
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristina Maria Marginean | -- | 2240 | 2023-02-16 09:34:48 | | | |
| 2 | Lindsay Dong | Meta information modification | 2240 | 2023-02-17 02:55:12 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2240 | 2023-02-17 02:56:22 | | |
Video Upload Options
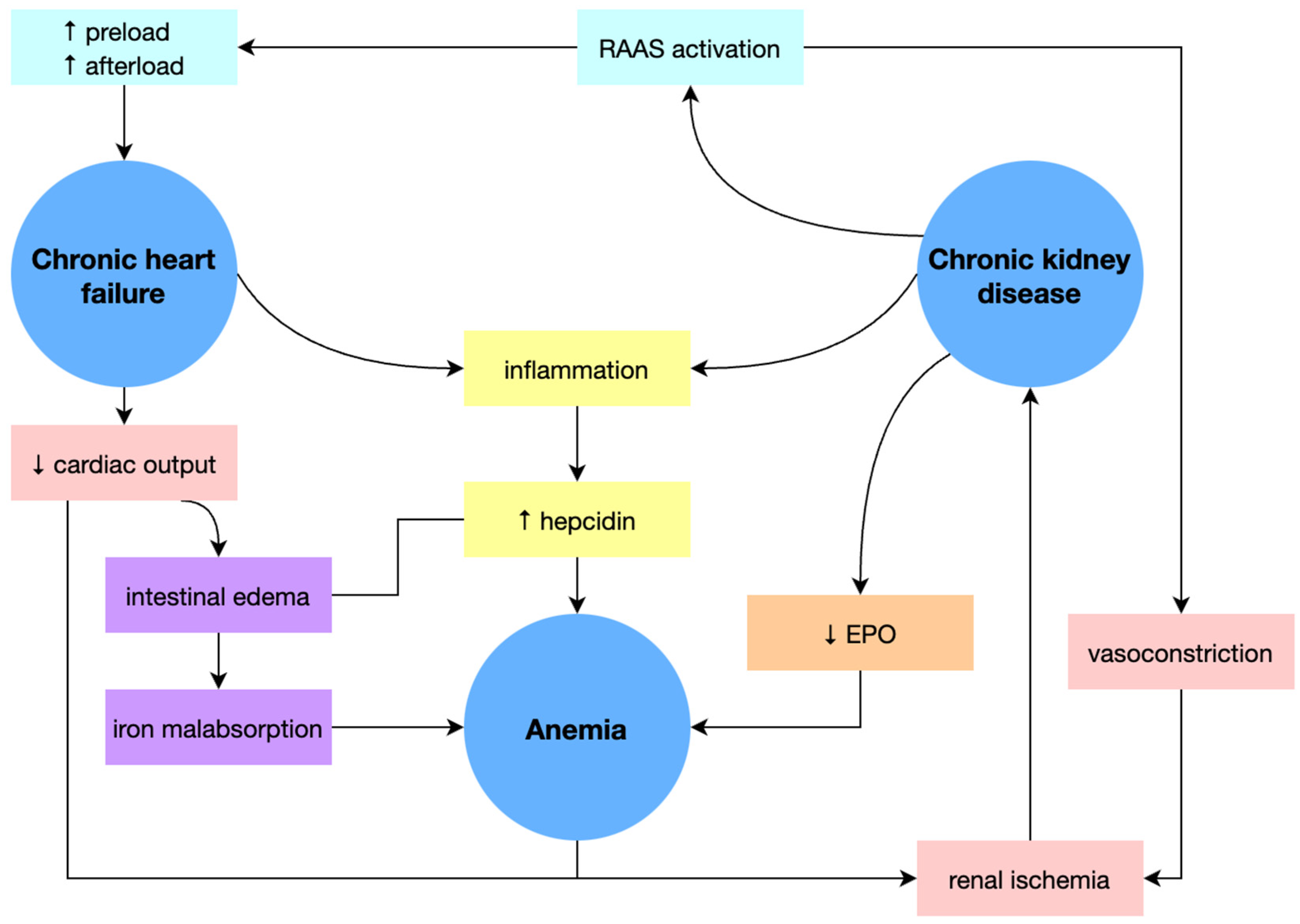
The association of chronic heart failure (CHF) and iron deficiency (ID) with or without anemia is frequently encountered in current medical practice and has a negative prognostic impact, worsening patients’ exercise capacity and increasing hospitalization costs. Moreover, anemia is common in patients with chronic kidney disease (CKD) and CHF, an association known as cardio-renal anemia syndrome (CRAS) possessing a significantly increased risk of death.
1. Introduction
2. Iron Deficiency Anemia
2.1. Physiology and Physiopathology
2.2. Clinical and Biological Features of Iron Deficiency Anemia
-
Iron depletion without anemia, characterized by exhaustion of iron stores, preserved serum iron, and hemoglobin levels.
-
Moderate normocytic normochromic anemia is preceded by lowering serum iron and increasing total iron-binding capacity (TIBC).
-
Severe microcytic hypochromic anemia, accompanied by characteristic clinical and biological features, facilitates the diagnosis.
2.3. Causes of Iron Deficiency
| Causes of Iron Deficiency Anemia | |
|---|---|
| 1. Anemia due to martial deficiency | |
| (a) insufficient reserves: | prematurity, twinship, neonatal hemorrhages, maternal anemia |
| (b) insufficient food intake: | diet with excess flour, exclusive diet with goat’s milk, protein and vitamin deficiencies, vegetarian diet |
| (c) deficient absorption: | presence of inhibitory factors (phytate, phosphates, carbonates), lack of reducing factors (vitamin C, hydrochloric acid, bile acids), celiac disease, gastrectomy, Helicobacter Pylori infection, intestinal resections, bacterial overgrowths |
| 2. Iron-loss anemia | |
| (a) gastro-intestinal hemorrhages: | esophageal varices (liver cirrhosis), diaphragmatic hernia, esophagitis, gastro-duodenal ulcer, cancer of the digestive tract (esophageal, gastric, colonic cancer), tumors of the small intestine, Vaterian ampulloma, hemorrhoids, rectal polyps, intestinal parasites, celiac disease, Crohn’s disease, ulcerative colitis, colonic angiodysplasia, bariatric surgery, NSAIDs consumption |
| (b) hemorrhages of respiratory origin: | epistaxis, pulmonary tuberculosis, lung cancer, bronchiectasis, pulmonary microinfarcts, alveolar hemorrhage |
| (c) genito-urinary hemorrhages: | prolonged menstrual cycle, metrorrhagia, renal tuberculosis, renovesical cancer, hemorrhagic nephritis, hemodialysis |
| (d) hemorrhagic diathesis: | alteration of the capillary wall, alteration of platelets, combined alterations |
| (f) hypersplenism: | |
| (g) genetic causes: | iron-refractory iron deficiency anemia |
| (h) mechanical fragmentation of RBCs: | prosthetic valves |
| (i) endocrine diseases: | hypothyroidism, pituitary insufficiency, autoimmune polyglandular syndromes |
| (j) autoimmune diseases: | scleroderma, rheumatoid arthritis, lupus |
| (k) drugs: | anticoagulants, antiaggregants, NSAIDs |
| (l) CHF, CKD. | |
3. Anemia and Iron Deficiency in Heart Failure
3.1. Causes and Etiopathogenic Mechanisms of Iron Deficiency and Anemia in Chronic Heart Failure
3.1.1. Absolute Iron Deficiency
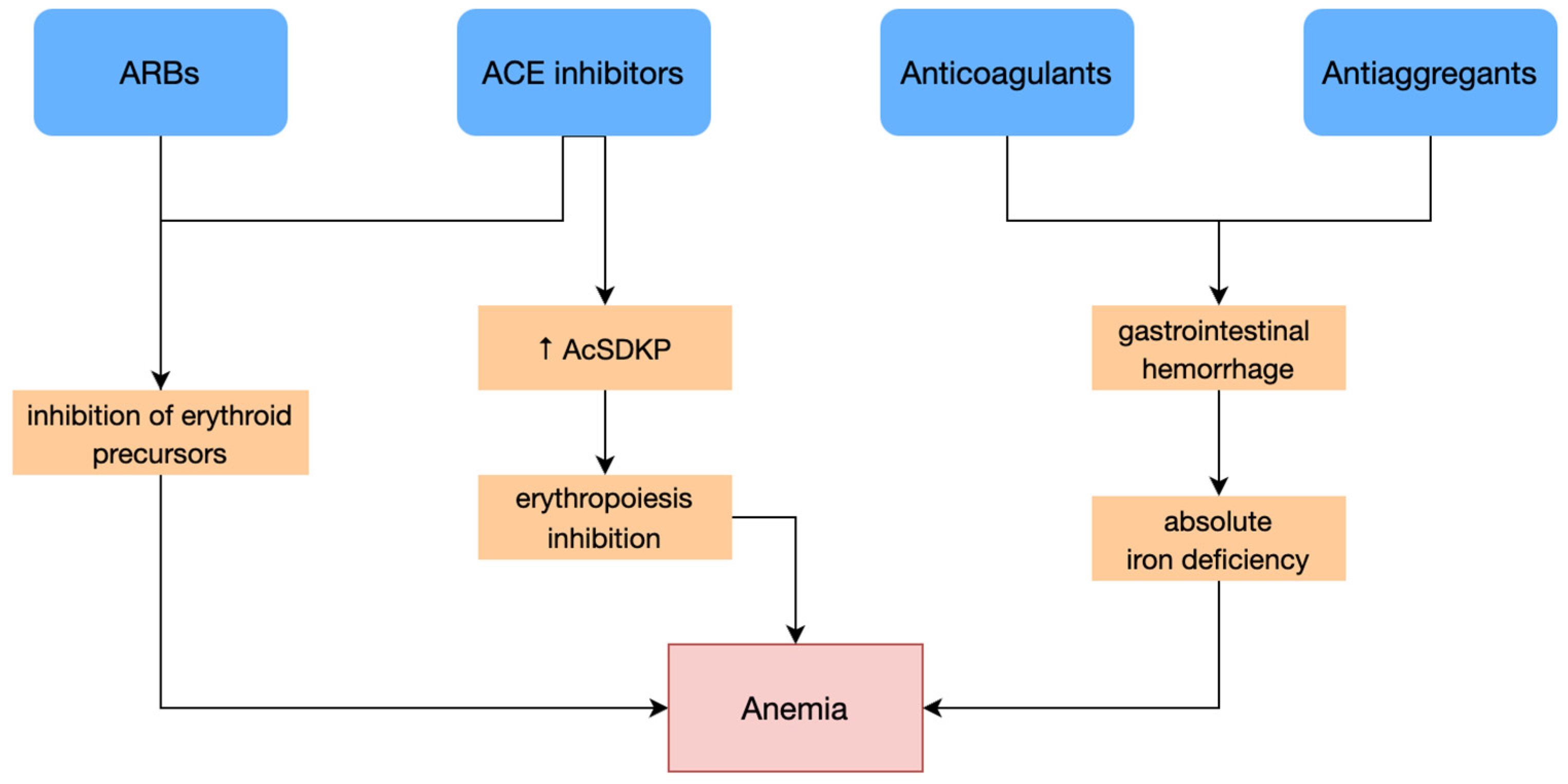
3.1.2. Functional Iron Deficiency
3.1.3. Cardio-Renal Anemia Syndrome
3.2. Diagnosis of Iron Deficiency
There is a relatively high rate of underdiagnosis of ID because anemia is often not clinically evident. Furthermore, anemia may be completely absent, as it needs a specific time frame to develop after the appearance of ID.
Diagnostic criteria for ID in CHF patients, with or without anemia, refer to serum ferritin <100 µg/L, or ferritin between 100 µg/L and 299 µg/L, and transferrin saturation <20% [19].
3.3. Clinical Consequences of Iron Deficiency in Heart Failure
3.4. Management of Iron Deficiency in Heart Failure
3.4.1. Intravenous Iron Supplementation Therapy

| Ferric Carboxymaltose | Parameter | Oral Iron Supplements |
|---|---|---|
| Significant | ↑ Hemoglobin levels | Minimal |
| + | NYHA class improvement | − |
| + | HRQL improvement | − |
| + | ↑ Exercise capacity | − |
| +/− | Gastro-intestinal adverse effects | + |
3.4.2. Oral Iron Supplementation Therapy
3.4.3. Blood Transfusion
3.4.4. Erythropoiesis-Stimulating Agents
References
- Dunn, L.L.; Rahmanto, Y.S.; Richardson, D.R. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007, 17, 93–100.
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006, 1, 25–39.
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98.
- Camaschella, C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017, 31, 225–233.
- DeLoughery, T.G. Iron Deficiency Anemia. Med. Clin. N. Am. 2017, 101, 319–332.
- Evstatiev, R.; Gasche, C. Iron sensing and signalling. Gut 2012, 61, 933–952.
- Chifman, J.; Laubenbacher, R.; Torti, S.V. A Systems Biology Approach to Iron Metabolism. Adv. Exp. Med. Biol. 2014, 844, 201–225.
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron deficiency anaemia revisited. J. Intern. Med. 2019, 287, 153–170.
- Felker, G.M.; Adams, K.F.; Gattis, W.A.; O’Connor, C.M. Anemia as a risk factor and therapeutic target in heart failure. J. Am. Coll. Cardiol. 2004, 44, 959–966.
- Jankowska, E.A.; Von Haehling, S.; Anker, S.D.; MacDougall, I.C.; Ponikowski, P. Iron deficiency and heart failure: Diagnostic dilemmas and therapeutic perspectives. Eur. Heart J. 2012, 34, 816–829.
- Silverberg, D.S.; Wexler, D.; Schwartz, D. Is Correction of Iron Deficiency a New Addition to the Treatment of the Heart Failure. Int. J. Mol. Sci. 2015, 16, 14056–14074.
- Sânpălean, R.M.; Petra, D.N. Actualizări privind anemia in insuficienţa cardiacă. Medic.ro 2020.
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.V.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093.
- von Haehling, S.; Anker, S.D. Cardio-Renal Anemia Syndrome. Contrib. Nephrol. 2011, 171, 266–273.
- Opasich, C.; Cazzola, M.; Scelsi, L.; De Feo, S.; Bosimini, E.; Lagioia, R.; Febo, O.; Ferrari, R.; Fucili, A.; Moratti, R.; et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur. Heart J. 2005, 26, 2232–2237.
- Comín-Colet, J.; Ruiz, S.; Cladellas, M.; Rizzo, M.; Torres, A.; Bruguera, J. A Pilot Evaluation of the Long-term Effect of Combined Therapy with Intravenous Iron Sucrose and Erythropoietin in Elderly Patients with Advanced Chronic Heart Failure and Cardio-Renal Anemia Syndrome: Influence on Neurohormonal Activation and Clinical Outcomes. J. Card. Fail. 2009, 15, 727–735.
- Ishani, A.; Weinhandl, E.; Zhao, Z.; Gilbertson, D.T.; Collins, A.J.; Yusuf, S.; Herzog, C.A. Angiotensin-converting enzyme inhibitor as a risk factor for the development of anemia, and the impact of incident anemia on mortality in patients with left ventricular dysfunction. J. Am. Coll. Cardiol. 2005, 45, 391–399.
- Luthi, J.-C.; Flanders, W.D.; Burnier, M.; Burnand, B.; McClellan, W.M. Anemia and chronic kidney disease are associated with poor outcomes in heart failure patients. BMC Nephrol. 2006, 7, 3.
- Jankowska, E.A.; Tkaczyszyn, M.; Drozd, M.; Ponikowski, P. Monitoring of iron status in patients with heart failure. Eur. Heart J. Suppl. 2019, 21, M32–M35.
- Toblli, J.E.; Lombraña, A.; Duarte, P.; Di Gennaro, F. Intravenous Iron Reduces NT-Pro-Brain Natriuretic Peptide in Anemic Patients with Chronic Heart Failure and Renal Insufficiency. J. Am. Coll. Cardiol. 2007, 50, 1657–1665.
- Enjuanes, C.; Klip, I.T.; Bruguera, J.; Cladellas, M.; Ponikowski, P.; Banasiak, W.; van Veldhuisen, D.J.; van der Meer, P.; Jankowska, E.A.; Comín-Colet, J. Iron deficiency and health-related quality of life in chronic heart failure: Results from a multicenter European study. Int. J. Cardiol. 2014, 174, 268–275.
- Fitzsimons, S.; Doughty, R.N. Iron deficiency in patients with heart failure. Eur. Heart J.-Cardiovasc. Pharmacother. 2015, 1, 58–64.
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; von Haehling, S.; Doehner, W.; Banasiak, W.; et al. Iron Deficiency Predicts Impaired Exercise Capacity in Patients with Systolic Chronic Heart Failure. J. Card. Fail. 2011, 17, 899–906.
- Beard, J.L. Iron Biology in Immune Function, Muscle Metabolism and Neuronal Functioning. J. Nutr. 2001, 131, 568S–580S.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200.
- Lewis, G.D.; Malhotra, R.; Hernandez, A.F.; McNulty, S.E.; Smith, A.; Felker, G.M.; Tang, W.H.W.; LaRue, S.J.; Redfield, M.M.; Semigran, M.J.; et al. Effect of Oral Iron Repletion on Exercise Capacity in Patients with Heart Failure with Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA 2017, 317, 1958–1966.
- Qaseem, A.; Humphrey, L.L.; Fitterman, N.; Starkey, M.; Shekelle, P.; for the Clinical Guidelines Committee of the American College of Physicians. Treatment of Anemia in Patients with Heart Disease: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med. 2013, 159, 770–779.
- Silverberg, D.S.; Wexler, D.; Iaina, A. The Role of Anemia in the Progression of Congestive Heart Failure. Is There a Place for Erythropoietin and Intravenous Iron. J. Nephrol. 2004, 17, 749–761.
- Ghali, J.K.; Anand, I.; Abraham, W.T.; Fonarow, G.; Greenberg, B.; Krum, H.; Massie, B.M.; Wasserman, S.M.; Trotman, M.-L.; Sun, Y.; et al. Randomized Double-Blind Trial of Darbepoetin Alfa in Patients with Symptomatic Heart Failure and Anemia. Circulation 2008, 117, 526–535.




