reactive oxygen species (ROS) [52]. Jacobsen et al. [53] used an in vitro barrier system analysis to study the Arabidopsis root response to short-term mechanical impedance (up to 30 h) through global transcriptional profiling. The results uncovered radially asymmetric changes in cell expansion and elongation and reduced root length in addition to a shorter distance of root hair emergence from the root tip (Figure 1F). The ROS, signaling genes linked to ethylene and auxin differential gradients, and transcriptional activation of ROS were all part of the early response of Arabidopsis roots [53]. The role of auxin in thigmotropism during plant–obstacle interactions has recently been established, where it was reported that PIN-FORMED (PIN)-mediated polar auxin transport enables root bending prior to obstacle avoidance [54]. Some authors suggest that in Arabidopsis, tension forces acting in the convex side of bent root induces an increase in Ca2+ levels in specific pericycle cells, becoming a ‘founder cell’ of a new lateral root [50][55] (Figure 1G). This Ca2+ increase leads to: (a) an alteration in ROS and cytosolic acidification, which is known to elicit signaling events; and (b) a cell wall alkalinization, known to rigidify the cell wall matrix (Figure 1E). Diaz-Sala [56] suggested that mechanosensitive ion channels that are present on the plasma membranes could generate electric action potentials that propagate on a short distance from cell to cell along with the plasma membrane network and through plasmodesmata (or alternatively through phloem cells over a longer distance) inducing modifications in the cell walls, creating specific interactions between the cell wall and cytoskeleton and alterations of the microtubule dynamics.
3.2. Woody Roots Response
In woody plants, very few studies are available with respect to the anatomical, morphological, biochemical, and molecular aspects of woody roots’ response to slope or bending stress [30][35][57][58] (Figure 1I). woody root growth on natural slope conditions produced an asymmetrical root system, designated as a ‘bilateral-fan shape’, in which lateral roots developed both downslope and upslope [59][60][61] (Figure 1J). However, mechanical constraints do not induce the same type of response in the stem and roots. Furthermore, roots subjected to similar mechanical constraints that are imposed to the stem may develop extremely dissimilar RW. Indeed, in poplar plants, bending induces TW formation on the ‘upper’ convex stretched side of the stem or branch, whereas a CW similar to gymnosperm stems is formed in the ‘lower’ concave compressed side after bending. Hellgren et al. [62] found that the formation of TW in poplar are not mediated by changes in the indole-3-acetic acid (IAA) level in the cambial tissues. On the contrary, a higher amount of endogenous IAA was detected at the side of the cambial region-forming CW, which could act as a spatial regulator of cambial activity, enhancing the cell division rate and conferring key positional information to the cells of the cambial zone’s surrounding tissues for differentiation/RW initiation [35][63][64].
The woody roots’ MS response was shown to be temporally and spatially modulated by an intricate interplay of different signal transduction pathways, involving reactive oxygen species (ROS), hormones (indole acetic acid, gibberellins, ABA, and ethylene), and specific molecular factors regulating lignin deposition, cell wall integrity, and lateral root formation [58][65][66][67][68]. Trupiano et al. [58] postulated a mechanical force distribution model where the convex and concave sides of each bent root sector are subjected to different mechanical force distributions, with the tension forces applied to the convex side and the compression forces concentrated on the concave side. Side- and sector-specific strategies were used by bent roots to maintain water uptake and transport in a deforming condition that was induced by tension and compression forces; this resulted in an increased xylem thickness on the compressed side and enhanced lateral root formation at the tension site (Figure 1K).
Following a 6-month root bending stress test, the woody roots of Populus nigra displayed a reaction wood (RW) formation due to compression forces at the concave side [35], showing root-specific characteristics in comparison to those produced in the bent stem. The woody roots’ RW is characterized by a low vessel density and high lignin content mainly triggered by auxin, and it is associated with the induction of cambium cell activity [35]. The research also provides some initial understanding of the mechanisms controlling this compression-induced wood on the concave side, characterized by the activation of specific proteins that govern cell wall deformation, lignification, and xylem differentiation [35]. A similar study applying a shorter (2 months) root bending stress test observed a bending sector-specific distribution of phytohormones (auxin, cytokinin, and abscisic acid) that reflected adaptations to compression- or tension-specific forces [69]. These changes were later confirmed in the mechanics of Arabidopsis seedling emergence. A recent study proposed a model for explaining the hypocotyl bending mechanics [70], reporting that auxin maxima are generated on the inner side of the bent by polar auxin transport through the auxin transport machinery components PIN3, PIN4, PIN7, and AUX1, promoting pectin with a high degree of methylesterification and therefore stiffening the wall and leading to a slower rate of cell elongation; this is in opposition to the outer side, which had low auxin levels favoring pectin demethylesterification, cell wall loosening, and faster cell elongation [70]. In another study on bent woody roots of poplar plants, De Zio et al. [30] observed the asymmetrical sector-specific response. These differences are expressed across measured parameters, with a higher lignin concentration in the above bending sector (ABS) and lower amount of carbohydrates on the concave side of the ABS, as well as a reduced amount of indole acetic acid (IAA) in the convex side of both the bending (BS) and below bending sector (BBS), and RW formation due to increased cambial cell activity on the concave side of the BS and BBS [30]. These changes were found to be strictly correlated to the ability of the vascular cambium cells to perceive specific signals and, in turn, to orchestrate specific genes leading to RW (towards the concave side) or lateral roots (towards the convex side) formation. Recently, Dimitrova et al. [71] provided novel information regarding the response coordination, communication, and potential signaling pathways that were asymmetrically activated along the main root axis, which were mainly delegated to Ca2+ (for new lateral root formation) and ROS (for gravitropic response and lignin accumulation) signatures. Furthermore, some of the data indicate that the concave side of the bent sector, where the mechanical forces are the most intense, communicates to the other (neighbor and distant) sectors, inducing spatially related strategies to ensure water uptake and accompanying cell modification [71]. The communication between these portions is supposed to engage in short distance signals, such as chemical and electrical signaling, plasma membrane hydraulic pulses, or plasmodesmata and meristematic connectomes [72], to cover long distances and adjust the root body to its surrounding environment.
During the past few decades, research efforts have provided a partial understanding of the response to MS in plant roots. However, large gaps remain, especially regarding the specific physiological, molecular, and genetic processes involved in mechanosensing and mechanotransduction. Thus, there remains a need for future technologically advanced research that is focused on the early events of the woody root bending response, as this would help in the understanding of plant tissues organization along with cell-to-cell communication between neighboring and distant cells.
References
- Love, J.; Björklund, S.; Vahala, J.; Hertzberg, M.; Kangasjärvi, J.; Sundberg, B. Ethylene Is an Endogenous Stimulator of Cell Division in the Cambial Meristem of Populus. Proc. Natl. Acad. Sci. USA 2009, 106, 5984–5989.
- Chaki, M.; Valderrama, R.; Fernández-Ocaña, A.M.; Carreras, A.; Gómez-Rodríguez, M.V.; Pedrajas, J.R.; Begara-Morales, J.C.; Sánchez-Calvo, B.; Luque, F.; Leterrier, M.; et al. Mechanical Wounding Induces a Nitrosative Stress by Down-Regulation of GSNO Reductase and an Increase in S-Nitrosothiols in Sunflower (Helianthus annuus) Seedlings. J. Exp. Bot. 2011, 62, 1803–1813.
- Bossdorf, O.; Pigliucci, M. Plasticity to Wind Is Modular and Genetically Variable in Arabidopsis thaliana. Evol. Ecol. 2009, 23, 669–685.
- Braam, J. In Touch: Plant Responses to Mechanical Stimuli. New Phytol. 2004, 165, 373–389.
- Hagihara, T.; Toyota, M. Mechanical Signaling in the Sensitive Plant Mimosa pudica L. Plants 2020, 9, 587.
- Hagihara, T.; Mano, H.; Miura, T.; Hasebe, M.; Toyota, M. Calcium-Mediated Rapid Movements Defend against Herbivorous Insects in Mimosa pudica. Nat. Commun. 2022, 13, 6412.
- Mitchell, C.A. Recent Advances in Plant Response to Mechanical Stress: Theory and Application. In Proceedings of the Recent Advances in Plant Response to Stress: Bridging the Gap between Science and Technology, Corvallis, OR, USA, 7 August 1994; Volume 31, pp. 31–35.
- Ennos, A.R. Wind as an Ecological Factor. Trends Ecol. Evol. 1997, 12, 108–111.
- Anten, N.P.R.; Casado-Garcia, R.; Pierik, R.; Pons, T.L. Ethylene Sensitivity Affects Changes in Growth Patterns, but Not Stem Properties, in Response to Mechanical Stress in Tobacco. Physiol. Plant. 2006, 128, 274–282.
- Lu, S.; Sun, Y.H.; Shi, R.; Clark, C.; Li, L.; Chiang, V.L. Novel and and Mechanical Stress-Responsive MicroRNAs in Populus trichocarpa That Are Absent from Arabidopsis. Plant Cell 2005, 17, 2186–2203.
- Lu, S.; Sun, Y.H.; Chiang, V.L. Stress-Responsive MicroRNAs in Populus. Plant J. 2008, 55, 131–151.
- Ko, J.H.; Han, K.H.; Park, S.; Yang, J. Plant Body Weight-Induced Secondary Growth in Arabidopsis and Its Transcription Phenotype Revealed by Whole-Transcriptome Profiling. Plant Physiol. 2004, 135, 1069–1083.
- Pigliucci, M. Touchy and Bushy: Phenotypic Plasticity and Integration in Response to Wind Stimulation in Arabidopsis thaliana. Int. J. Plant Sci. 2002, 163, 399–408.
- Braam, J.; Sistrunk, M.L.; Polisensky, D.H.; Xu, W.; Purugganan, M.M.; Antosiewicz, D.M.; Campbell, P.; Johnson, K.A. Plant Responses to Environmental Stress: Regulation and Functions of the Arabidopsis TCH Genes. Planta 1997, 203, 35–41.
- Vogel, S. Life in Moving Fluids: The Physical Biology of Flow—Revised and Expanded Second Edition; NED-New edition; Princeton University Press: Princeton, NJ, USA, 1994.
- Hamant, O. Widespread Mechanosensing Controls the Structure behind the Architecture in Plants. Curr. Opin. Plant Biol. 2013, 16, 654–660.
- Sehr, E.M.; Agusti, J.; Lehner, R.; Farmer, E.E.; Schwarz, M.; Greb, T. Analysis of Secondary Growth in the Arabidopsis Shoot Reveals a Positive Role of Jasmonate Signalling in Cambium Formation. Plant J. 2010, 63, 811–822.
- Chehab, E.W.; Yao, C.; Henderson, Z.; Kim, S.; Braam, J. Arabidopsis Touch-Induced Morphogenesis Is Jasmonate Mediated and Protects against Pests. Curr. Biol. 2012, 22, 701–706.
- Uyttewaal, M.; Burian, A.; Alim, K.; Landrein, B.; Borowska-Wykrt, D.; Dedieu, A.; Peaucelle, A.; Ludynia, M.; Traas, J.; Boudaoud, A.; et al. Mechanical Stress Acts via Katanin to Amplify Differences in Growth Rate between Adjacent Cells in Arabidopsis. Cell 2012, 149, 439–451.
- Ragni, L.; Hardtke, C.S. Small but Thick Enough—The Arabidopsis Hypocotyl as a Model to Study Secondary Growth. Physiol. Plant. 2014, 151, 164–171.
- Lundgren, M.R.; Des Marais, D.L. Life History Variation as a Model for Understanding Trade-Offs in Plant–Environment Interactions. Curr. Biol. 2020, 30, R180–R189.
- Anten, N.P.R.; Casado-Garcia, R.; Nagashima, H. Effects of Mechanical Stress and Plant Density on Mechanical Characteristics, Growth, and Lifetime Reproduction of Tobacco Plants. Am. Nat. 2005, 166, 650–660.
- Felten, J.; Sundberg, B. Biology, Chemistry and Structure of Tension Wood. In Cellular Aspects of Wood Formation; Springer: Berlin, Germany, 2013; pp. 203–224.
- Pilate, G.; Chabbert, B.; Cathala, B.; Yoshinaga, A.; Leplé, J.-C.; Laurans, F.; Lapierre, C.; Ruel, K. Lignification and Tension Wood. C. R. Biol. 2004, 327, 889–901.
- Ruelle, J. Morphology, Anatomy and Ultrastructure of Reaction Wood. In The Biology of Reaction Wood; Springer: Berlin, Germany, 2014; pp. 13–35.
- Badel, E.; Ewers, F.W.; Cochard, H.; Telewski, F.W. Acclimation of Mechanical and Hydraulic Functions in Trees: Impact of the Thigmomorphogenetic Process. Front. Plant Sci. 2015, 6, 266.
- Peng, H.; Salmén, L.; Jiang, J.; Lu, J. Creep Properties of Compression Wood Fibers. Wood Sci. Technol. 2020, 54, 1497–1510.
- Donaldson, L.A.; Singh, A.P. Formation and Structure of Compression Wood. In Cellular Aspects of Wood Formation; Springer: Berlin, Germany, 2013; pp. 225–256.
- Miodek, A.; Gizińska, A.; Włoch, W.; Kojs, P. What Do We Know about Growth of Vessel Elements of Secondary Xylem in Woody Plants? Biol. Rev. 2021, 96, 2911–2924.
- De Zio, E.; Montagnoli, A.; Karady, M.; Terzaghi, M.; Sferra, G.; Antoniadi, I.; Scippa, G.S.; Ljung, K.; Chiatante, D.; Trupiano, D. Reaction Wood Anatomical Traits and Hormonal Profiles in Poplar Bent Stem and Root. Front. Plant Sci. 2020, 11, 590985.
- Purusatama, B.D.; Kim, N.H. Quantitative Anatomical Characteristics of Compression Wood, Lateral Wood, and Opposite Wood in the Stem Wood of Ginkgo biloba L. BioResources 2018, 13, 8076–8088.
- Plomion, C.; Pionneau, C.; Brach, J.; Costa, P.; Baillères, H. Compression Wood-Responsive Proteins in Developing Xylem of Maritime Pine (Pinus pinaster Ait.). Plant Physiol. 2000, 123, 959–969.
- Wang, D.; Lin, L.; Fu, F. Deformation Mechanisms of Wood Cell Walls under Tensile Loading: A Comparative Study of Compression Wood (CW) and Normal Wood (NW). Cellulose 2020, 27, 4161–4172.
- Lopez, D.; Franchel, J.; Venisse, J.-S.; Drevet, J.R.; Label, P.; Coutand, C.; Roeckel-Drevet, P. Early Transcriptional Response to Gravistimulation in Poplar without Phototropic Confounding Factors. AoB Plants 2021, 13, plaa071.
- De Zio, E.; Trupiano, D.; Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Grosso, A.; Marra, M.; Scaloni, A.; Scippa, G.S. Poplar Woody Taproot under Bending Stress: The Asymmetric Response of the Convex and Concave Sides. Ann. Bot. 2016, 118, 865–883.
- Ghislain, B.; Clair, B. Diversity in the Organisation and Lignification of Tension Wood Fibre Walls—A Review. IAWA J. 2017, 38, 245–265.
- Du, S.; Yamamoto, F. An Overview of the Biology of Reaction Wood Formation. J. Integr. Plant Biol. 2007, 49, 131–143.
- Funada, R.; Miura, T.; Shimizu, Y.; Kinase, T.; Nakaba, S.; Kubo, T.; Sano, Y. Gibberellin-Induced Formation of Tension Wood in Angiosperm Trees. Planta 2008, 227, 1409–1414.
- Pilate, G.; Déjardin, A.; Laurans, F.; Leplé, J.C. Tension Wood as a Model for Functional Genomics of Wood Formation. New Phytol. 2004, 164, 63–72.
- Bedon, F.; Grima-Pettenati, J.; Mackay, J. Conifer R2R3-MYB Transcription Factors: Sequence Analyses and Gene Expression in Wood-Forming Tissues of White Spruce (Picea glauca). BMC Plant Biol. 2007, 7, 1–17.
- Pomiès, L.; Decourteix, M.; Franchel, J.; Moulia, B.; Leblanc-Fournier, N. Poplar Stem Transcriptome Is Massively Remodelled in Response to Single or Repeated Mechanical Stimuli. BMC Genomics 2017, 18, 1–16.
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardising Root Classification, Sampling, Processing and Trait Measurements. New Phytol. 2021, 232, 973–1122.
- Ditengou, F.A.; Teale, W.D.; Kochersperger, P.; Flittner, K.A.; Kneuper, I.; Van Der Graaff, E.; Nziengui, H.; Pinosa, F.; Li, X.; Nitschke, R.; et al. Mechanical Induction of Lateral Root Initiation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 18818–18823.
- De Smet, I.; Tetsumura, T.; De Rybel, B.; dit Frey, N.F.; Laplaze, L.; Casimiro, I.; Swarup, R.; Naudts, M.; Vanneste, S.; Audenaert, D. Auxin-Dependent Regulation of Lateral Root Positioning in the Basal Meristem of Arabidopsis. Development 2007, 134, 681–690.
- Laskowski, M.; Grieneisen, V.A.; Hofhuis, H.; Ten Hove, C.A.; Hogeweg, P.; Marée, A.F.M.; Scheres, B. Root System Architecture from Coupling Cell Shape to Auxin Transport. PLoS Biol. 2008, 6, e307.
- Lucas, M.; Godin, C.; Jay-Allemand, C.; Laplaze, L. Auxin Fluxes in the Root Apex Co-Regulate Gravitropism and Lateral Root Initiation. J. Exp. Bot. 2008, 59, 55–66.
- Lucas, M.; Guédon, Y.; Jay-Allemand, C.; Godin, C.; Laplaze, L. An Auxin Transport-Based Model of Root Branching in Arabidopsis thaliana. PLoS ONE 2008, 3, e3673.
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602.
- Geldner, N.; Richter, S.; Vieten, A.; Marquardt, S.; Torres-Ruiz, R.A.; Mayer, U.; Jürgens, G. Partial Loss-of-Function Alleles Reveal a Role for GNOM in Auxin Transport-Related, Post-Embryonic Development of Arabidopsis. Development 2004, 131, 389–400.
- Richter, G.L.; Monshausen, G.B.; Krol, A.; Gilroy, S. Mechanical Stimuli Modulate Lateral Root Organogenesis. Plant Physiol. 2009, 151, 1855–1866.
- Basu, D.; Haswell, E.S. Plant Mechanosensitive Ion Channels: An Ocean of Possibilities. Curr. Opin. Plant Biol. 2017, 40, 43–48.
- Monshausen, G.B.; Gilroy, S. The Exploring Root—Root Growth Responses to Local Environmental Conditions. Curr. Opin. Plant Biol. 2009, 12, 766–772.
- Jacobsen, A.G.R.; Jervis, G.; Xu, J.; Topping, J.F.; Lindsey, K. Root Growth Responses to Mechanical Impedance Are Regulated by a Network of ROS, Ethylene and Auxin Signalling in Arabidopsis. New Phytol. 2021, 231, 225–242.
- Lee, H.; Kim, H.; Park, J.M.; Cho, H.S.; Jeon, J.H. PIN-mediated Polar Auxin Transport Facilitates Root−Obstacle Avoidance. New Phytol. 2020, 225, 1285–1296.
- Monshausen, G.B.; Bibikova, T.N.; Weisenseel, M.H.; Gilroy, S. Ca2+ Regulates Reactive Oxygen Species Production and PH during Mechanosensing in Arabidopsis Roots. Plant Cell 2009, 21, 2341–2356.
- Díaz-Sala, C. A Perspective on Adventitious Root Formation in Tree Species. Plants 2020, 9, 1789.
- Scippa, G.S.; Trupiano, D.; Rocco, M.; Di Iorio, A.; Chiatante, D. Unravelling the Response of Poplar (Populus nigra) Roots to Mechanical Stress Imposed by Bending. Plant Biosyst. 2008, 142, 401–413.
- Trupiano, D.; Rocco, M.; Renzone, G.; Scaloni, A.; Viscosi, V.; Chiatante, D.; Scippa, G.S. The Proteome of Populus nigra Woody Root: Response to Bending. Ann. Bot. 2012, 110, 415–432.
- Van Moerkercke, A.; Duncan, O.; Zander, M.; Šimura, J.; Broda, M.; Vanden Bossche, R.; Lewsey, M.G.; Lama, S.; Singh, K.B.; Ljung, K. A MYC2/MYC3/MYC4-Dependent Transcription Factor Network Regulates Water Spray-Responsive Gene Expression and Jasmonate Levels. Proc. Natl. Acad. Sci. USA 2019, 116, 23345–23356.
- Kloth, K.J.; Dicke, M. Rapid Systemic Responses to Herbivory. Curr. Opin. Plant Biol. 2022, 68, 102242.
- Walling, L.L. The Myriad Plant Responses to Herbivores. J. Plant Growth Regul. 2000, 19, 195–216.
- Hellgren, J.M.; Olofsson, K.; Sundberg, B. Patterns of Auxin Distribution during Gravitational Induction of Reaction Wood in Poplar and Pine. Plant Physiol. 2004, 135, 212–220.
- Funada, R.; Mizukami, E.; Kubo, T.; Fushitani, M.; Sugiyama, T. Distribution of Indole-3-Acetic Acid and Compression Wood Formation in the Stems of Inclined Cryptomeria Japonica. IAA Inclin. Cryptomeria Jpn. 1990, 44, 331–334.
- Du, S.; Uno, H.; Yamamoto, F. Roles of Auxin and Gibberellin in Gravity-Induced Tension Wood Formation in Aesculus turbinata Seedlings. IAWA J. 2004, 25, 337–347.
- Trupiano, D.; Di Iorio, A.; Montagnoli, A.; Lasserre, B.; Rocco, M.; Grosso, A.; Scaloni, A.; Marra, M.; Chiatante, D.; Scippa, G.S. Involvement of Lignin and Hormones in the Response of Woody Poplar Taproots to Mechanical Stress. Physiol. Plant. 2012, 146, 39–52.
- Trupiano, D.; Rocco, M.; Renzone, G.; Scaloni, A.; Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Chiatante, D.; Scippa, G.S. Poplar Woody Root Proteome during the Transition Dormancy-Active Growth. Plant Biosyst. 2013, 147, 1095–1100.
- Trupiano, D.; Rocco, M.; Renzone, G.; Scaloni, A.; Rossi, M.; Viscosi, V.; Chiatante, D.; Scippa, G.S. Temporal Analysis of Poplar Woody Root Response to Bending Stress. Physiol. Plant. 2014, 150, 174–193.
- Rossi, M.; Trupiano, D.; Tamburro, M.; Ripabelli, G.; Montagnoli, A.; Chiatante, D.; Scippa, G.S. MicroRNAs Expression Patterns in the Response of Poplar Woody Root to Bending Stress. Planta 2015, 242, 339–351.
- De Zio, E.; Trupiano, D.; Karady, M.; Antoniadi, I.; Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Ljung, K.; Scippa, G.S. Tissue-Specific Hormone Profiles from Woody Poplar Roots under Bending Stress. Physiol. Plant. 2019, 165, 101–113.
- Jonsson, K.; Lathe, R.S.; Kierzkowski, D.; Routier-Kierzkowska, A.-L.; Hamant, O.; Bhalerao, R.P. Mechanochemical Feedback Mediates Tissue Bending Required for Seedling Emergence. Curr. Biol. 2021, 31, 1154–1164.
- Dimitrova, A.; Sferra, G.; Scippa, G.S.; Trupiano, D. Network-Based Analysis to Identify Hub Genes Involved in Spatial Root Response to Mechanical Constrains. Cells 2022, 11, 3121.
- Chiatante, D.; Montagnoli, A.; Trupiano, D.; Sferra, G.; Bryant, J.; Rost, T.L.; Scippa, G.S. Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals? Cells 2021, 10, 2544.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
21 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




