
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fangyuan Hu | -- | 5482 | 2023-02-17 02:05:00 | | | |
| 2 | Lindsay Dong | -1 word(s) | 5481 | 2023-02-17 06:31:33 | | | | |
| 3 | Lindsay Dong | -6 word(s) | 5475 | 2023-02-20 08:26:27 | | |
Video Upload Options
Lithium–sulfur (Li-S) batteries are considered as among the most promising electrochemical energy storage devices due to their high theoretical energy density and low cost. However, the inherently complex electrochemical mechanism in Li-S batteries leads to problems such as slow internal reaction kinetics and a severe shuttle effect, which seriously affect the practical application of batteries.
1. Introduction
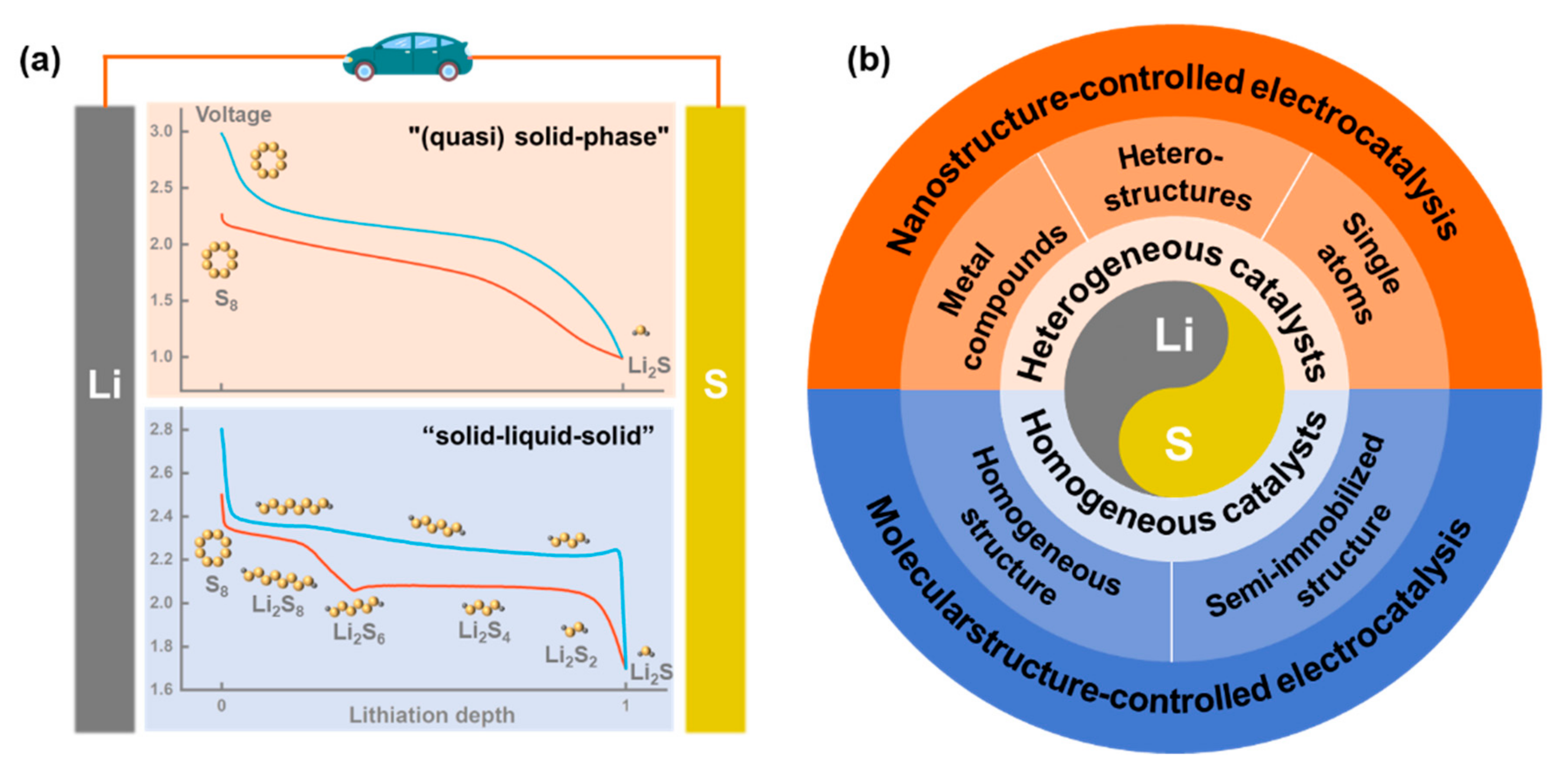
2. Heterogeneous Catalysts
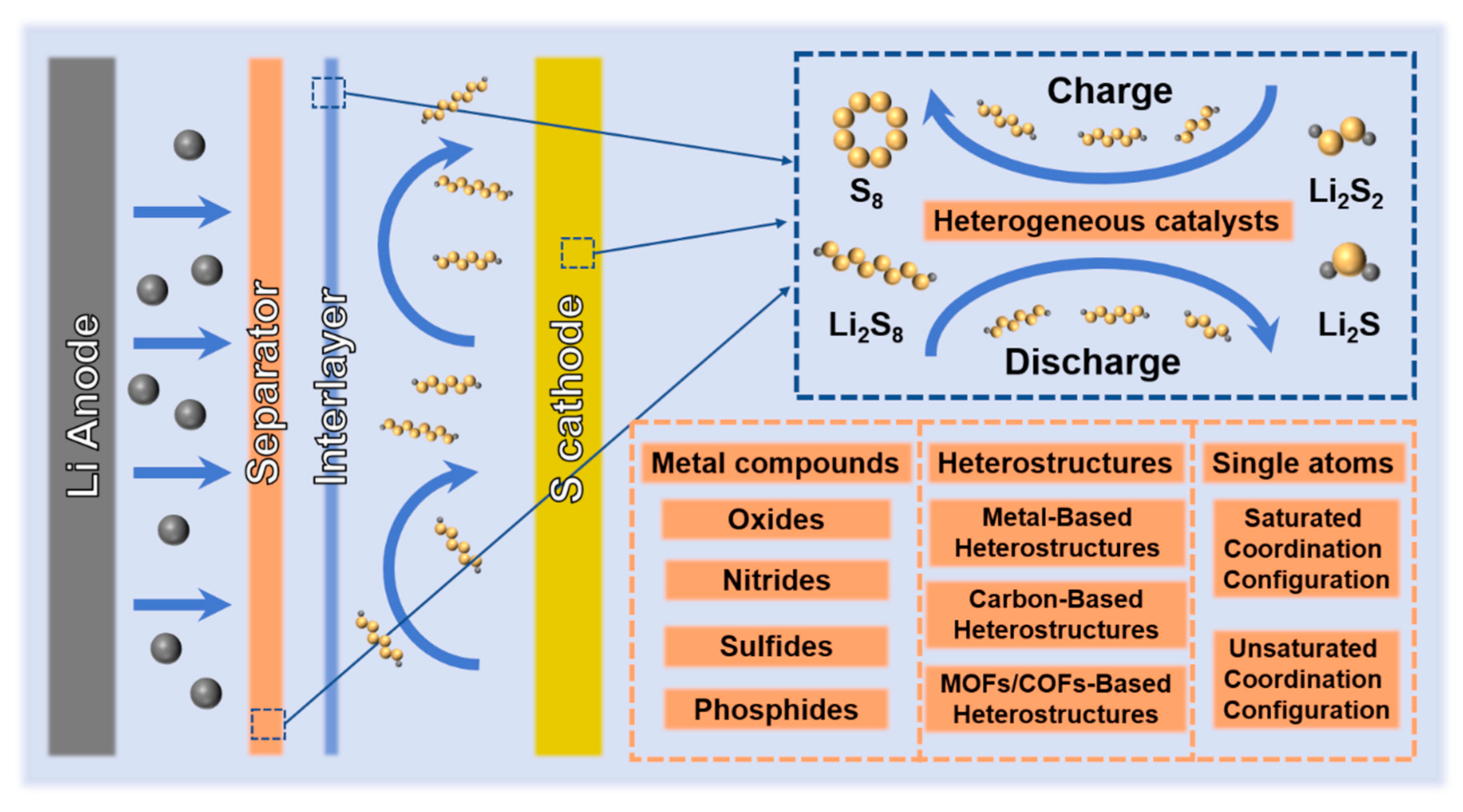
2.1. Metal Compounds
2.1.1. Metal Oxide
2.1.2. Metal Nitride
2.1.3. Metal Sulfides
2.1.4. Metal Phosphides
2.2. Heterostructures
2.2.1. Metal-Based Heterostructures
2.2.2. Carbon-Based Heterostructures
Carbon materials have become among the most used substrates in heterostructures because of their diverse types, rich structures, good conductivity, and large specific surface area [37][91]. Common carbon-based heterostructures refer to hybrid materials that combine carbon materials with other materials (mainly metal compounds). Commonly used carbon materials mainly include CNTs, graphene and activated carbon [92][93]. Compared with metal-based heterostructures, carbon-based heterostructures have more abundant synthesis methods and more diverse structures. In addition, the good processability of carbon materials also provides a broader space for the application of carbon-based heterostructures. Aiming at the problem of good adsorption but poor conductivity of VO2, in situ growth of VO2 on the surface of 2D reduced graphene oxide (rGO) by solvothermal method to construct heterostructures is a good solution [94]. Compared with the single component, the reaction kinetics of VO2@rGO is significantly improved, and the diffusion of Li+ is smoother. This is achieved by VO2 with catalytic properties and 2D rGO with good conductivity, which reflects the superiority of the heterostructure. A heterostructure composed of CNTs and MoP2 NPs was combined and applied to the interlayer of Li-S batteries [95]. Benefit from the synergistic effect of the heterostructure, the cycle performance of batteries is improved.
2.2.3. MOFs/COFs-Based Heterostructures
2.3. Single Atoms
2.3.1. Saturated Coordination Configuration
3. Effect of Nanostructure on Heterogeneous Electrocatalysts
4. Homogeneous Electrocatalysts
4.1. Homogeneous-Type Electrocatalysts
4.2. Semi-Immobilized-Type Electrocatalysts
5. Effect of Molecular Structure on Homogeneous Electrocatalysts
5.1. Building Fast Electronic Pathway Types
5.2. Change the Electrochemical Reaction Mechanism Types
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22.
- Bruce, D.; Haresh, K.; Jean, M.T. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
- Schmidt, O.; Hawkes, A.; Gambhir, A.; Staffell, I. The future cost of electrical energy storage based on experience rates. Nat. Energy 2017, 2, 17110.
- Alexandra, K.S. The age of Li-ion batteries. Joule 2019, 3, 2583–2584.
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494.
- Zhang, M.; Chen, W.; Xue, L.; Jiao, Y.; Lei, T.; Chu, J.; Huang, J.; Gong, C.; Yan, C.; Yan, Y.; et al. Adsorption-catalysis design in the lithium-sulfur battery. Adv. Energy Mater. 2020, 10, 1903008.
- Liu, T.F.; Hu, H.L.; Ding, X.F.; Yuan, H.D.; Jin, C.B.; Nai, J.W.; Liu, Y.J.; Wang, Y.; Wan, Y.H.; Tao, X.Y. 12 years roadmap of the sulfur cathode for lithium sulfur batteries (2009–2020). Energy Storage Mater. 2020, 30, 346–366.
- Kaiser, M.R.; Han, Z.J.; Liang, J.; Dou, S.X.; Wang, J.Z. Lithium sulfide-based cathode for lithium-ion/sulfur battery: Recent progress and challenges. Energy Storage Mater. 2019, 19, 1–15.
- Harks, P.P.R.M.L.; Robledo, C.B.; Verhallen, T.W.; Notten, P.H.L.; Mulder, F.M. The significance of elemental sulfur dissolution in liquid electrolyte lithium sulfur batteries. Adv. Energy Mater. 2017, 7, 1601635.
- Wang, J.L.; He, Y.S.; Yang, J. Sulfur-based composite cathode materials for high-energy rechargeable lithium batteries. Adv. Mater. 2015, 27, 569–575.
- Zhao, X.H.; Wang, C.L.; Li, Z.W.; Hu, X.C.; Razzaq, A.A.; Deng, Z. Sulfurized polyacrylonitrile for high-performance lithium sulfur batteries: Advances and prospects. J. Mater. Chem. A 2021, 9, 19282–19297.
- Yin, L.C.; Wang, J.L.; Lin, F.J.; Yang, J.; Nuli, Y. Polyacrylonitrile/graphene composite as a precursor to a sulfur-based cathode material for high-rate rechargeable Li–S batteries. Energy Environ. Sci. 2012, 5, 6966–6972.
- Wang, X.; Qian, Y.; Wang, L.; Yang, H.; Li, H.; Zhao, Y.; Liu, T. Sulfurized polyacrylonitrile cathodes with high compatibility in both ether and carbonate electrolytes for ultrastable lithium–sulfur batteries. Adv. Funct. Mater. 2019, 29, 1902929.
- He, B.; Rao, Z.; Cheng, Z.; Liu, D.; He, D.; Chen, J.; Miao, Z.; Yuan, L.; Li, Z.; Huang, Y. Rationally design a sulfur cathode with solid-phase conversion mechanism for high cycle-stable Li–S batteries. Adv. Energy Mater. 2021, 11, 2003690.
- Zhang, X.; Xie, H.; Kim, C.S.; Zaghib, K.; Mauger, A.; Julien, C.M. Advances in lithium—Sulfur batteries. Mater. Sci. Eng. R Rep. 2017, 121, 1–29.
- Tang, T.; Hou, Y. Chemical confinement and utility of lithium polysulfides in lithium sulfur batteries. Small Methods 2019, 4, 1900001.
- Guo, J.L.; Pei, H.Y.; Dou, Y.; Zhao, S.Y.; Shao, G.S.; Liu, J.P. Rational designs for lithium-sulfur batteries with low electrolyte/sulfur ratio. Adv. Funct. Mater. 2021, 31, 2010499.
- Xu, Z.L.; Kim, J.K.; Kang, K. Carbon nanomaterials for advanced lithium sulfur batteries. Nano Today 2018, 19, 84–107.
- Fan, L.L.; Deng, N.P.; Yan, J.; Li, Z.H.; Kang, W.M.; Cheng, B.W. The recent research status quo and the prospect of electrolytes for lithium sulfur batteries. Chem. Eng. J. 2019, 369, 874–897.
- Chung, S.H.; Manthiram, A. Current status and future prospects of metal–sulfur batteries. Adv. Mater. 2019, 31, 1901125.
- Huang, L.; Li, J.; Liu, B.; Li, Y.H.; Shen, S.H.; Deng, S.J.; Lu, C.W.; Zhang, W.K.; Xia, Y.; Pan, G.X.; et al. Electrode design for lithium–sulfur batteries: Problems and solutions. Adv. Funct. Mater. 2020, 30, 1910375.
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162.
- Li, F.; Liu, Q.H.; Hu, J.W.; Feng, Y.Z.; He, P.B.; Ma, J.M. Recent advances in cathode materials for rechargeable lithium–sulfur batteries. Nanoscale 2019, 11, 15418–15439.
- Fan, X.J.; Sun, W.W.; Meng, F.C.; Xing, A.M.; Liu, J.H. Advanced chemical strategies for lithium–sulfur batteries: A review. Green Energy Environ. 2018, 3, 2–19.
- Hu, A.; Zhou, M.; Lei, T.; Hu, Y.; Du, X.; Gong, C.; Shu, C.; Long, J.; Zhu, J.; Chen, W.; et al. Optimizing redox reactions in aprotic lithium–sulfur batteries. Adv. Energy Mater. 2020, 10, 2002180.
- Miao, Z.Y.; Li, Y.P.; Xiao, X.P.; Sun, Q.Z.; He, B.; Chen, X.; Liao, Y.Q.; Zhang, Y.; Yuan, L.X.; Yan, Z.J.; et al. Direct optical fiber monitor on stress evolution of the sulfur-based cathodes for lithium–sulfur batteries. Energy Environ. Sci. 2022, 15, 2029–2038.
- Zhang, S.S. Understanding of sulfurized polyacrylonitrile for superior performance lithium/sulfur battery. Energies 2014, 7, 4588–4600.
- Li, G.R.; Wang, S.; Zhang, Y.N.; Li, M.; Chen, Z.W.; Lu, J. Revisiting the role of polysulfides in lithium–sulfur batteries. Adv. Mater. 2018, 30, 1705590.
- Li, T.; Bai, X.; Gulzar, U.; Bai, Y.J.; Capiglia, C.; Deng, W.; Zhou, X.F.; Liu, Z.P.; Feng, Z.F.; Zaccaria, R.P. A comprehensive understanding of lithium–sulfur battery technology. Adv. Funct. Mater. 2019, 29, 1901730.
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon materials for lithium sulfur batteries—Ten critical questions. Chem. Eur. J. 2016, 22, 7324.
- Chung, S.H.; Chang, C.H.; Manthiram, A. Progress on the critical parameters for lithium–sulfur batteries to be practically viable. Adv. Funct. Mater. 2018, 28, 1801188.
- Shen, X.; Liu, H.; Cheng, X.B.; Yan, C.; Huang, J.Q. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes. Energy Storage Mater. 2018, 12, 161–175.
- Wei, Z.H.; Ren, Y.Q.; Sokolowski, J.; Zhu, X.D.; Wu, G. Mechanistic understanding of the role separators playing in advanced lithium-sulfur batteries. InfoMat 2020, 2, 483–508.
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in lithium–sulfur batteries: From academic research to commercial viability. Adv. Mater. 2021, 33, 2003666.
- Zhang, L.L.; Wang, Y.J.; Niu, Z.Q.; Chen, J. Advanced nanostructured carbon-based materials for rechargeable lithium-sulfur batteries. Carbon 2019, 141, 400–416.
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L.Q. Nanostructured metal oxides and sulfides for lithium–sulfur batteries. Adv. Mater. 2017, 29, 1601759.
- Huang, S.Z.; Wang, Z.H.; Lim, Y.V.; Wang, Y.; Li, Y.; Zhang, D.H.; Yang, H.Y. Recent advances in heterostructure engineering for lithium–sulfur batteries. Adv. Energy Mater. 2021, 11, 2003689.
- Li, S.Q.; Fan, Z.Y. Encapsulation methods of sulfur particles for lithium-sulfur batteries: A review. Energy Storage Mater. 2021, 34, 107–127.
- Versaci, D.; Cozzarin, M.; Amici, J.; Francia, C.; Leiva, E.P.M.; Visintin, A.; Bodoardo, S. Influence of synthesis parameters on g-C3N4 polysulfides trapping: A systematic study. Appl. Mater. Today 2021, 25, 101169.
- Versaci, D.; Canale, I.; Goswami, S.; Amici, J.; Francia, C.; Fortunato, E.; Martins, R.; Pereira, L.; Bodoardo, S. Molybdenum disulfide/polyaniline interlayer for lithium polysulphide trapping in lithium-sulphur batteries. J. Power Sources 2022, 521, 230945.
- Chen, W.; Lei, T.Y.; Wu, C.Y.; Deng, M.; Gong, C.H.; Hu, K.; Ma, Y.C.; Dai, L.P.; Lv, W.Q.; He, W.D.; et al. Designing safe electrolyte systems for a high-stability lithium–sulfur battery. Adv. Energy Mater. 2018, 8, 1702348.
- Umeshbabu, E.; Zheng, B.Z.; Yang, Y. Recent progress in all-solid-state lithium−sulfur batteries using high Li-ion conductive solid electrolytes. Electrochem. Energy Rev. 2019, 2, 199–230.
- Zhao, H.J.; Deng, N.P.; Yan, J.; Kang, W.M.; Ju, J.G.; Ruan, Y.L.; Wang, X.Q.; Zhuang, X.P.; Li, Q.X.; Cheng, B.W. A review on anode for lithium-sulfur batteries: Progress and prospects. Chem. Eng. J. 2018, 347, 343–365.
- Bonnick, P.; Muldoon, J. The Dr Jekyll and Mr Hyde of lithium sulfur batteries. Energy Environ. Sci. 2020, 13, 4808–4833.
- Lim, W.G.; Kim, S.; Jo, C.; Lee, J. A comprehensive review of materials with catalytic effects in Li–S batteries: Enhanced redox kinetics. Angew. Chem. Int. Ed. 2019, 58, 18746.
- Shi, Z.X.; Li, M.; Sun, J.Y.; Chen, Z.W. Defect engineering for expediting Li–S chemistry: Strategies, mechanisms, and perspectives. Adv. Energy Mater. 2021, 11, 2100332.
- Wang, P.; Xi, B.J.; Huang, M.; Chen, W.H.; Feng, J.K.; Xiong, S.L. Emerging catalysts to promote kinetics of lithium–sulfur batteries. Adv. Energy Mater. 2021, 11, 2002893.
- Zhang, Z.W.; Peng, H.J.; Zhao, M.; Huang, J.Q. Heterogeneous/homogeneous mediators for high-energy-density lithium–sulfur batteries: Progress and prospects. Adv. Funct. Mater. 2018, 28, 1707536.
- Teixeira, M.F.S.; Olean-Oliveira, A.; Anastácio, F.C.; David-Parra, D.N.; Cardoso, C.X. Electrocatalytic reduction of CO2 in water by a palladium-containing metallopolymer. Nanomaterials 2022, 12, 1193.
- Wang, X.Q.; Li, Z.J.; Qu, Y.T.; Yuan, T.W.; Wang, W.Y.; Wu, Y.; Li, Y.D. Review of metal catalysts for oxygen reduction reaction: From nanoscale engineering to atomic design. Chem 2019, 5, 1486–1511.
- Peng, Y.; Liao, Y.; Ye, D.; Meng, Z.; Wang, R.; Zhao, S.; Tian, T.; Tang, H. Recent advances regarding precious metal-based electrocatalysts for acidic water splitting. Nanomaterials 2022, 12, 2618.
- Al Salem, H.; Babu, G.; Rao, C.V.; Arava, L.M.R. Electrocatalytic polysulfide traps for controlling redox shuttle process of Li-S batteries. J. Am. Chem. Soc. 2015, 137, 11542–11545.
- Fan, C.Y.; Xiao, P.; Li, H.G.; Wang, H.F.; Zhang, L.L.; Sun, H.Z.; Wu, X.L.; Xie, H.M.; Zhang, J.P. Nanoscale polysulfides reactors achieved by chemical Au–S interaction: Improving the performance of Li–S batteries on the electrode level. ACS Appl. Mater. Interfaces 2015, 7, 27959–27967.
- Mei, J.; Liao, T.; Kou, L.Z.; Sun, Z.Q. Two-dimensional metal oxide nanomaterials for next-generation rechargeable batteries. Adv. Mater. 2017, 29, 1700176.
- Liu, Y.T.; Liu, S.; Li, G.R.; Yan, T.Y.; Gao, X.P. High volumetric energy density sulfur cathode with heavy and catalytic metal oxide host for lithium–sulfur battery. Adv. Sci. 2020, 7, 1903693.
- Zheng, C.; Niu, S.Z.; Lv, W.; Zhou, G.M.; Li, J.; Fan, S.X.; Deng, Y.Q.; Pan, Z.Z.; Li, B.H.; Kang, F.Y.; et al. Propelling polysulfides transformation for high-rate and long-life lithium–sulfur batteries. Nano Energy 2017, 33, 306–312.
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759.
- Han, H.; Wang, T.; Zhang, Y.; Nurpeissova, A.; Bakenov, Z. Three-dimensionally ordered macroporous ZnO framework as dual-functional sulfur host for high-efficiency lithium–sulfur batteries. Nanomaterials 2020, 10, 2267.
- Zhang, Z.; Yi, Z.; Liu, L.; Yang, J.; Zhang, C.; Pan, X.; Chi, F. 3D hollow rGO microsphere decorated with ZnO nanoparticles as efficient sulfur host for high-performance Li-S battery. Nanomaterials 2020, 10, 1633.
- Dong, C.W.; Gao, W.; Jin, B.; Jiang, Q. Advances in cathode materials for high-performance lithium-sulfur batteries. iScience 2018, 6, 151–198.
- He, B.W.; Wang, Z.Y.; Li, G.R.; Liu, S.; Gao, X.P. Perovskite transition metal oxide of nanofibers as catalytic hosts for lithium–sulfur battery. J. Alloys Compd. 2022, 918, 165660.
- Zhang, Y.P.; Gu, R.; Zheng, S.; Liao, K.X.; Shi, P.H.; Fan, J.C.; Xu, Q.J.; Min, Y.L. Long-life Li–S batteries based on enabling the immobilization and catalytic conversion of polysulfides. J. Mater. Chem. A 2019, 7, 21747–21758.
- Ni, L.B.; Wu, Z.; Zhao, G.J.; Sun, C.Y.; Zhou, C.Q.; Gong, X.X.; Diao, G.W. Core–shell structure and interaction mechanism of γ-MnO2 coated sulfur for improved lithium-sulfur batteries. Small 2017, 13, 1603466.
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682.
- Guo, X.; Bi, X.; Zhao, J.; Yu, X.; Dai, H. Tunnel structure enhanced polysulfide conversion for inhibiting “shuttle effect” in lithium-sulfur battery. Nanomaterials 2022, 12, 2752.
- Zukalová, M.; Vinarčíková, M.; Bouša, M.; Kavan, L. Nanocrystalline TiO2/carbon/sulfur composite cathodes for lithium–sulfur battery. Nanomaterials 2021, 11, 541.
- Yang, J.B.; Xu, L.Y.; Li, S.Z.; Peng, C. The role of titanium-deficient anatase TiO2 interlayers in boosting lithium–sulfur battery performance: Polysulfide trapping, catalysis and enhanced lithium ion transport. Nanoscale 2020, 12, 4645–4654.
- Chen, Z.J.; Lv, W.; Kang, F.Y.; Li, J. Theoretical investigation of the electrochemical performance of transition metal nitrides for lithium–sulfur batteries. J. Phys. Chem. C 2019, 123, 25025.
- Zhong, Y.; Xia, X.H.; Shi, F.; Zhan, J.Y.; Tu, J.P.; Fan, H.J. Transition metal carbides and nitrides in energy storage and conversion. Adv. Sci. 2016, 3, 1500286.
- Wang, H.; Li, J.M.; Li, K.; Lin, Y.P.; Chen, J.M.; Gao, L.J.; Nicolosi, V.; Xiao, X.; Lee, J.M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390.
- Sun, Z.H.; Zhang, J.Q.; Yin, L.C.; Hu, G.J.; Fang, R.P.; Cheng, H.M.; Li, F. Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium-sulfur batteries. Nat. Commun. 2017, 8, 14627.
- Cui, Z.M.; Zu, C.X.; Zhou, W.D.; Manthiram, A.; Goodenough, J.B. Mesoporous titanium nitride-enabled highly stable lithium-sulfur batteries. Adv. Mater. 2016, 28, 6926–6931.
- Liu, H.H.; Shen, H.J.; Li, R.R.; Liu, S.Q.; Turak, A.; Yang, M.H. Tungsten-nitride-coated carbon nanospheres as a sulfur host for high-performance lithium-sulfur batteries. ChemElectroChem 2019, 6, 2074.
- Zubair, U.; Bianco, S.; Amici, J.; Francia, C.; Bodoardo, S. Probing the interaction mechanism of heterostructured VOxNy nanoparticles supported in nitrogen-doped reduced graphene oxide aerogel as an efficient polysulfide electrocatalyst for stable sulfur cathodes. J. Power Sources 2020, 461, 228144.
- Bonaccorso, F.; Colombo, L.; Yu, G.H.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501.
- Ma, Z.L.; Li, Z.; Hu, K.; Liu, D.D.; Huo, J.; Wang, S.Y. The enhancement of polysulfide absorbsion in LiS batteries by hierarchically porous CoS2/carbon paper interlayer. J. Power Sources 2016, 325, 71–78.
- Zhang, S.S.; Tran, D.T. Pyrite FeS2 as an efficient adsorbent of lithium polysulphide for improved lithium–sulphur batteries. J. Mater. Chem. A 2016, 4, 4371–4374.
- Chandrasekaran, S.; Yao, L.; Deng, L.B.; Bowen, C.; Zhang, Y.; Chen, S.M.; Lin, Z.Q.; Peng, F.; Zhang, P.X. Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem. Soc. Rev. 2019, 48, 4178–4280.
- Pang, Q.; Kundu, D.; Nazar, L.F. A graphene-like metallic cathode host for long-life and high-loading lithium–sulfur batteries. Mater. Horiz. 2016, 3, 130–136.
- Wang, H.T.; Zhang, Q.F.; Yao, H.B.; Liang, Z.; Lee, H.W.; Hsu, P.C.; Zheng, G.Y.; Cui, Y. High electrochemical selectivity of edge versus terrace sites in two-dimensional layered MoS2 materials. Nano Lett. 2014, 14, 7138–7144.
- Sun, M.; Liu, H.J.; Qu, J.H.; Li, J.H. Earth-rich transition metal phosphide for energy conversion and storage. Adv. Energy Mater. 2016, 6, 1600087.
- Peng, L.S.; Shah, S.S.A.; Wei, Z.D. Recent developments in metal phosphide and sulfide electrocatalysts for oxygen evolution reaction. Chin. J. Catal. 2018, 39, 1575.
- Ng, S.F.; Lau, M.Y.L.; Ong, W.J. Lithium–sulfur battery cathode design: Tailoring metal-based nanostructures for robust polysulfide adsorption and catalytic conversion. Adv. Mater. 2021, 33, 2008654.
- Zhong, Y.R.; Yin, L.C.; He, P.; Liu, W.; Wu, Z.S.; Wang, H.L. Surface chemistry in cobalt phosphide-stabilized lithium–sulfur batteries. J. Am. Chem. Soc. 2018, 140, 1455–1459.
- Shen, J.D.; Xu, X.J.; Liu, J.; Liu, Z.B.; Li, F.K.; Hu, R.Z.; Liu, J.W.; Hou, X.H.; Feng, Y.Z.; Yu, Y.; et al. Mechanistic understanding of metal phosphide host for sulfur cathode in high-energy-density lithium–sulfur batteries. ACS Nano 2019, 13, 8986–8996.
- Chen, H.; Wang, J.; Zhao, Y.; Zeng, Q.; Zhou, G.; Jin, M. Three-dimensionally ordered macro/mesoporous Nb2O5/Nb4N5 heterostructure as sulfur host for high-performance lithium/sulfur batteries. Nanomaterials 2021, 11, 1531.
- Ye, C.; Jiao, Y.; Jin, H.Y.; Slattery, A.D.; Davey, K.; Wang, H.H.; Qiao, S.Z. 2D MoN-VN heterostructure to regulate polysulfides for highly efficient lithium-sulfur batteries. Angew. Chem. Int. Ed. 2018, 57, 16703.
- Wang, S.Z.; Feng, S.P.; Liang, J.W.; Su, Q.M.; Zhao, F.P.; Song, H.J.; Zheng, M.; Sun, Q.; Song, Z.X.; Jia, X.H.; et al. Insight into MoS2–MoN heterostructure to accelerate polysulfide conversion toward high-energy-density lithium–sulfur batteries. Adv. Energy Mater. 2021, 11, 2003314.
- Zhou, T.H.; Lv, W.; Li, J.; Zhou, G.M.; Zhao, Y.; Fan, S.X.; Liu, B.L.; Li, B.H.; Kang, F.Y.; Yang, Q.H. Twinborn TiO2–TiN heterostructures enabling smooth trapping–diffusion–conversion of polysulfides towards ultralong life lithium–sulfur batteries. Energy Environ. Sci. 2017, 10, 1694–1703.
- Xue, Y.F.; Luo, D.; Yang, N.; Ma, G.; Zhang, Z.; Hou, J.F.; Wang, J.T.; Ma, C.Y.; Wang, X.; Jin, M.L.; et al. Engineering checkerboard-like heterostructured sulfur electrocatalyst towards high-performance lithium sulfur batteries. Chem. Eng. J. 2022, 440, 135990.
- Artchuea, T.; Srikhaow, A.; Sriprachuabwong, C.; Tuantranont, A.; Tang, I.-M.; Pon-On, W. Copper zinc sulfide (CuZnS) quantum dot-decorated (NiCo)–S/conductive carbon matrix as the cathode for Li–S batteries. Nanomaterials 2022, 12, 2403.
- Wang, H.; Song, Y.; Zhao, Y.; Zhao, Y.; Wang, Z. CuCo2S4 nanoparticles embedded in carbon nanotube networks as sulfur hosts for high performance lithium-sulfur batteries. Nanomaterials 2022, 12, 3104.
- Zhou, L.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. Host materials anchoring polysulfides in Li–S batteries reviewed. Adv. Energy Mater. 2021, 11, 2001304.
- Li, S.; Cen, Y.; Xiang, Q.; Aslam, M.K.; Hu, B.B.; Li, W.; Tang, Y.; Yu, Q.; Liu, Y.P.; Chen, C.G. Vanadium dioxide-reduced graphene oxide binary host as an efficient polysulfide plague for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 1658–1668.
- Luo, Y.F.; Luo, N.N.; Kong, W.B.; Wu, H.C.; Wang, K.; Fan, S.S.; Duan, W.H.; Wang, J.P. Multifunctional interlayer based on molybdenum diphosphide catalyst and carbon nanotube film for lithium–sulfur batteries. Small 2018, 14, 1702853.
- Liang, Z.B.; Qu, C.; Guo, W.H.; Zou, R.Q.; Xu, Q. Pristine metal–organic frameworks and their composites for energy storage and conversion. Adv. Mater. 2018, 30, 1702891.
- Zheng, Y.; Zheng, S.S.; Xue, H.G.; Pang, H. Metal–organic frameworks for lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 3469–3491.
- Han, G.; Wang, X.; Yao, J.; Zhang, M.; Wang, J. The application of indium composite material derived from MOF in cathode material of lithium sulfur batteries. Nanomaterials 2020, 10, 177.
- Huang, S.Z.; Wang, Y.; Hu, J.P.; Lim, Y.V.; Kong, D.; Zheng, Y.; Ding, M.; Pam, M.E.; Yang, H.Y. Mechanism investigation of high-performance Li-polysulfide batteries enabled by tungsten disulfide nanopetals. ACS Nano 2018, 12, 9504–9512.
- He, J.R.; Bhargav, A.; Asl, H.Y.; Chen, Y.F.; Manthiram, A. 1T′-ReS2 nanosheets in situ grown on carbon nanotubes as a highly efficient polysulfide electrocatalyst for stable Li-S batteries. Adv. Energy Mater. 2020, 10, 202001017.
- Yang, J.L.; Liu, T.; Zhao, S.X. Interfaces-dominated Li2S nucleation behavior enabled by heterostructure catalyst for fast kinetics Li-S batteries. Nano Energy 2021, 89, 106452.
- Zhang, H.B.; Liu, G.G.; Shi, L.; Ye, J.H. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Funct. Mater. 2018, 8, 1701343.
- Zhang, J.; You, C.Y.; Lin, H.Z.; Wang, Z. Electrochemical kinetic modulators in lithium–sulfur batteries: From defect-rich catalysts to single atomic catalysts. Energy Environ. Mater. 2021, 5, 731–750.
- Shi, Z.X.; Ding, Y.F.; Zhang, Q.; Sun, J.Y. Electrocatalyst modulation toward bidirectional sulfur redox in Li-S batteries: From strategic probing to mechanistic understanding. Adv. Energy Mater. 2022, 12, 2201056.
- Xu, Y.S.; Zheng, W.; Liu, X.H.; Zhang, L.Q.; Zheng, L.L.; Yang, C.; Pinna, N.; Zhang, J. Platinum single atoms on tin oxide ultrathin films for extremely sensitive gas detection. Mater. Horiz. 2020, 7, 1519–1527.
- Jones, J.; Xiong, H.F.; DeLaRiva, A.T.; Peterson, E.J.; Hien, P.; Challa, S.R.; Qi, G.S.; Oh, S.; Wiebenga, M.H.; Hernandez, X.I.P.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154.
- Qiao, B.T.; Liu, J.X.; Wang, Y.G.; Lin, Q.Q.; Liu, X.Y.; Wang, A.Q.; Li, J.; Zhang, T.; Liu, J.Y. Highly efficient catalysis of preferential oxidation of CO in H-2-rich stream by gold single-atom catalysts. ACS Catal. 2015, 5, 6249–6254.
- Kaden, W.E.; Wu, T.P.; Kunkel, W.A.; Anderson, S.L. Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science 2009, 326, 826–829.
- Yang, M.; Allard, L.F.; Flytzani-Stephanopoulos, M. Atomically dispersed Au-(OH)x species bound on titania catalyze the low-temperature water-gas shift reaction. J. Am. Chem. Soc. 2013, 135, 3768–3771.
- Liu, W.G.; Zhang, L.L.; Yan, W.S.; Liu, X.Y.; Yang, X.F.; Miao, S.; Wang, A.Q.; Zhang, T. Single-atom dispersed Co-N-C catalyst: Structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 2016, 7, 5758–5764.
- Liu, W.G.; Chen, Y.J.; Qi, H.F.; Zhang, L.L.; Yan, W.S.; Liu, X.Y.; Yang, X.F.; Miao, S.; Wang, W.T.; Liu, C.G. A durable nickel single-atom catalyst for hydrogenation reactions and cellulose valorization under harsh conditions. Angew. Chem. Int. Ed. 2018, 57, 7071–7075.
- Liu, Z.Z.; Zhou, L.; Ge, Q.; Chen, R.J.; Ni, M.; Utetiwabo, W.; Zhang, X.L.; Yang, W. Atomic iron catalysis of polysulfide conversion in lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 19311–19317.
- Wang, J.; Jia, L.J.; Zhong, J.; Xiao, Q.B.; Wang, C.; Zang, K.T.; Lin, H.T.; Zheng, H.C.; Luo, J.; Yang, J. Single-atom catalyst boosts electrochemical conversion reactions in batteries. Energy Storage Mater. 2019, 18, 246–252.
- Wang, S.Y.; Li, J.Q.; Li, Q.; Bai, X.W.; Wang, J.L. Metal single-atom coordinated graphitic carbon nitride as an efficient catalyst for CO oxidation. Nanoscale 2020, 12, 364–371.
- Lu, B.Z.; Liu, Q.M.; Chen, S.W. Electrocatalysis of single-atom sites: Impacts of atomic coordination. ACS Catal. 2020, 10, 7584–7618.
- Zhou, G.M.; Zhao, S.Y.; Wang, T.S.; Yang, S.Z.; Johannessen, B.; Chen, H.; Liu, C.W.; Ye, Y.S.; Wu, Y.C.; Peng, Y.C.; et al. Theoretical calculation guided design of single-atom catalysts toward fast kinetic and long-life Li-S batteries. Nano Lett. 2020, 20, 1252–1261.
- Ma, F.; Wan, Y.Y.; Wang, X.M.; Wang, X.C.; Liang, J.S.; Miao, Z.P.; Wang, T.Y.; Ma, C.; Lu, G.; Han, J.T.; et al. Bifunctional atomically dispersed Mo-N2/C nanosheets boost lithium sulfide deposition/decomposition for stable lithium-sulfur batteries. ACS Nano 2020, 14, 10115–10126.
- Wang, Y.Z.; Adekoya, D.; Sun, J.Q.; Tang, T.Y.; Qiu, H.L.; Xu, L.; Zhang, S.Q.; Hou, Y.L. Manipulation of edge-site Fe-N2 moiety on holey Fe, N codoped graphene to promote the cycle stability and rate capacity of Li-S batteries. Adv. Funct. Mater. 2019, 29, 1807485.
- Luo, C.; Liang, X.; Sun, Y.F.; Lv, W.; Sun, Y.W.; Lu, Z.Y.; Hua, W.X.; Yang, H.T.; Wang, R.C.; Yan, C.L.; et al. An organic nickel salt-based electrolyte additive boosts homogeneous catalysis for lithium-sulfur batteries. Energy Storage Mater. 2020, 33, 290–297.
- Xie, J.; Peng, H.J.; Song, Y.W.; Li, B.Q.; Xiao, Y.; Zhao, M.; Yuan, H.; Huang, J.Q.; Zhang, Q. Spatial and kinetic regulation of sulfur electrochemistry on semi-immobilized redox mediators in working batteries. Angew. Chem. Int. Ed. 2020, 59, 17670–17675.
- Zhao, C.X.; Li, X.Y.; Zhao, M.; Chen, Z.X.; Song, Y.W.; Chen, W.J.; Liu, J.N.; Wang, B.; Zhang, X.Q.; Chen, C.M.; et al. Semi-immobilized molecular electrocatalysts for high-performance lithium-sulfur batteries. J. Am. Chem. Soc. 2021, 143, 19865–19872.
- Gerber, L.C.H.; Frischmann, P.D.; Fan, F.Y.; Doris, S.E.; Qu, X.H.; Scheuermann, A.M.; Persson, K.; Chiang, Y.M.; Helms, B.A. Three-dimensional growth of Li2S in lithium-sulfur batteries promoted by a redox mediator. Nano Lett. 2016, 16, 549–554.
- Tsao, Y.C.; Lee, M.; Miller, E.C.; Gao, G.P.; Park, J.; Chen, S.C.; Katsumata, T.; Tran, H.; Wang, L.W.; Toney, M.F.; et al. Designing a quinone-based redox mediator to facilitate Li2S oxidation in Li-S batteries. Joule 2019, 3, 872–884.
- Gao, X.; Zheng, X.L.; Tsao, Y.C.; Zhang, P.; Xiao, X.; Ye, Y.S.; Li, J.; Yang, Y.F.; Xu, R.; Bao, Z.N.; et al. All-solid-state lithium–sulfur batteries enhanced by redox mediators. J. Am. Chem. Soc. 2021, 143, 18188–18195.
- Jiao, L.; Jiang, H.; Lei, Y.C.; Wu, S.L.; Gao, Q.L.; Bu, S.Y.; Kong, X.; Yang, S.; Shu, D.K.; Li, C.Y.; et al. “Dual mediator system” enables efficient and persistent regulation toward sulfur redox conversion in lithium-sulfur batteries. ACS Nano 2022, 16, 14262–14273.
- Zhao, M.; Peng, H.J.; Wei, J.Y.; Huang, J.Q.; Li, B.Q.; Yuan, H.; Zhang, Q. Dictating high-capacity lithium–sulfur batteries through redox-mediated lithium sulfide growth. Small Methods 2020, 4, 1900344.
- Wang, Z.K.; Ji, H.Q.; Zhou, L.Z.; Shen, X.W.; Gao, L.H.; Liu, J.; Yang, T.Z.; Qian, T.; Yan, C.L. All-liquid-phase reaction mechanism enabling cryogenic Li-S batteries. ACS Nano 2021, 15, 13847–13856.
- Zhao, M.; Chen, X.; Li, X.Y.; Li, B.Q.; Huang, J.Q. An organodiselenide comediator to facilitate sulfur redox kinetics in lithium–sulfur batteries. Adv. Mater. 2021, 33, 2007298.
- Ye, H.L.; Sun, J.G.; Lim, X.F.; Zhao, Y.; Lee, J.Y. Mediator-assisted catalysis of polysulfide conversion for high-loading lithium-sulfur batteries operating under the lean electrolyte condition. Energy Storage Mater. 2021, 38, 338–343.




