
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | BENETTAYEB AS | -- | 1317 | 2023-02-17 17:35:55 | | | |
| 2 | BENETTAYEB AS | Meta information modification | 1317 | 2023-02-17 17:38:01 | | | | |
| 3 | Conner Chen | Meta information modification | 1317 | 2023-02-20 03:34:01 | | |
Video Upload Options
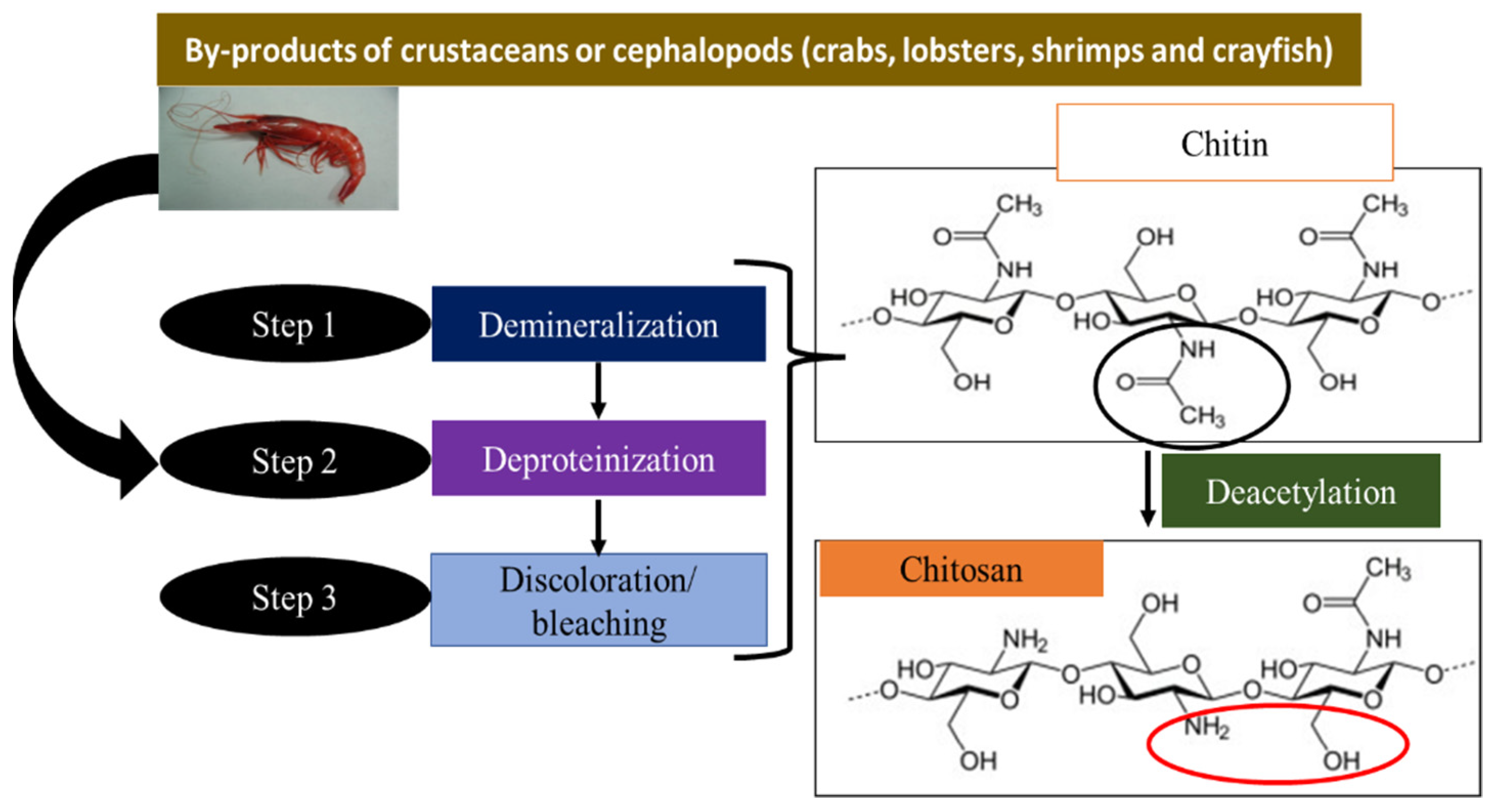
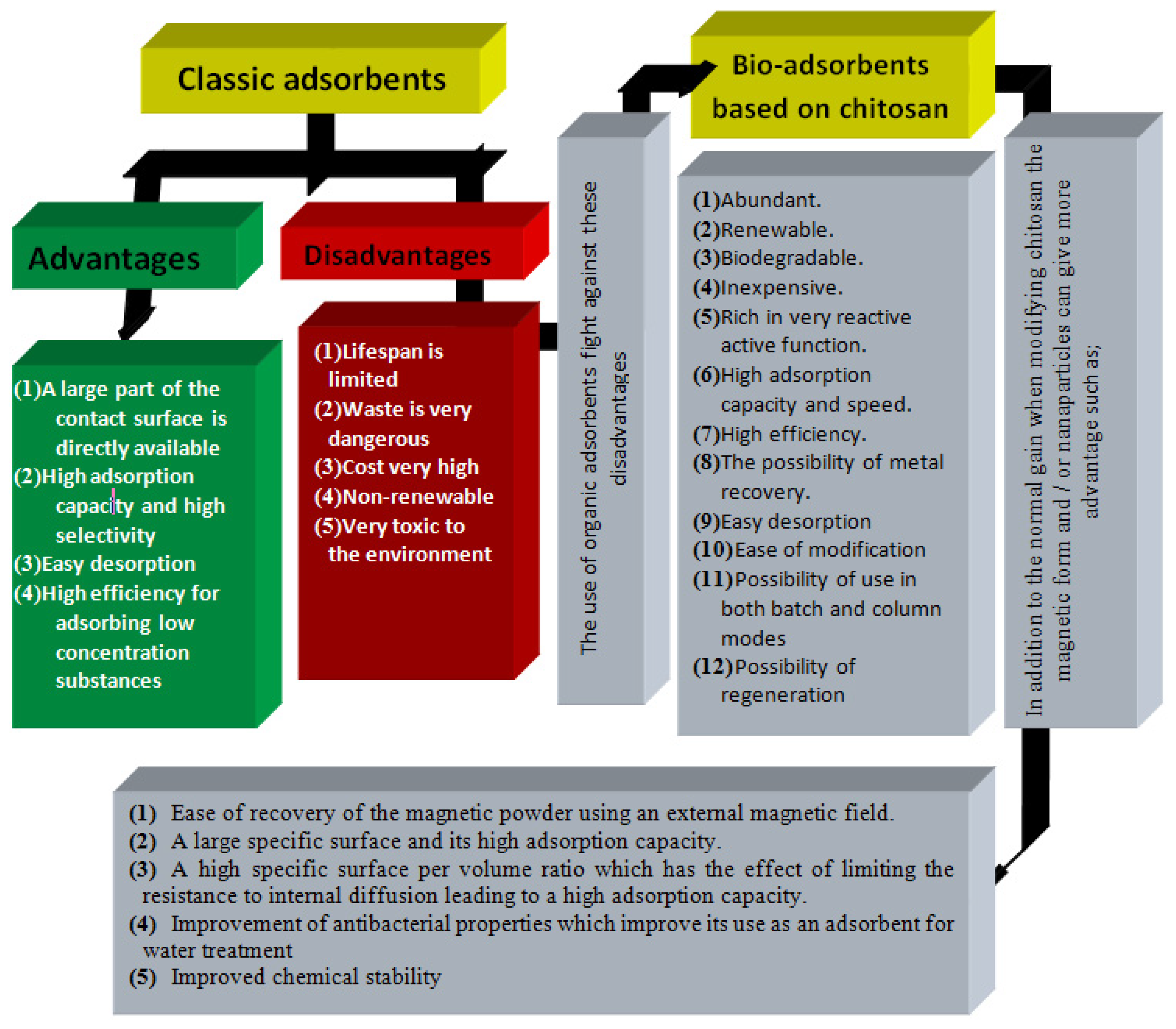
Adsorption is the most widely used technique for advanced wastewater treatment. The preparation and application of natural renewable and environmentally friendly materials makes this process easier and more profitable. Chitosan is often used as an effective biomaterial in the adsorption world because of its numerous functional applications. Chitosan is one of the most suitable and functionally flexible adsorbents because it contains hydroxyl (-OH) and amine (-NH2) groups. The adsorption capacity and selectivity of chitosan can be further improved by introducing additional functions into its basic structure. Owing to its unique surface properties and adsorption ability of chitosan, the development and application of chitosan nanomaterials has gained significant attention. The recovery of pollutants using magnetic nanoparticles is an important treatment process that has contributed to additional development and sustainable growth.
1. Introduction
2. Unique Properties of Chitosan Nanomaterials and Magnetic Chitosan
- (i)
-
The size of the nanomaterial is comparable to the Bohr radius of the excitons. This dramatically alters the optical, luminescent, and redox properties of nanomaterials compared to their bulk counterparts.
- (ii)
-
The surface area atom largely determined the thermodynamic properties of solids and also determines the melting temperature and structural transitions of nanomaterials.
- (iii)
-
When the particle size is decreased, the net internal cohesive forces increase. Thus, reducing the particle size increases the surface area to bulk volume ratio, i.e., particle size is inversely proportional to the surface area to bulk volume ratio [52].
References
- Usman, M.; Waseem, M.; Mani, N.; Andiego, G. Optimization of soil aquifer treatment by chemical oxidation with hydrogen peroxide addition. Pollution 2018, 4, 369–379.
- Gao, J.; Yuan, Y.; Yu, Q.; Yan, B.; Qian, Y.; Wen, J.; Ma, C.; Jiang, S.; Wang, X.; Wang, N. Bio-inspired antibacterial cellulose paper-poly(amidoxime) composite hydrogel for highly efficient uranium(vi) capture from seawater. Chem. Commun. 2020, 56, 3935–3938.
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322.
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115.
- Ahila, K.G.; Vasanthy, M.; Thamaraiselvi, C. Green Synthesis of Magnetic Iron Nanoparticle Using Moringa oleifera Lam Seeds and Its Application in Textile Effluent Treatment. In Utilization and Management of Bioresources; Springer: Singapore, 2018; pp. 315–324.
- Hamza, M.F.; Wei, Y.; Benettayeb, A.; Wang, X.; Guibal, E. Efficient removal of uranium, cadmium and mercury from aqueous solutions using grafted hydrazide-micro-magnetite chitosan derivative. J. Mater. Sci. 2019, 55, 4193–4212.
- Zhang, W.X. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332.
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414.
- Osagie, C.; Othmani, A.; Ghosh, S.; Malloum, A.; Kashitarash Esfahani, Z.; Ahmadi, S. Dyes adsorption from aqueous media through the nanotechnology: A review. J. Mater. Res. Technol. 2021, 14, 2195–2218.
- Rashtbari, Y.; Sher, F.; Afshin, S.; Hamzezadeh bahrami, A.; Ahmadi, S.; Azhar, O.; Rastegar, A.; Ghosh, S.; Poureshgh, Y. Green synthesis of zero-valent iron nanoparticles and loading effect on activated carbon for furfural adsorption. Chemosphere 2022, 287, 132114.
- Igwegbe, C.A.; Ighalo, J.O.; Ghosh, S.; Ahmadi, S.; Ugonabo, V.I. Pistachio (Pistacia vera) waste as adsorbent for wastewater treatment: A review. Biomass Convers. Biorefin. 2021.
- Thirunavukkarasu, A.; Nithya, R.; Sivashankar, R. A review on the role of nanomaterials in the removal of organic pollutants from wastewater. Rev. Environ. Sci. Biotechnol. 2020, 19, 751–778.
- Kurniawan, T.A.; Sillanpää, M.E.T.; Sillanpää, M. Nanoadsorbents for Remediation of Aquatic Environment: Local and Practical Solutions for Global Water Pollution Problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295.
- Ghosh, S.; Malloum, A.; Bornman, C.; Othmani, A.; Osagie, C.; Esfahani, Z.K.; Khanday, W.A.; Ahmadi, S.; Dehghani, M.H. Novel green adsorbents for removal of aniline from industrial effluents: A review. J. Mol. Liq. 2022, 345, 118167.
- Usman, M.; Belkasmi, A.I.; Katsoyiannis, I.A.; Ernst, M. Pre-deposited dynamic membrane adsorber formed of microscale conventional iron oxide-based adsorbents to remove arsenic from water: Application study and mathematical modeling. J. Chem. Technol. Biotechnol. 2021, 96, 1504–1514.
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance evaluation of small sized powdered ferric hydroxide as arsenic adsorbent. Water 2018, 10, 957.
- Benettayeb, A.; Morsli, A.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Recovery of Heavy Metal Ions Using Magnetic Glycine—Modified Chitosan—Application to Aqueous Solutions and Tailing Leachate. Appl. Sci. 2021, 11, 8377.
- Ghosh, S.; Malloum, A.; Igwegbe, C.A.; Ighalo, J.O.; Ahmadi, S.; Dehghani, M.H.; Othmani, A.; Gökkuş, Ö.; Mubarak, N.M. New generation adsorbents for the removal of fluoride from water and wastewater: A review. J. Mol. Liq. 2022, 346, 118257.
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z. Some Well-Known Alginate and Chitosan Modifications Used in Adsorption: A Review. Water J. 2022, 14, 1353.
- Benettayeb, A.; Usman, M.; Tinashe, C.C.; Haddou, B. A critical review with emphasis on recent pieces of evidence of Moringa oleifera biosorption in water and wastewater treatment. Environ. Sci. Pollut. Res. 2022, 29, 48185–48209.
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074.
- Shiju, N.R.; Guliants, V.V. Recent developments in catalysis using nanostructured materials. Appl. Catal. A Gen. 2009, 356, 1–17.
- Kim, K.; Jung, B.; Kim, J.; Kim, W. Effects of embedding non-absorbing nanoparticles in organic photovoltaics on power conversion efficiency. Sol. Energy Mater. Sol. Cells 2010, 94, 1835–1839.
- Phillips, J.; Bowen, W.; Cagin, E.; Wang, W. Electronic and Optoelectronic Devices Based on Semiconducting Zinc Oxide. In Comprehensive Semiconductor Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1–6, pp. 101–127. ISBN 9780444531537.
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130.
- Benettayeb, A.; Guibal, E.; Morsli, A.; Kessas, R. Chemical modification of alginate for enhanced sorption of Cd(II), Cu(II) and Pb(II). Chem. Eng. J. 2017, 316, 704–714.
- Benettayeb, A.; Guibal, E.; Bhatnagar, A.; Morsli, A.; Kessas, R. Effective removal of nickel (II) and zinc (II) in mono-compound and binary systems from aqueous solutions by application of alginate-based materials. Int. J. Environ. Anal. Chem. 2021, 1–22.
- Benettayeb, A.; Morsli, A.; Guibal, E.; Kessas, R. New derivatives of urea-grafted alginate for improving the sorption of mercury ions in aqueous solutions. Mater. Res. Express 2021, 8, 035303.
- Benettayeb, A.; Haddou, B. New biosorbents based on the seeds, leaves and husks powder of Moringa oleifera for the effective removal of various toxic pollutants. Int. J. Environ. Anal. Chem. 2021, 1–26.
- Hamza, M.F.; Fouda, A.; Wei, Y.; El Aassy, I.E.; Alotaibi, S.H.; Guibal, E.; Mashaal, N.M. Functionalized biobased composite for metal decontamination–Insight on uranium and application to water samples collected from wells in mining areas (Sinai, Egypt). Chem. Eng. J. 2022, 431, 133967.
- Hamza, M.F.; Khalafalla, M.S.; Wei, Y.; Hamad, N.A. Effect of bi-functionalization silica micro beads on uranium adsorption from synthetic and washing pregnant uranyl solutions. J. Radioanal. Nucl. Chem. 2021, 330, 191–206.
- Wang, J.; Sun, Y.; Zhao, X.; Chen, L.; Peng, S.; Ma, C.; Duan, G.; Liu, Z.; Wang, H.; Yuan, Y.; et al. A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater. E-Polymers 2022, 22, 399–410.
- Chen, Y.; Li, S.; Li, X.; Mei, C.; Zheng, J.; Shiju, E.; Duan, G.; Liu, K.; Jiang, S. Liquid Transport and Real-Time Dye Purification via Lotus Petiole-Inspired Long-Range-Ordered Anisotropic Cellulose Nanofibril Aerogels. ACS Nano 2021, 15, 20666–20677.
- Chen, Y.; Hanshe, M.; Sun, Z.; Zhou, Y.; Mei, C.; Duan, G.; Zheng, J.; Shiju, E.; Jiang, S. Lightweight and anisotropic cellulose nanofibril/rectorite composite sponges for efficient dye adsorption and selective separation. Int. J. Biol. Macromol. 2022, 207, 130–139.
- Gregorio-Jauregui, K.M.; Pineda, M.G.; Rivera-Salinas, J.E.; Hurtado, G.; Saade, H.; Martinez, J.L.; Ilyina, A.; López, R.G. One-step method for preparation of magnetic nanoparticles coated with chitosan. J. Nanomater. 2012, 2012, 4.
- Ngah, W.S.W.; Fatinathan, S. Pb(II) biosorption using chitosan and chitosan derivatives beads: Equilibrium, ion exchange and mechanism studies. J. Environ. Sci. 2010, 22, 338–346.
- Fan, L.; Zhang, Y.; Luo, C.; Lu, F.; Qiu, H.; Sun, M. Synthesis and characterization of magnetic β-cyclodextrin-chitosan nanoparticles as nano-adsorbents for removal of methyl blue. Int. J. Biol. Macromol. 2012, 50, 444–450.
- Chen, W.; Mo, J.; Du, X.; Zhang, Z.; Zhang, W. Biomimetic dynamic membrane for aquatic dye removal. Water Res. 2019, 151, 243–251.
- Pal, P.; Pal, A. Methylene blue removal: An approach towards sludge management after adsorption of cadmium onto surfactant modified chitosan beads. J. Indian Chem. Soc. 2018, 95, 357–364.
- Fu, C.C.; Tran, H.N.; Chen, X.H.; Juang, R.S. Preparation of polyaminated Fe3O4@chitosan core-shell magnetic nanoparticles for efficient adsorption of phosphate in aqueous solutions. J. Ind. Eng. Chem. 2020, 83, 235–246.
- Pap, S.; Kirk, C.; Bremner, B.; Turk Sekulic, M.; Gibb, S.W.; Maletic, S.; Taggart, M.A. Synthesis optimisation and characterisation of chitosan-calcite adsorbent from fishery-food waste for phosphorus removal. Environ. Sci. Pollut. Res. 2020, 27, 9790–9802.
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281.
- Qiu, M.; Hu, B.; Chen, Z.; Yang, H.; Zhuang, L.; Wang, X. Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 2021, 3, 117–123.
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent advances in metal-organic framework membranes for water treatment: A review. Sci. Total Environ. 2021, 800, 149662.
- Saxena, R.; Saxena, M.; Lochab, A. Recent Progress in Nanomaterials for Adsorptive Removal of Organic Contaminants from Wastewater. ChemistrySelect 2020, 5, 335–353.
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242.
- Moradi Dehaghi, S.; Rahmanifar, B.; Moradi, A.M.; Azar, P.A. Removal of permethrin pesticide from water by chitosan-zinc oxide nanoparticles composite as an adsorbent. J. Saudi Chem. Soc. 2014, 18, 348–355.
- Crini, G. Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour. Technol. 2003, 90, 193–198.
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70.
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447.
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25.
- Azzaza, S.; Kumar, R.T.; Vijaya, J.J.; Bououdina, M. CHAPTER 7: Nanomaterials for Heavy Metal Removal. In Biological Fluid-Surface Interactions in Detection and Medical Devices; Royal Society of Chemistry: London, UK, 2017; ISBN 9781782620976.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074.
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49.
- Tuutijärvi, T.; Lu, J.; Sillanpää, M.; Chen, G. As(V) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009, 166, 1415–1420.
- Zhou, L.; Liu, Z.; Liu, J.; Huang, Q. Adsorption of Hg(II) from aqueous solution by ethylenediamine-modified magnetic crosslinking chitosan microspheres. Desalination 2010, 258, 41–47.
- Chang, Y.C.; Chang, S.W.; Chen, D.H. Magnetic chitosan nanoparticles: Studies on chitosan binding and adsorption of Co(II) ions. React. Funct. Polym. 2006, 66, 335–341.
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10.
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chemie-Int. Ed. 2007, 46, 1222–1244.




