Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rebecca Wingert | -- | 1401 | 2023-02-16 13:45:58 | | | |
| 2 | Dean Liu | -6 word(s) | 1395 | 2023-02-17 01:41:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chambers, B.E.; Weaver, N.E.; Wingert, R.A. The “3Ds” of Growing Kidney Organoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/41307 (accessed on 07 February 2026).
Chambers BE, Weaver NE, Wingert RA. The “3Ds” of Growing Kidney Organoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/41307. Accessed February 07, 2026.
Chambers, Brooke E., Nicole E. Weaver, Rebecca A. Wingert. "The “3Ds” of Growing Kidney Organoids" Encyclopedia, https://encyclopedia.pub/entry/41307 (accessed February 07, 2026).
Chambers, B.E., Weaver, N.E., & Wingert, R.A. (2023, February 16). The “3Ds” of Growing Kidney Organoids. In Encyclopedia. https://encyclopedia.pub/entry/41307
Chambers, Brooke E., et al. "The “3Ds” of Growing Kidney Organoids." Encyclopedia. Web. 16 February, 2023.
Copy Citation
A kidney organoid is a three-dimensional (3D) cellular aggregate grown from stem cells in vitro that undergoes self-organization, recapitulating aspects of normal renal development to produce nephron structures that resemble the native kidney organ. These miniature kidney-like structures can also be derived from primary patient cells and thus provide simplified context to observe how mutations in kidney-disease-associated genes affect organogenesis and physiological function.
kidney
nephron
organoid
stem cell
nephron progenitor
1. Introduction
Organoids are products of recent advances in stem cell biology and 3D tissue culture research, where miniaturized structures that resemble human organs can be grown in vitro from stem cells [1]. Generating these mini-organs begins with the culturing of human embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), or tissue-resident adult stem cells (ASCs), which, when exposed to the correct nutrients, growth factors, and instructive signals, are induced to undergo self-organization and differentiation [2]. The ability to recapitulate aspects of human development in vitro marks a major milestone, as nearly all of the current understanding of embryogenesis has been drawn from landmark studies conducted in animal models [3]. Not only does the study of human organoids help address gaps in developmental biology knowledge related to species divergence, but it offers substantial opportunities for disease modeling and preclinical studies [4]. To this point, organoids derived from an individual’s cells may offer a scalable system for personalized drug screening and show the potential to enhance cellular therapies and tissue grafts [5].
The surge of interest in organoid research began about fifteen years ago when labs began reporting the ability to grow ‘in a dish’ miniature versions of the mammalian eye [6][7][8], the intestine [9][10], and the brain [11]. The sensation has only expanded over time as researchers successfully created methods to grow organoids of the liver, stomach, retina, prostate, lung, and kidney, among others [12][13].
2. Ongoing Limitations in Renal Organoid Research
Traditional kidney organoid protocols begin with growing human iPSCs in a monolayer, or two-dimensional (2D) culture, for 7 days to induce intermediate mesoderm by the addition of two main molecules: CHIR99201 and FGF9 [14][15][16]. After a week of monolayer culture, the cells are dissociated, plated in 3D culture conditions, and subjected to APEL media supplemented with FGF9 and heparin. Within the next few days, the cells will aggregate, self-organize, and undergo nephrogenesis (Figure 1, top panel). From day 12 on, the maturing kidney organoids are subjected to media without FGF9 and heparin. This protocol produces ‘mini-kidneys’ that have up to several hundred nephron-like structures with multiple cell lineages, including glomeruli, tubule segments (e.g., proximal, loop of Henle, distal), and various stromal cells.
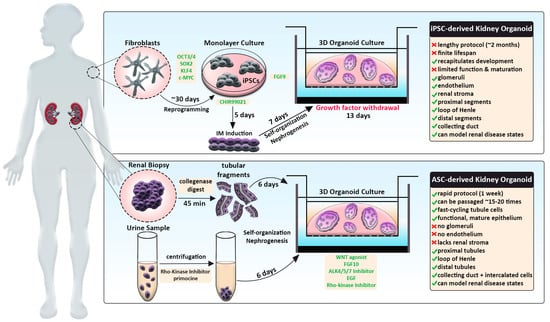
Figure 1. Schematic comparing human iPSC-derived and ASC-derived kidney organoid strategies. iPSC-derived kidney organoid protocol (top) typically entails reprogramming patient fibroblasts (grey), induction of IM in monolayer culture conditions and directed differentiation by timed addition of certain factors (green). This process is relatively lengthy but more accurately recapitulates processes taking place during embryonic kidney development. ASC-derived kidney organoid protocol (bottom), also termed ‘tubuloids,’ involves the isolation of renal cells via biopsy or urine sample (purple). Upon processing, tubular fragments or urine-derived cells are directly seeded into 3D culture conditions and treated with growth factors (green) to induce rapid differentiation of nephron tubule structures. Resulting tubuloids constitute mature epithelium, can be passaged many times, but lack certain kidney cell types. Both systems promote the 3D assembly of organoids by employing Matrigel-coated transwell culture apparatuses.
In addition, kidney organoids that are termed ‘tubuloids’ can be produced easily and rapidly by culturing ASCs obtained from a renal biopsy (Figure 1, bottom panel). By comparison, tubuloids lack glomeruli, endothelium, and stroma but contain proximal, intermediate, and distal segments along with collecting ducts.
2.1. Technical Challenges of Reproducibility and Cellular Heterogeneity
Both kidney organoid protocols generate renal organoids that exhibit variations in cell type composition, as briefly described. Interestingly, within a given experiment using any of the established protocols to grow renal organoids, the renal organoids are highly similar. However, there is high technical variability or so-called ‘batch-to-batch’ variation between different experiments using the same protocol. This is thought to partly reflect differences in the groups of reagents used, such as growth factors. There are also variability differences based on the cell line source and even from different stocks of the same cell line. Current protocols also can elicit different total percentages of nephron segment populations, e.g., more tubular cells. Thus, it remains a challenge how to tailor conditions in the right manner to create nephrons with the proper segment ratios.
2.2. Architectural Simplicity and Abnormal Nephrogenesis
Renal organoids display notable anatomical and compositional differences compared to the naturally developed mammalian kidney. At birth, the final human kidney form encompasses from ~200,000 to upwards of 1–2 million nephrons arranged in cortical and medullary zones, with each individual nephron being connected on one end to the systemic vasculature and the other to a central, arborized collecting duct system [17][18]. The elaborate structure of the human kidney develops over the course of approximately 200 days [19]. In contrast, most kidney organoid protocols occur over a 2–4-week time span.
For the most part, renal organoids have lacked a higher order/complex organization, with nephrons randomly arranged and intermingled, sometimes with various overt malformations such as nephron-nephron connections and branched nephrons. However, some protocols have been able to foster the cultivation of a clearly defined ureteric bud/collecting duct-like structure [20].
2.3. Generation of Off-Target Cell Types, While Some Renal Cell Types Are Missing Altogether
In addition to the heterogeneity of renal organoids, current methods produce nephrons and surrounding interstitium with missing or underrepresented cell types. For example, macrophages and other immune cells are absent, as well as mesangial cells within glomeruli [14][15][16]. Renal organoids lack vasculature, which limits how much they can grow in vitro. Further, they contain varying percentages of ‘off-target’ populations such as neurons, skeletal muscle, satellite cells, and melanocytes, which expand over prolonged culture times [14][15][16].
2.4. Renal Organoids Show Limited Lifespan and Lack Stem/Progenitor Self-Renewal Characteristics
In addition to reduced nephron numbers, kidney organoids have a finite lifespan with limited nephrogenesis capability. To date, the interval over which nephrogenesis occurs in these cultures is also quite limited. During the culture process, there is a single bout of nephron formation but no ongoing rounds over time. The growth conditions have not supported the emergence of the proper microenvironment for the lengthy survival and/or self-renewal of NPCs. In part, this barrier likely exists because of differences in the stromal cell populations within current renal organoids. In support of this notion, a recently reported induced stroma protocol with mouse ES cells has supported the generation of more advanced higher-order organoids [20]. Nevertheless, escalating nephron numbers within culture remains an aspect to address.
The limited culture longevity of realized renal organoids is also impacted by the absence of functional vasculature in this setting. So far, the best methods to achieve vascularization have been to transplant the renal organoid into another host system, such as a mouse, to co-culture on the chick chorioallantoic membrane or to culture under sheer stress in a microfluidic device [21][22][23][24][25][26][27][28][29][30]. Interestingly, transplanted human renal organoids are vascularized through angiogenesis of murine host cells [24][31]. Such organoids can undergo successful blood perfusion and glomerular filtration [24][31].
2.5. The Renal Organoid ‘Fetal State’—A Lack of Maturity in Renal Cell Types
Nephrons in renal organoids also exhibit features of an immature fetal state which has been likened to the first or second-trimester human fetal kidney [32][33][34] and shows limited functional characteristics. For example, proximal tubules produced via organoid culture express decreased levels of anionic, amino acid, and glucose transport proteins, which are inherent for the physiological function of this nephron compartment. However, a recent report has shown enhanced mature features by inhibition of Wnt signaling [34]. Further, there has been remarkable progress in achieving the recipe for cultivating the pattern formation and subsequent differentiation of some structures and their composite lineages—such as the 3D glomeruli with podocytes and parietal epithelial cells that form the Bowman’s capsule [35]. Podocytes display hallmark differentiated features: ultrastructural traits such as foot processes, gene expression characteristics such as collagen and laminin switching, and autonomous calcium signaling [28][32][36][37][38]. Though, as previously noted, mesangial cells are absent within in vitro conditions.
While kidney organoids are a simplified representation of the human kidney, many researchers continue to improve these models by employing innovative technologies such as single-cell RNA sequencing and microbioreactors to optimize differentiation protocols. In the following sections, researchers discuss how the application of several such technologies has led to advancements in the kidney organoid model.
References
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and diseases using organoid technologies. Science 2014, 345, 1247125.
- Clevers, H. Modeling development, and disease with organoids. Cell 2016, 165, 1586–1597.
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941.
- Huch, M.; Koo, B.K. Modeling mouse and human development using organoid cultures. Development 2015, 142, 3113–3125.
- Schutgens, F.; Clevers, H. Human organoids: Tools for understanding biology and treating disease. Annu. Rev. Pathol. 2020, 15, 211–234.
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008, 3, 519–532.
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56.
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 2012, 10, 771–785.
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265.
- Dekkers, J.F.; Wiegerinck, C.L.; de Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; de Winter-de Groot, K.M.; Brandsma, A.M.; de Jong, N.W.; Bijvelds, M.J.; Scholte, B.J.; et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013, 19, 939–945.
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379.
- Artegiani, B.; Clevers, H. Use and application of 3D-organoid technology. Hum. Mol. Genet. 2018, 27, R99–R107.
- Corro, C.; Novellasdemunt, L.; Li, V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165.
- Trush, O.; Takasato, M. Kidney organoid research: Current status and applications. Curr. Opin. Genet. Dev. 2022, 75, 101944.
- Dorison, A.; Forbes, T.A.; Little, M.H. What can we learn from kidney organoids? Kidney Int. 2022, 102, 1013–1029.
- Sharmin, S.; Taguchi, A.; Kaku, Y.; Yoshimura, Y.; Ohmori, T.; Sakuma, T.; Mukoyama, M.; Yamamoto, T.; Kurihara, H.; Nishinakamura, R. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J. Am. Soc. Nephrol. 2016, 27, 1778–1791.
- Bertram, J.F.; Douglas-Denton, R.N.; Diouf, B.; Hughson, M.D.; Hoy, W.E. Human nephron number: Implications for health and disease. Pediatr. Nephrol. 2011, 26, 1529–1533.
- Perl, A.J.; Schuh, M.P.; Kopan, R. Regulation of nephron progenitor cell lifespan and nephron endowment. Nat. Rev. Nephrol. 2022, 18, 683–695.
- McMahon, A.P. Development of the mammalian kidney. Curr. Top. Dev. Biol. 2016, 117, 31.
- Tanigawa, S.; Tanaka, E.; Miike, K.; Ohmori, T.; Inoue, D.; Cai, C.L.; Taguchi, A.; Kobayashi, A.; Nishinakamura, R. Generation of the organotypic kidney structure by integrating pluripotent stem cell-derived renal stroma. Nat. Commun. 2022, 13, 611.
- Taguchi, A.; Nishinakamura, R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 2017, 21, 730–746.
- Bantounas, I.; Ranjzad, P.; Tengku, F.; Silajdžić, E.; Forster, D.; Asselin, M.C.; Lewis, P.; Lennon, R.; Plagge, A.; Wang, Q.; et al. Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Rep. 2018, 10, 766–779.
- Tanigawa, S.; Islam, M.; Sharmin, S.; Naganuma, H.; Yoshimura, Y.; Haque, F.; Era, T.; Nakazato, H.; Nakanishi, K.; Sakuma, T. Organoids from nephrotic disease-derived iPSCs identify impaired NEPHRIN localization and slit diaphragm formation in kidney podocytes. Stem Cell Rep. 2018, 11, 727–740.
- van den Berg, C.W.; Ritsma, L.; Avramut, M.C.; Wiersma, L.E.; van den Berg, B.M.; Leuning, D.G.; Lievers, E.; Koning, M.; Vanslambrouck, J.M.; Koster, A.J.; et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo- vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep. 2018, 10, 751–765.
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405.
- Gupta, A.K.; Coburn, J.M.; Davis-Knowlton, J.; Kimmerling, E.; Kaplan, D.L.; Oxburgh, L. Scaffolding kidney organoids on silk. J. Tissue Eng. Regen. Med. 2019, 13, 812–822.
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262.
- Tran, T.; Lindström, N.O.; Ransick, A.; De Sena Brandine, G.; Guo, Q.; Kim, A.D.; Der, B.; Peti-Peterdi, J.; Smith, A.D.; Thornton, M.; et al. In vivo developmental trajectories of human podocyte inform in vitro differentiation of pluripotent stem cell-derived podocytes. Dev. Cell. 2019, 50, 102–116.e6.
- Tsujimoto, H.; Kasahara, T.; Sueta, S.I.; Araoka, T.; Sakamoto, S.; Okada, C.; Mae, S.I.; Nakajima, T.; Okamoto, N.; Taura, D.; et al. A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep. 2020, 31, 107476.
- Wu, H.; Uchimura, K.; Donnelly, E.L.; Kirita, Y.; Morris, S.A.; Humphreys, B.D. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 2018, 23, 869–881.e8.
- van den Berg, C.W.; Koudijs, A.; Ritsma, L.; Rabelink, T.J. In vivo assessment of size-selective glomerular sieving in transplanted human induced pluripotent stem cell-derived kidney organoids. J. Am. Soc. Nephrol. 2020, 31, 921–929.
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568.
- Combes, A.N.; Zappia, L.; Er, P.X.; Oshlack, A.; Little, M.H. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med. 2019, 11, 3.
- Vanslambrouck, J.M.; Wilson, S.B.; Tan, K.S.; Groenewegen, E.; Rudraraju, R.; Neil, J.; Lawlor, K.T.; Mah, S.; Scurr, M.; Howden, S.E.; et al. Enhanced metanephric specification to functional proximal tubule enables toxicity screening and infectious disease modelling in kidney organoids. Nat. Commun. 2022, 13, 5943.
- Djenoune, L.; Tomar, R.; Dorison, A.; Ghobrial, I.; Schenk, H.; Hegermann, J.; Beverly-Staggs, L.; Hidalgo-Gonzalez, A.; Little, M.H.; Drummond, I.A. Autonomous calcium signaling in human and zebrafish podocytes controls kidney filtration barrier morphogenesis. J. Am. Soc. Nephrol. 2021, 32, 1697–1712.
- Hale, L.J.; Howden, S.E.; Phipson, B.; Lonsdale, A.; Er, P.X.; Ghobrial, I.; Hosawi, S.; Wilson, S.; Lawlor, K.T.; Khan, S.; et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat. Commun. 2018, 9, 5167.
- Yoshimura, Y.; Taguchi, A.; Tanigawa, S.; Yatsuda, J.; Kamba, T.; Takahashi, S.; Kurihara, H.; Mukoyama, M.; Nishinakamura, R. Manipulation of nephron-patterning signals enables selective induction of podocytes from human pluripotent stem cells. J. Am. Soc. Nephrol. 2019, 30, 304–321.
- Wu, H.; Humphreys, B.D. Single cell sequencing and kidney organoids generated from pluripotent stem cells. Clin J. Am. Soc. Nephrol. 2020, 15, 550–556.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
17 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




