| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ahmed Wadaa-Allah | -- | 2456 | 2023-02-16 13:27:22 | | | |
| 2 | Peter Tang | + 1 word(s) | 2457 | 2023-02-17 04:41:10 | | |
Video Upload Options
microRNAs (miRNAs) are small single-stranded, non-coding RNAs that are 22–23 nucleotides in length. More than 2000 miRNA genes were identified. The last step in the processing of miRNAs is the Dicer-mediated cleavage. This final step is considered the interface link between miRNA and its regulators (e.g., E2 and androgens) on one hand and neurons on the other. Any disruption in the dicer-mediated cleavage of pre-miRNAs will affect mature miRNA production, which could propagate a negative effect on both cortical neurogenesis and the embryonic development of the nervous system. Several studies concluded that the disruption of mature miRNAs would probably affect the function of the nervous system by causing a reduction in neural progenitor cells’ proliferation, a delay in the cell cycle, a disturbance in neural migration, an induction of apoptosis by activation of caspase 3, the stimulation of astrocyte differentiation, and the inhibition of neuronal differentiation.
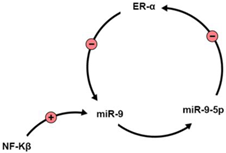
1. miR-9 Activity and Regulation by Estrogens (Table 1)
|
Molecule |
Interaction with miR-9 |
Reference |
|---|---|---|
|
NF-Kβ |
|
[3] |
|
NEFH |
miR-9 binds to the 3′UTR region of NEFH. miR-9-5p is significantly downregulated in ALS. |
[14] |
|
Serine Palmitoyltransferase [SPT] |
SPT regulates Aβ in Alzheimer’s disease, and is correlated with miR-9 serum and cortical levels. |
[15] |
|
SIRT1 |
Negative correlation with miR-9 levels. SIRT1 gene is a target of E2. |
|
|
E2 |
|
|
|
REST/CoREST |
They regulate, and are regulated by miR-9. In HD, mutant huntingtin fails to regulate REST/CoREST, disrupting miR-9 activity. |
2. miR-29 Family and Neurodegenerative Disorders (Table 2)
|
Molecule |
Interaction with miR-29 |
Reference |
|---|---|---|
|
Doublecortin |
miR-29a targets doublecortin expression, reducing axonal branching |
[26] |
|
Voltage-dependent anion channel 1 |
miR-29a regulates this molecule, reducing apoptosis |
|
|
BH3-only family |
miR-29b silences this proapoptotic gene family |
[30] |
|
Wnt/βcatenin signaling |
miR-29b regulates this pathway, hereby affecting embryonic proliferation and neurogenesis |
|
|
BACE |
Negative correlation with miR-29a, miR-29b and miR-29c-3p expression in AD |
[37] |
|
DNA methyltransferase III beta (DNMT3B) |
miR-29c acts on DNMT3B to reduce BDNF levels in AD invitro models. |
[38] |
|
Parkinsonism-associated Deglycase (PARK7) |
miR-29 regulates this molecule. It is also regulated by estrogens and is implicated in PD pathology. |
|
|
BcI2L2 |
BcI2L2 gene, which is antiapoptotic, is regulated by miR-29b. |
3. Alzheimer’s Disease
4. Parkinson Disease
5. Huntington’s Disease
6. Other Brain Diseases
References
- Power, B.D.; Mitrofanis, J. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J. Comp. Neurol. 2001, 436, 64–81.
- Shima, N.; Yamaguchi, Y.; Yuri, K. Distribution of estrogen receptor β mRNA-containing cells in ovariectomized and estrogen-treated female rat brain. Anat. Sci. Int. 2003, 78, 85–97.
- Barbano, R.; Pasculli, B.; Rendina, M.; Fontana, A.; Fusilli, C.; Copetti, M.; Castellana, S.; Valori, V.M.; Morritti, M.; Graziano, P.; et al. Stepwise analysis of MIR9 loci identifies MIR-9-5p to be involved in Oestrogen regulated pathways in breast cancer patients. Sci. Rep. 2017, 7, 45283.
- Zhang, Q.; Raz, L.; Wang, R.; Han, D.; De Sevilla, L.; Yang, F.; Vadlamudi, R.K.; Brann, D.W. Estrogen Attenuates Ischemic Oxidative Damage via an Estrogen Receptor -Mediated Inhibition of NADPH Oxidase Activation. J. Neurosci. 2009, 29, 13823–13836.
- Hsu, P.Y.; Deatherage, D.E.; Rodriguez, B.A.T.; Liyanarachchi, S.; Weng, Y.I.; Zuo, T.; Liu, J.; Cheng, A.S.L.; Huang, T.H.M. Xenoestrogen-induced epigenetic repression of microRNA-9-3 in breast epithelial cells. Cancer Res. 2009, 69, 5936–5945.
- Pillai, M.M.; Gillen, A.E.; Yamamoto, T.M.; Kline, E.; Brown, J.; Flory, K.; Hesselberth, J.R.; Kabos, P. HITS-CLIP reveals key regulators of nuclear receptor signaling in breast cancer. Breast Cancer Res. Treat. 2014, 146, 85–97.
- Chang, K.H.; Wu, Y.R.; Chen, C.M. Down-regulation of miR-9∗ in the peripheral leukocytes of Huntington’s disease patients. Orphanet J. Rare Dis. 2017, 12, 1–5.
- Rao, Y.S.; Mott, N.N.; Wang, Y.; Chung, W.C.J.; Pak, T.R. MicroRNAs in the aging female brain: A putative mechanism for age-specific estrogen effects. Endocrinology 2013, 154, 2795–2806.
- Lin, S.J.; Defossez, P.A.; Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in saccharomyces cerevisiae. Science 2000, 289, 2126–2128.
- Longo, V.D.; Kennedy, B.K. Sirtuins in Aging and Age-Related Disease. Cell 2006, 126, 257–268.
- Gao, J.; Wang, W.Y.; Mao, Y.W.; Gräff, J.; Guan, J.S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 2010, 466, 1105–1109.
- Michan, S.; Li, Y.; Chou, M.M.-H.; Parrella, E.; Ge, H.; Long, J.M.; Allard, J.S.; Lewis, K.; Miller, M.; Xu, W.; et al. SIRT1 Is Essential for Normal Cognitive Function and Synaptic Plasticity. J. Neurosci. 2010, 30, 9695–9707.
- Schonrock, N.; Humphreys, D.T.; Preiss, T.; Götz, J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J. Mol. Neurosci. 2012, 46, 324–335.
- Campos-Melo, D.; Hawley, Z.C.E.; Strong, M.J. Dysregulation of human NEFM and NEFH mRNA stability by ALS-linked miRNAs. Mol. Brain 2018, 11, 43.
- Geekiyanage, H.; Upadhye, A.; Chan, C. Inhibition of serine palmitoyltransferase reduces Aβ and tau hyperphosphorylation in a murine model: A safe therapeutic strategy for Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2037–2051.
- Packer, A.N.; Xing, Y.; Harper, S.Q.; Jones, L.; Davidson, B.L. The Bifunctional microRNA miR-9/miR-9* Regulates REST and CoREST and Is Downregulated in Huntington’s Disease. J. Neurosci. 2008, 28, 14341–14346.
- Giusti, S.A.; Vogl, A.M.; Brockmann, M.M.; Vercelli, C.A.; Rein, M.L.; Trümbach, D.; Wurst, W.; Cazalla, D.; Stein, V.; Deussing, J.M.; et al. MicroRNA-9 controls dendritic development by targeting REST. Elife 2014, 3, e02755.
- Guo, X.; Liu, T.; Zhao, D.; Wang, X.; Liu, D.; He, Y.; Shan, C.; Kong, Y.; Hu, W.; Tao, B.; et al. FGF18 protects against 6-hydroxydopamine-induced nigrostriatal damage in a rat model of Parkinson’s disease. Neuroscience 2017, 356, 229–241.
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588.
- Juźwik, C.A.; Drake, S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog. Neurobiol. 2019, 182, 101664.
- Khanna, S.; Rink, C.; Ghoorkhanian, R.; Gnyawali, S.; Heigel, M.; Wijesinghe, D.S.; Chalfant, C.E.; Chan, Y.C.; Banerjee, J.; Huang, Y.; et al. Loss of miR-29b following Acute Ischemic Stroke Contributes to Neural Cell Death and Infarct Size. J. Cereb. Blood Flow Metab. 2013, 33, 1197–1206.
- Shi, Z.; Zhou, H.; Lu, L.; Pan, B.; Wei, Z.; Liu, J.; Li, J.; Yuan, S.; Kang, Y.; Liu, L.; et al. MicroRNA-29a regulates neural stem cell neuronal differentiation by targeting PTEN. J. Cell. Biochem. 2018, 119, 5813–5820.
- Chen, L.; Guo, D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell. Mol. Immunol. 2017, 14, 581–589.
- Waite, K.A.; Eng, C. Protean PTEN: Form and function. Am. J. Hum. Genet. 2002, 70, 829–844.
- Gregorian, C.; Nakashima, J.; Belle, J.L.; Ohab, J.; Kim, R.; Liu, A.; Smith, K.B.; Groszer, M.; Garcia, A.D.; Sofroniew, M.V.; et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J. Neurosci. 2009, 29, 1874–1886.
- Bilimoria, P.M.; De La Torre-Ubieta, L.; Ikeuchi, Y.; Becker, E.B.E.; Reiner, O.; Bonni, A. A JIP3-regulated GSK3β/DCX signaling pathway restricts axon branching. J. Neurosci. 2010, 30, 16766–16776.
- Li, H.; Mao, S.; Wang, H.; Zen, K.; Zhang, C.; Li, L. MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons. Protein Cell 2014, 5, 160–169.
- Bargaje, R.; Gupta, S.; Sarkeshik, A.; Park, R.; Xu, T.; Sarkar, M.; Halimani, M.; Roy, S.S.; Yates, J.; Pillai, B. Identification of novel targets for miR-29a using miRNA proteomics. PLoS ONE 2012, 7, e43243.
- Roshan, R.; Shridhar, S.; Sarangdhar, M.A.; Banik, A.; Chawla, M.; Garg, M.; Singh, V.P.; Pillai, B. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA 2014, 20, 1287–1297.
- Kole, A.J.; Swahari, V.; Hammond, S.M.; Deshmukh, M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011, 25, 125–130.
- Shin, J.; Shin, Y.; Oh, S.M.; Yang, H.; Yu, W.J.; Lee, J.P.; Huh, S.O.; Lee, S.H.; Suh, Y.H.; Chung, S.; et al. MiR-29b controls fetal mouse neurogenesis by regulating ICAT-mediated Wnt/β-catenin signaling. Cell Death Dis. 2014, 5, e1473.
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414.
- Morgan, C.P.; Bale, T.L. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J. Neurosci. 2011, 31, 11748–11755.
- Morgan, C.P.; Bale, T.L. Sex differences in microRNA regulation of gene expression: No smoke, just miRs. Biol. Sex Differ. 2012, 3, 22.
- Piscopo, P.; Bellenghi, M.; Manzini, V.; Crestini, A.; Pontecorvi, G.; Corbo, M.; Ortona, E.; Carè, A.; Confaloni, A. A sex perspective in neurodegenerative diseases: Micrornas as possible peripheral biomarkers. Int. J. Mol. Sci. 2021, 22, 4423.
- Ashraf, G.M.; Ebada, M.A.; Suhail, M.; Ali, A.; Uddin, M.S.; Bilgrami, A.L.; Perveen, A.; Husain, A.; Tarique, M.; Hafeez, A.; et al. Dissecting Sex-Related Cognition between Alzheimer’s Disease and Diabetes: From Molecular Mechanisms to Potential Therapeutic Strategies. Oxid. Med. Cell. Longev. 2021, 2021, 4572471.
- Rademakers, R.; Rovelet-Lecrux, A. Recent insights into the molecular genetics of dementia. Trends Neurosci. 2009, 32, 451–461.
- Yang, G.; Song, Y.; Zhou, X.; Deng, Y.; Liu, T.; Weng, G.; Yu, D.; Pan, S. DNA methyltransferase 3, a target of microRNA-29c, contributes to neuronal proliferation by regulating the expression of brain-derived neurotrophic factor. Mol. Med. Rep. 2015, 12, 1435–1442.
- Fernández-Santiago, R.; Iranzo, A.; Gaig, C.; Serradell, M.; Fernández, M.; Tolosa, E.; Santamaría, J.; Ezquerra, M. MicroRNA association with synucleinopathy conversion in rapid eye movement behavior disorder. Ann. Neurol. 2015, 77, 895–901.
- Zhou, Z.; Ribas, V.; Rajbhandari, P.; Drew, B.G.; Moore, T.M.; Fluitt, A.H.; Reddish, B.R.; Whitney, K.A.; Georgia, S.; Vergnes, L.; et al. Estrogen receptor α protects pancreatic β-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J. Biol. Chem. 2018, 293, 4735–4751.
- Shi, G.; Liu, Y.; Liu, T.; Yan, W.; Liu, X.; Wang, Y.; Shi, J.; Jia, L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp. Brain Res. 2012, 216, 225–230.
- Mott, J.L.; Kobayashi, S.; Bronk, S.F.; Gores, G.J. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007, 26, 6133–6140.
- Ma, Y.-H.; Deng, W.-J.; Luo, Z.-Y.; Jing, J.; Pan, P.-W.; Yao, Y.-B.; Fang, Y.-B.; Teng, J.-F. Inhibition of microRNA-29b suppresses oxidative stress and reduces apoptosis in ischemic stroke. Neural Regen. Res. 2022, 17, 433–439.
- Ouerdane, Y.; El-Nahas, Z.S.; Ouerdane, F.; Hamam, K.M.; Ebada, M.A. Gut–Brain Axis in Alzheimer’s Disease: Interplay Between Cholecystokinin, Dysbiosis, and Brain-Derived Neurotrophic Factor. In Current Thoughts on Dementia; Springer Nature: Singapore, 2022; pp. 311–353.
- Benmelouka, A.; Sherif, A.M.; Ebada, M.A. A Review of the Relationship Between Gut Microbiota and Memory. In Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease; Springer: Singapore, 2019; pp. 151–165.
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. MicroRNAs in plasma and cerebrospinal fluid as potential markers for Alzheimer’s disease. J. Alzheimers. Dis. 2014, 39, 253–259.
- Müller, M.; Jäkel, L.; Bruinsma, I.B.; Claassen, J.A.; Kuiperij, H.B.; Verbeek, M.M. MicroRNA-29a Is a Candidate Biomarker for Alzheimer’s Disease in Cell-Free Cerebrospinal Fluid. Mol. Neurobiol. 2016, 53, 2894–2899.
- Liang, Y.; Wang, L. Inflamma-MicroRNAs in Alzheimer’s Disease: From Disease Pathogenesis to Therapeutic Potentials. Front. Cell. Neurosci. 2021, 15, 785433.
- Vest, R.S.; Pike, C.J. Gender, sex steroid hormones, and Alzheimer’s disease. Horm. Behav. 2013, 63, 301–307.
- Pan, Q.; Guo, K.; Xue, M.; Tu, Q. Estradiol exerts a neuroprotective effect on SH-SY5Y cells through the miR-106b-5p/TXNIP axis. J. Biochem. Mol. Toxicol. 2021, 35, e22861.
- Guglielmotto, M.; Manassero, G.; Vasciaveo, V.; Venezia, M.; Tabaton, M.; Tamagno, E. Estrogens Inhibit Amyloid-β-Mediated Paired Helical Filament-Like Conformation of Tau Through Antioxidant Activity and miRNA 218 Regulation in hTau Mice. J. Alzheimers. Dis. 2020, 77, 1339–1351.
- Bonetti, L.; Bruzzone, S.E.P.; Sedghi, N.A.; Haumann, N.T.; Paunio, T.; Kantojarvi, K.; Kliuchko, M.; Vuust, P.; Brattico, E. Brain predictive coding processes are associated to COMT gene Val158Met polymorphism. Neuroimage 2021, 233, 117954.
- Tatura, R.; Kraus, T.; Giese, A.; Arzberger, T.; Buchholz, M.; Höglinger, G.; Müller, U. Parkinson’s disease: SNCA-, PARK2-, and LRRK2- targeting microRNAs elevated in cingulate gyrus. Park. Relat. Disord. 2016, 33, 115–121.
- Chatterjee, P.; Roy, D. Comparative analysis of RNA-Seq data from brain and blood samples of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017, 484, 557–564.
- Goh, S.Y.; Chao, Y.X.; Dheen, S.T.; Tan, E.-K.; Tay, S.S.-W. Role of MicroRNAs in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 5649.
- Steiner, D.F.; Thomas, M.F.; Hu, J.K.; Yang, Z.; Babiarz, J.E.; Allen, C.D.C.; Matloubian, M.; Blelloch, R.; Ansel, K.M. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity 2011, 35, 169–181.
- Bai, X.; Tang, Y.; Yu, M.; Wu, L.; Liu, F.; Ni, J.; Wang, Z.; Wang, J.; Fei, J.; Wang, W.; et al. Downregulation of blood serum microRNA 29 family in patients with Parkinson’s disease. Sci. Rep. 2017, 7, 5411.
- Liu, X.; Chen, J.; Guan, T.; Yao, H.; Zhang, W.; Guan, Z.; Wang, Y. miRNAs and target genes in the blood as biomarkers for the early diagnosis of Parkinson’s disease. BMC Syst. Biol. 2019, 13, 10.
- Martinez, B.; Peplow, P. V Altered microRNA expression in animal models of Huntington’s disease and potential therapeutic strategies. Neural Regen. Res. 2021, 16, 2159–2169.
- Nampoothiri, S.S.; Fayaz, S.M.; Rajanikant, G.K. A Novel Five-Node Feed-Forward Loop Unravels miRNA-Gene-TF Regulatory Relationships in Ischemic Stroke. Mol. Neurobiol. 2018, 55, 8251–8262.
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell. Neurosci. 2013, 7, 220.
- Wei, N.; Xiao, L.; Xue, R.; Zhang, D.; Zhou, J.; Ren, H.; Guo, S.; Xu, J. MicroRNA-9 Mediates the Cell Apoptosis by Targeting Bcl2l11 in Ischemic Stroke. Mol. Neurobiol. 2016, 53, 6809–6817.
- Chen, S.; Wang, M.; Yang, H.; Mao, L.; He, Q.; Jin, H.; Ye, Z.-m.; Luo, X.-y.; Xia, Y.-p.; Hu, B. LncRNA TUG1 sponges microRNA-9 to promote neurons apoptosis by up-regulated Bcl2l11 under ischemia. Biochem. Biophys. Res. Commun. 2017, 485, 167–173.
- Nampoothiri, S.S.; Rajanikant, G.K. miR-9 Upregulation Integrates Post-ischemic Neuronal Survival and Regeneration In Vitro. Cell. Mol. Neurobiol. 2019, 39, 223–240.
- Wang, Q.; Wang, F.; Fu, F.; Liu, J.; Sun, W.; Chen, Y. Diagnostic and prognostic value of serum miR-9-5p and miR-128-3p levels in early-stage acute ischemic stroke. Clinics 2021, 76, e2958.
- Wu, Z.; Wang, L.; Li, G.; Liu, H.; Fan, F.; Li, Z.; Li, Y.; Gao, G. Increased expression of microRNA-9 predicts an unfavorable prognosis in human glioma. Mol. Cell. Biochem. 2013, 384, 263–268.
- Tan, X.; Wang, S.; Yang, B.; Zhu, L.; Yin, B.; Chao, T.; Zhao, J.; Yuan, J.; Qiang, B.; Peng, X. The CREB-miR-9 Negative Feedback Minicircuitry Coordinates the Migration and Proliferation of Glioma Cells. PLoS ONE 2012, 7, e49570.
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 99.
- Ye, X.; Wei, W.; Zhang, Z.; He, C.; Yang, R.; Zhang, J.; Wu, Z.; Huang, Q.; Jiang, Q. Identification of MicroRNAs Associated with Glioma Diagnosis and Prognosis. Oncotarget 2017, 8, 26394–26403.
- Liu, Y.; Guo, L. MicroRNA-9 regulates the proliferation, migration and invasion of human glioma cells by targeting CDH1. J. B.U.ON. 2020, 25, 1091–1097.
- Shi, C.; Rao, C.; Sun, C.; Yu, L.; Zhou, X.; Hua, D.; Wang, R.; Luo, W.; Jiang, Z.; Zhou, J.; et al. miR-29s function as tumor suppressors in gliomas by targeting TRAF4 and predict patient prognosis. Cell Death Dis. 2018, 9, 1078.
- Madeddu, P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp. Physiol. 2005, 90, 315–326.
- Ning, R.; Xiong, Y.; Mahmood, A.; Zhang, Y.; Meng, Y.; Qu, C.; Chopp, M. Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury. Brain Res. 2011, 1384, 140–150.
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.C.; Wu, C.C.; Chuang, D.M.; Chiang, Y.H.; Chen, K.Y. Transplantation of mesenchymal stem cells overexpressing fibroblast growth factor 21 facilitates cognitive recovery and enhances neurogenesis in a mouse model of traumatic brain injury. J. Neurotrauma 2020, 37, 14–26.
- Wu, J.; He, J.; Tian, X.; Li, H.; Wen, Y.; Shao, Q.; Cheng, C.; Wang, G.; Sun, X. Upregulation of miRNA-9-5p Promotes Angiogenesis after Traumatic Brain Injury by Inhibiting Ptch-1. Neuroscience 2020, 440, 160–174.
- Zhang, A.; Lu, Y.; Yuan, L.; Zhang, P.; Zou, D.; Wei, F.; Chen, X. MiR-29a-5p Alleviates Traumatic Brain Injury-(TBI-) Induced Permeability Disruption via Regulating NLRP3 Pathway. Dis. Markers 2021, 2021, 9556513.