A biocatalyst is an enzyme that speeds up or slows down the rate at which a chemical reaction occurs and speeds up certain processes by 108 times. It is used as an anticancer agent because it targets drug activation inside the tumor microenvironment while limiting damage to healthy cells. Biocatalysts have been used for the synthesis of different heterocyclic compounds and is also used in the nano drug delivery systems. The use of nano-biocatalysts for tumor-targeted delivery not only aids in tumor invasion, angiogenesis, and mutagenesis, but also provides information on the expression and activity of many markers related to the microenvironment. Iosmapinol, moclobemide, cinepazide, lysine dioxygenase, epothilone, 1-homophenylalanine, and many more are only some of the anticancer medicines that have been synthesised using biocatalysts.
1. Introduction
Biocatalysis has become an essential component of modern organic synthesis, both in the commercial and academic spheres of activity and in green synthesis. Its success may be attributed in large part to the creation of cutting-edge methodologies for the discovery of novel enzymes and the application of a high-throughput laboratory. Biocatalysts can be used for preparation on a grammes (g) to kilogrammes (kg) scale. It has also become an alternative to chemical catalysis in recent years, and it is currently being used in a diverse field
[1][2][3][4][5]. Over the last two decades, the number of biocatalytic tools, such as newly developed catalysts with specifically tuned features and novel ideas for reaction, has expanded tremendously
[5][6]. The initial stages in biocatalysis include identifying the target reaction, searching for biocatalysts, characterizing those biocatalysts, and using them in different fields. Biocatalysts are also used in recombinant enzyme systems; from the extraction of natural sources to gene mining using bioinformatics techniques, the screening of biocatalysts has evolved rapidly. They are effective in reactions having multiple steps as they offer an environment that is protective of enzymes (
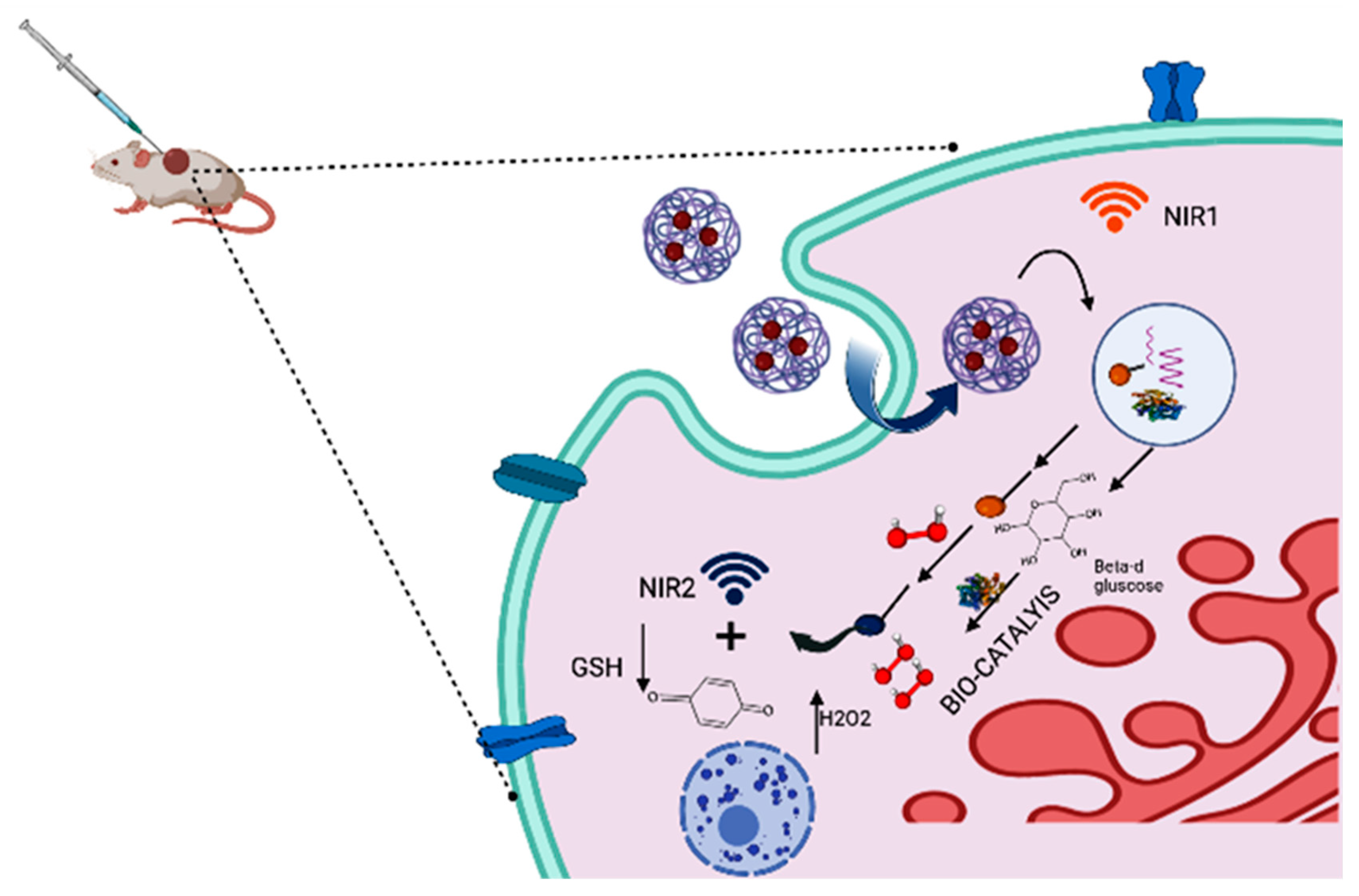
Figure 1)
[7][8][9][10].
Figure 1. Mechanistic pathways of biocatalyst.
Biocatalysts can be identified using genomic and metagenomic techniques, such as sequence-based methods that search for biocatalysts homologous to presently known biocatalysts, and PCR-based techniques that use primers designed in accordance with methods to conserve regions of known enzymes or functional metagenomics, where genomic libraries are constructed, such as fosmid- and cosmid-based repositories with 25–40 kb DNA inserts, and screened with the DNA used directly from environmental metagenomes. In order to successfully construct biocatalysts, it is necessary to engage in rational planning and selectivity
[8][11] in enzymes to catalyse chemical reactions in industrial processes such as the manufacture of drug substances, flavours, fragrances, chemicals, and polymers. The incorporation of biocatalysis into conventional chemical manufacturing will result in increased longevity of the process, less environmental toxicity, and a decrease in the production cost
[12][13][14].
2. Role of Biocatalyst in Tumor Tissue
Tumor tissue has a variety of enzyme expression patterns, which is useful for creating effective enzyme-responsive nano-drug delivery systems. Designing a stimulus-responsive mechanism that can only deliver the potential drug in the tumor microenvironment (TME) is one way to get a drug to accumulate in tumors. The enzyme substrate which is found in the TME can be used to connect the drug to its carrier
[15][16][17]. An alternative is to encapsulate the drug in a carrier that can be precisely broken down by these enzymes in the TME. The benefit of this approach includes fast-released drug molecules, superior tumor tissue penetration, and effectiveness. The GPX4 enzyme can be inhibited by the Fe3+ ion and tannic acid to initiate ferroptosis and inhibit tumors
[18]. Carboxyl-esterase-responsive albumins coated with folate form a nanocluster (FHP) useful for precision cancer theranostics and is enzyme-triggered
[19].
2.1. Matrix metalloproteinases as Biocatalyst
Matrix metalloproteinase (MMP) enzyme can be used as a tumor-specific catalyst to promote the release of active drugs in prostate tumor tissues since they are overexpressed in a wide range of tumors. Formononetin, an isoflavone, is found to have anti-migration properties when tested on MDA-MB-231 and 4T1 breast cancer cells. MMP-2 and MMP-9 might be inhibited by formononetin via the PI3K/Akt pathways. This research’s findings demonstrated improved perfusion and hypoxia reduction by removing tumor microenvironment barriers in the tumor location
[20][21][22]. Using the pancreatic stellate cell type in BALB/c nude mice, matrix-metallopeptidase-2-sensitive and RGD-peptide-modified liposomes are found to contain gemcitabine and pirfenidone
[23]. In another finding, the treatment of pancreatic-tumor-bearing animals with an intravenous injection of a therapeutic drug carrier containing monomethyl auristatin E, which was aimed at albumin where β-glucuronidase was highly expressed, produced a remarkable effect
[24].
2.2. Polysaccharides
A significant role is played by polysaccharides during enzyme-targeted treatment of colorectal cancer. These polymers are natural, biodegradable, and biocompatible, and also function as drug-delivery systems. Colonic enzyme-responsive oligoesters and cross-linked nanoparticles that are based on dextran and transport 5-FU were created. It was observed that the compound released no drug under the pH conditions of the small intestine and stomach but released 75% of the drug after 12 h of glucanase incubation
[25].
2.3. Prostate-Specific Antigen
Prostate-specific antigen (PSA) is a 33 kDa mono-chain glycoprotein and a serine protease that is androgen-regulated and a member of the glandular Kallikrein family. The FDA authorized a PSA test for prostate cancer progression assessment in 1986. A novel target drug delivery method for prostate cancer treatment might be created using a substrate-drug combination. The 7-mer peptide group (His-Ser-Ser-Lys-Leu-Gln-Leu) could be cleaved by PSA, and a prodrug was designed by conjugating this peptide to doxorubicin
[26][27][28][29]. Cathepsins A, B, C, D, E, F, G, H, L, K, O, S, V, and W are overexpressed in various forms of human cancer and belong to the family of endopeptidases. Cathepsins B, C, F, H, L, K, O, S, V, W, and X are cysteine proteases; cathepsins D and E are aspartic proteases, and cathepsins A and G are serine carboxypeptidases
[30][31]. Prostate cancer cells overexpress the protein sub-MMPs, and the amount of expression is connected with the development of tumors. All cathepsins are developed in an inactive state, and the low pH of lysosomes can activate the majority of its members
[32].
2.4. Indoleamine 2,3-Dioxygenase as Biocatalyst
With an inherent biocompatibility, absence of toxicity, and specific catalysis against -d-glucose, glucose oxidase (GOx) has garnered an increased amount of attention as a biocatalyst in the field of biomedicine. It does this by catalyzing the transformation of glucose into hydrogen peroxide and gluconic acid in an effective manner, hence increasing the concentrations of these chemicals in the microenvironment of the tumors
[33]. Influencing immunological responses and contributing to the progression of cancer, indoleamine 2,3-dioxygenase (IDO) is an essential enzyme in the breakdown of tryptophan. In the preclinical research phase, increased efforts have been made to construct IDO-inhibitor nanomedicines for tumor-targeted delivery. In the clinical research phase, efforts have also been increased to optimise the IDO-inhibitor-based combination therapy
[34][35]. Because they play such an important part in tumor invasion, angiogenesis, and metastasis, proteases are a good candidate for use as a target for imaging probes in the early identification and treatment of cancer but also provide information on the activity and expression of a variety of markers linked with the microenvironment of the tumors
[35][36].
3. Biocatalysts Used in Nanoparticles for Tumor-Targeted Delivery
Different biological characteristics are significantly impacted in tumorous tissues compared to normal tissues that can detect these changes and deliver the drug to a particular site
[37]. Achieving successful precise therapeutic administration and reducing the toxicity of traditional chemotherapeutic drugs in off-target tissues are both benefits of the drug-delivery systems’ tumor-targeting and stimulus-response behavior. The administration of drugs using nanoparticles is a new method. The nanoparticle’s hydrophilic nature helps them to avoid detection by the reticuloendothelial systems, their submicrometric size promotes their uptake by cells via phagocytosis, and their intrinsic stability avoids breakdown in the blood circulatory system.
In addition, they may have a large surface area, a precise distribution of pore sizes, and, if necessary, specialized surface acid–base characteristics for site-specific adaptation. Docetaxel, gemcitabine, paclitaxel, and carboplatin can be quite successful. Cisplatin has the drawback of causing nephrotoxicity, ototoxicity, hepatotoxicity, cardiotoxicity, and neurotoxicity in non-target tissues, necessitating tumor-site-specific drug administration to prevent this toxicity from occurring. By reacting with carboxyl ions such as cisplatin-poly (methacrylic acid), cisplatin-PEOpoly (aspartic acid or glutamic acid), or cisplatin-PEG-b-poly (aspartic acid), researchers have created a variety of complexes with cisplatin
[38][39][40][41].
3.1. Site-Specific Drug Delivery
Nanomedicine-based drug delivery systems are becoming more popular for the treatment of cancer
[42]. This is due to the fact that these systems have stimuli-responsive smart nanocarriers, which result in better site-specific drug delivery and enhanced drug solubility. The drug within the nanocarriers is shielded from the physiological environment while it is being transported to the target location by the nanocarriers
[43].
There are a number of different approaches that have been taken to allow receptor targeting and intelligent drug release by nanocarriers with stimulus sensitivity. The levels of numerous matrix metalloproteinase (MMP) enzymes are much greater in tumorous tissue. These enzymes are helpful for the regulated release of drugs from nanocarriers and may be found in higher concentrations in tumorous tissue. There is an overexpression of MMP enzymes, which are proteases that govern metastasis, tumor invasiveness, and angiogenic pathways in tumor cells. The MMP enzymes have the potential to degrade a wide variety of proteins that are found in biological systems, including gelatin, collagen, fibronectin, and others. For the purpose of developing a therapeutic strategy for the treatment of lung cancer, gelatin nanoparticles that are sensitive to MMP and mannose receptors were synthesized and tested for their capacity to target particular receptors and release medicines at the appropriate time. Cisplatin was complexed with the gelatin matrix (CG-NP) and surface-decorated with con-A with the aim of assessing how effectively lung cancer cells react to stimuli and the pattern of their release. Cisplatin was also used to determine the pattern of their release (CCG-NP)
[44][45][46].
3.2. Enzyme-Responsive Drug Delivery System
In order to target cancer, the nanosystem will become an enzyme-responsive drug delivery system to selectively target tumor cells. A class of heparanase-based, systematically released nanoparticles were enhanced with β-cyclodextrin-grafted heparin (NLC/H (D + F + S) NPs) and co-loaded with the TGF-β receptor inhibitor, doxorubicin, and ferrocene. Breast cancer metastasis was prevented by intracellular and extracellular hybrid mechanisms of the produced nanoparticles. In order to activate the ferroptosis pathway, doxorubicin and ferrocene loaded in NLC/H (D + F + S) NPs efficiently increase intracellular ROS levels. The augmented ROS also stimulated the apoptosis pathway and lowered MMP-9 expression to work in conjunction with ferroptosis for tumor treatment
[47][48][49].
Mesoporous silica nanoparticles (MSNs) produce a matrix-metalloproteinase-responsive drug-delivery system. MSNs were immobilized with the substrate peptide PLGLAR by an amidation process. Additionally, to prevent the mesopores of polysulfonic mucopolysaccharide, bovine serum albumin was employed as an end cap. When combined with immunotherapy, MSNs reacted to the overexpressed matrix metalloproteinases in the tumor resulting in the regulated release of the loaded drug and adverse effects
[50].
3.3. Photothermal Therapy
Photothermal treatment (PTT) is a strong and non-invasive therapeutic alternative for treating many different forms of cancer. It is an acid-triggered self-destructing nano-biocatalyst for triple therapy (starvation, chemical, and photothermal combination), and these techniques are used to kill cancer cells. An enzyme-responsive nanomedicine called Pd-DOX@TGMs is injected in the microenvironment of the tumors with palladium and doxorubicin nanoparticles. The combination of chemotherapy and photothermal treatment resulted in an increase in the release of molecules such as adenosine triphosphate, calreticulin, and high mobility group box 1 protein. These chemicals increased the immunogenicity of the tumor cells that had died. More notably, the combination treatment activated the immune checkpoint defense (ICD), which in turn successfully inhibited the PD-L1 checkpoint and effectively reversed the immunosuppressive microenvironment
[51][52].
The biocatalyst used in different drugs and their combination in various drug delivery system are summarized in Table 1.