Adsorption is the most widely used technique for advanced wastewater treatment. The preparation and application of natural renewable and environmentally friendly materials makes this process easier and more profitable. Chitosan is often used as an effective biomaterial in the adsorption world because of its numerous functional applications. Chitosan is one of the most suitable and functionally flexible adsorbents because it contains hydroxyl (-OH) and amine (-NH2) groups. The adsorption capacity and selectivity of chitosan can be further improved by introducing additional functions into its basic structure. Owing to its unique surface properties and adsorption ability of chitosan, the development and application of chitosan nanomaterials has gained significant attention. The recovery of pollutants using magnetic nanoparticles is an important treatment process that has contributed to additional development and sustainable growth.
1. Introduction
The diversity and complexity of pollutants greatly affect the efficiency of wastewater treatment
[1]. To overcome this limitation, extensive research has focused on the development of biosorbents
[2][3][2,3] and their variable applications with the help of nanotechnology
[3][4][3,4]. Nanotechnology exploits the properties of any material at the nanoscale. The materials of this new technology are termed nanoparticles (NPs)
[5]. Nanotechnology is an ideal solution to ensure high quality water. It can be considered a powerful 21st century tool for protecting the environment and improving environmental quality
[6]. The application of nanotechnology in water purification and environmental sanitation has potential, as conventional methods do not always provide cost-effective solutions for the removal of common pollutants. Conventional technologies have a limited lifetime, generate hazardous and toxic environmental wastes, and are non-renewable. For several years, NPs have been the subject of numerous research publications, and patents, because of their large surface area, high resistance to heat and chemicals, and high adsorption capacity for the removal of organic and inorganic contaminants
[7][8][9][10][11][7,8,9,10,11].
Adsorption technology is one of the most reliable strategies in wastewater treatment, and the use of a variety of nanosized adsorbents enables preferential surface adsorption
[12][13][14][15][16][12,13,14,15,16]. The increase in surface area can increase the sorption capacity towards pollutants on the surface of NPs
[6][17][18][19][20][6,17,18,19,20]. In addition to wastewater treatment, NPs are used as antimicrobial agents
[21], as catalysts
[22], in biomedicine, energy conversion
[23], agriculture, electronics, and optoelectronics industries
[24]. According to Vakili et al. (2014), nano-chitosan is one such nanomaterial that is a natural substance with excellent physicochemical properties and is harmless to humans
[25]. Therefore, chitosan biopolymer has become the environmentally friendly substance of choice. Several modifications have been carried out on the alginate for the introduction of the amine functional group (-NH
2) on its surface
[26][27][28][26,27,28], or other biosorbents by introducing other actives functions
[29][30][31][32][33][34][29,30,31,32,33,34], this active site is found naturally in chitosan. Chitosan is rich in amino (-NH
2) and hydroxyl (-OH) groups, which gives it a powerful adsorption capacity and reactivity to most pollutants
[35][36][37][38][35,36,37,38]. Thus, chitosan is an excellent natural adsorbent that can be modified to increase its efficiency and improve its basic properties
[6][17][39][40][41][6,17,39,40,41].
The main problem with using chitosan in its natural form is its low adsorption capacity, which can be improved by physical or chemical modification. Hence, researchers have developed more effective chitosan-based adsorbents. Chitosan NPs are among the best nano-adsorbents due to their large surface area, high adsorption capacity, and environmental friendliness. Chitosan is abundant in nature, reusable, and can be easily modified with various chemical and biological agents so that it can be regenerated and reused over several cycles. Chitosan NPs can be categorised as nano adsorbents that meet the essential criteria for use in wastewater treatment. Chitosan NPs can be chemically inert, and their morphology resists various complex conditions.
The preparation of chitosan NPs cannot only improve the surface area and adsorption capacity, but the presence of functional groups also makes it selective
[42][43][44][42,43,44]. Since chitosan is biodegradable, it does not cause additional environmental pollution. Apart from its ecological nature, it also has antibacterial properties that enhance its use as an adsorbent for water treatment. According to Saxena et al. (2020), it needs chemical modification using chemical cross-linking to increase its stability over time
[45].
2. Unique Properties of Chitosan Nanomaterials and Magnetic Chitosan
Chitosan (poly [β-(1-4)-2-amino-2-deoxy-D-glucopyranose]) is a linear cationic biopolymer with high molecular weight. The primary source of usable chitosan is the deacetylation of chitin obtained from the shells of crustaceans (crabs, lobsters, shrimp, and crayfish)
[46], which are an abundant natural resource. The natural material is commercially obtained from the deacetylation of chitin by thermochemical treatment
[35]. Natural chitosan is non-toxic, non-allergenic, biodegradable, biocompatible, inexpensive, hydrophilic, biologically active, and can form fibers and films
[47][48][49][50][47,48,49,50].
Figure 1 summarizes the chemical process to obtain chitosan from chitin. Chitosan is a semi-crystalline cationic polysaccharide that attracts positively charged molecules and enhances bonding due to the presence of the –NH
2 group. In addition, the –OH group is also present in the structure and helps to increase the adsorption capacity
[51].
Figure 1.
Different stages of the chitosan manufacturing process.
Nano-sized particles have characteristics that cannot be achieved with solid, normal-sized material. For example, the electronic and optical properties of metallic materials can be modified by controlling their size below the Bohr radius (usually between 1–10 nm). The interest in NPs is due to their ability to act as an effective bridge between solid materials and atomic structures. Solid materials exhibit constant physical properties, regardless of their size and mass. However, NPs have properties that depend on their size due to the large proportion of atoms on their surfaces relative to their volume, resulting in a large specific surface area. In view of this, the electronic, optical, and magnetic properties of materials change as their size decreases towards the nanoscale. Therefore, controlling the size of NPs is of particular interest because it can influence their properties. The exceptional physicochemical properties of nanomaterials are due to three main reasons:
- (i)
-
The size of the nanomaterial is comparable to the Bohr radius of the excitons. This dramatically alters the optical, luminescent, and redox properties of nanomaterials compared to their bulk counterparts.
-
- (ii)
-
The surface area atom largely determined the thermodynamic properties of solids and also determines the melting temperature and structural transitions of nanomaterials.
-
- (iii)
-
When the particle size is decreased, the net internal cohesive forces increase. Thus, reducing the particle size increases the surface area to bulk volume ratio, i.e., particle size is inversely proportional to the surface area to bulk volume ratio
[52].
-
In general, nanotechnology is used to produce materials with specific properties and a high degree of reproducibility. In this regard, researchers are currently focusing on the synthesis of new nanostructured materials capable of cleaning the environment; they know that nanomaterials are entirely or partially composed of nano-objects, which gives them improved or specific properties in nanometric dimensions. In the family of nanomaterials, there are three types, namely NPs, nano-fibers, and nano-films. NPs are elements with a nanometric size between 1–100 nm
[53] and are used daily in products such as cosmetics, paints, electronics, and computers. NPs can be in the form of powders, suspensions, solutions, or gels from which other physical forms, such as nano-beads are formed.
In recent years, researchers have focused on the use of NPs, particularly magnetic NPs, as effective adsorbents for the treatment of pollutants present in wastewater
[54][55][54,55].
Magnetic NPs have been used as adsorbents for water treatment. These adsorbents have remarkable properties such as nanometric size, high specific surface area per volume ratio, and resistance to internal diffusion leading to a high adsorption capacity
[56][57][56,57], biocompatibility, biodegradability and low toxicity
[58], low cost of fabrication, green sources, magnetic intensity, so, for these reasons that chitosan magnetic received considerable attention.
In addition, iron oxide NPs have the advantage of being superpara-magnetic
[59] and the powder can be easily recovered using an external magnetic field. Magnetic separation technology is easy to use and preferable to avoid slow separation techniques such as filtration, centrifugation, and precipitation. Some important characteristics of nano-adsorbents compared to classical ones are summarized in
Figure 2.
Figure 2. Advantages and disadvantages of NPs and use of chitosan NPs to overcome the disadvantages of traditional NPs in wastewater treatment.
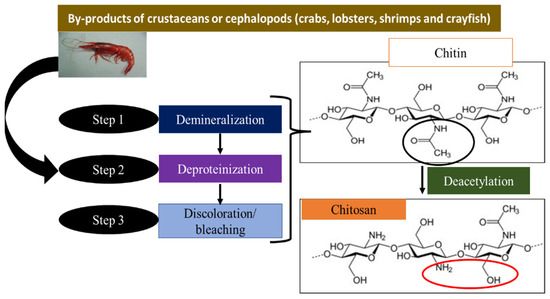
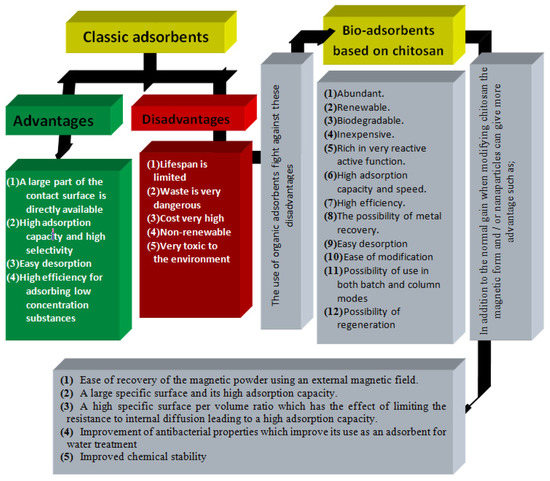 Figure 2. Advantages and disadvantages of NPs and use of chitosan NPs to overcome the disadvantages of traditional NPs in wastewater treatment.
Figure 2. Advantages and disadvantages of NPs and use of chitosan NPs to overcome the disadvantages of traditional NPs in wastewater treatment.