Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yaqian Zhao | -- | 2717 | 2023-02-14 02:14:35 | | | |
| 2 | Sirius Huang | Meta information modification | 2717 | 2023-02-14 02:19:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, A.; Zhao, Y.; Cai, Y.; Kang, P.; Huang, Y.; Li, M.; Yang, A. Hospital Wastewater Treatment Processes. Encyclopedia. Available online: https://encyclopedia.pub/entry/41182 (accessed on 08 February 2026).
Liu A, Zhao Y, Cai Y, Kang P, Huang Y, Li M, et al. Hospital Wastewater Treatment Processes. Encyclopedia. Available at: https://encyclopedia.pub/entry/41182. Accessed February 08, 2026.
Liu, Ang, Yaqian Zhao, Yamei Cai, Peiying Kang, Yulong Huang, Min Li, Anran Yang. "Hospital Wastewater Treatment Processes" Encyclopedia, https://encyclopedia.pub/entry/41182 (accessed February 08, 2026).
Liu, A., Zhao, Y., Cai, Y., Kang, P., Huang, Y., Li, M., & Yang, A. (2023, February 14). Hospital Wastewater Treatment Processes. In Encyclopedia. https://encyclopedia.pub/entry/41182
Liu, Ang, et al. "Hospital Wastewater Treatment Processes." Encyclopedia. Web. 14 February, 2023.
Copy Citation
Hospital wastewater contains a considerably higher concentration of drug residues (antibiotics, β-receptor blockers, analgesics, etc.) than municipal wastewater. Each hospital wastewater treatment process has its own merits, but combinatory processes frequently achieve higher levels of treatment effectiveness.
hospital wastewater
constructed wetlands
MBR
Fenton oxidation
1. Introduction
Hospitals offer patients medical exams, therapy, nursing, and consultations, while a hospital’s treatment department, laboratories, wards, and living facilities for administrative employees all generate wastewater [1]. Due to its varied sources, hospital wastewater contains a high organic load, heavy metals, bacteria, and viruses [2]. During the era of epidemics, medical resources have been constrained, resulting in a substantial quantity of hospital wastewater [1]. Hence, the safe treatment of hospital wastewater is particularly important.
During the evolution of wastewater treatment technology, the activated sludge process was the first to emerge. It was the treatment process most commonly employed in wastewater treatment plants (WWTPs) [3]. The activated sludge process (ASP) effectively removes the majority of macromolecular pollutants but is ineffective against bacteria and viruses. Thus, membrane bioreactors (MBRs) were employed to aid sludge–water separation. An MBR’s built-in filter membrane has a small pore size and can filter the majority of pollutants in hospital wastewater [4]. Advanced techniques, such as Fenton oxidation, electrocoagulation, and the electro-peroxone process, can be used to successfully remove organic matter and drugs. However, such techniques are rarely employed in actual engineering due to technical problems and expenses [5]. Constructed wetlands (CWs) constitute a sustainable treatment process with low cost, simple operation, and landscape value [6]. In recent years, there have been a number of reports regarding the treatment of hospital wastewater by CWs systems. A brief summary of the features of hospital wastewater and its treatment processes is illustrated in Figure 1.
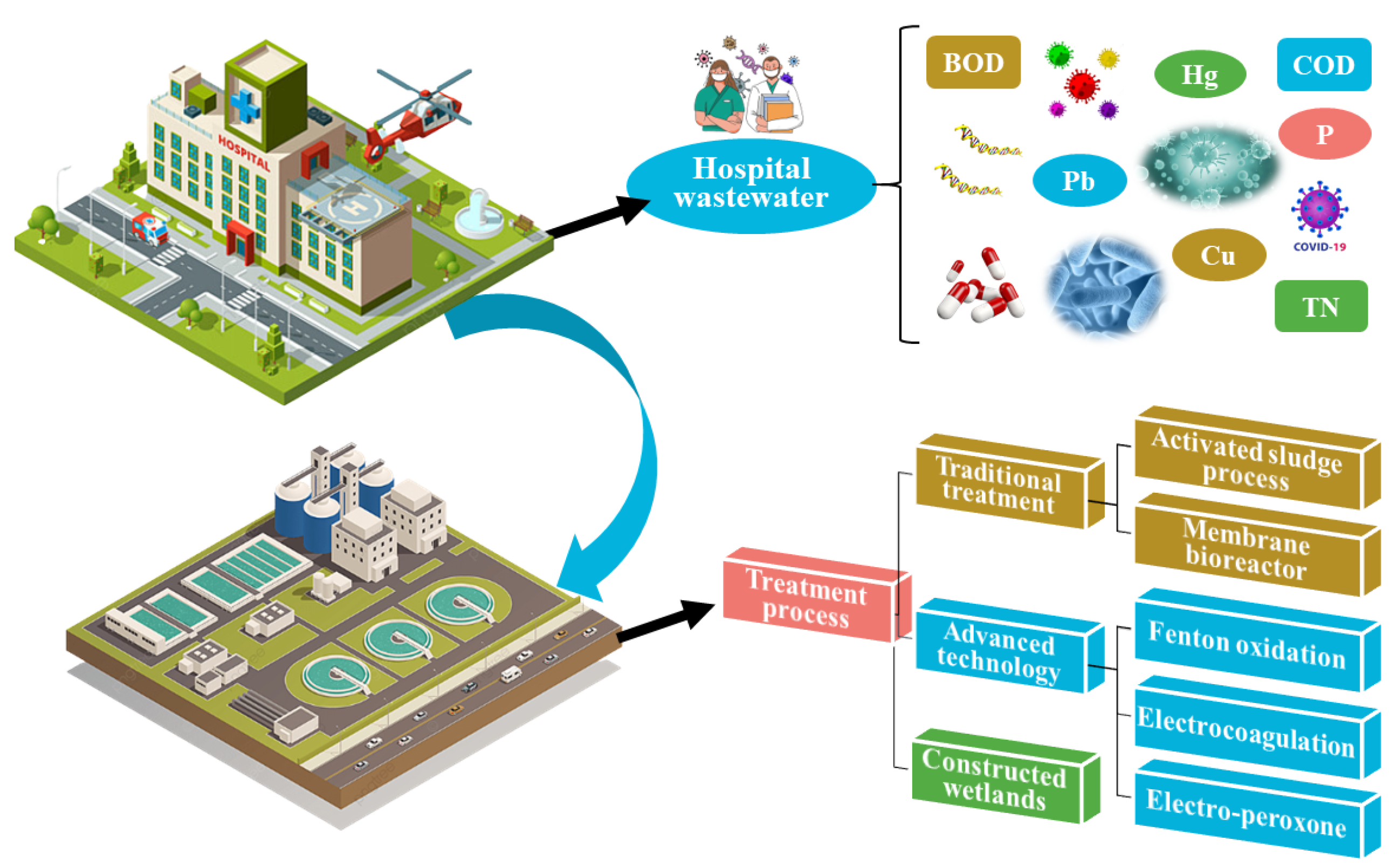
Figure 1. A schematic summary of hospital wastewater and its treatment processes.
The information and summary of the traditional treatments, advanced technology, and CWs regarding hospital wastewater from the literature are listed in Table 1. Each of the techniques is introduced from a technical perspective as well as with respect to their pros and cons for the purpose of identifying the best solution for hospital wastewater treatment.
Table 1. Traditional treatment, advanced technology, and application of CWs in hospital wastewater treatment.
| Number | Country | Details | Treatment | Water Quality Indexes | Reference |
|---|---|---|---|---|---|
| 1 | Spain | The pretreatment employed is coagulation, followed by the activated sludge process and UV/H2O2 disinfection Volume: 1 L HRT: 48 h SRT: 20–22 d |
ASP combined with UV/H2O2 | antibiotics and other drugs | [7] |
| 2 | Iran | An aeration tank equipped with an extended aeration device and a submerged biological filter. ASP Type: plug flow Size: L × W × H = 7 × 6.5 × 4 m. Biological filter: Size: L × W × H = 22 × 12 × 1.2 m Substrate: slag (specific surface area 2.49 m2/g, porosity 36%,density 2.96 g/cm3) |
ASP combined with Biological filter | COD, TSS, amoxicillin, ceftriaxone | [8] |
| 3 | Vietnam | MBR: Material: glass Size: L × W × H = 0.28 × 0.08 × 0.6 m Membrane modules: hollow fiber Pore size: 0.2 μm Flux: 10/15/20 LMH Ozone reactor: Size: W × H = 8×42 cm Working volume: 2 L Contact time: 20 min Supply rate: (20–40) mgO3/h The device runs for: 20 d HRT: 10/6.7/5 h; SRT: 20 d |
MBR combined with ozone oxidation | Norfloxacin, ciprofloxacin, ofloxacin, sulfamethoxazole, erythromycin, tetracycline, and trimethoprim | [9] |
| 4 | Vietnam | Volume: 8 L Size: L × W × H = 0.28 × 0.08 × 0.6 m Membrane modules: hollow fibers (surface area 0.05 m2) Pore size: 0.4 μm Flux: 20 LMH HRT: 8 h; SRT: 20 d |
MBR | COD; BOD; ciprofloxacin | [10] |
| 5 | Spain | Fe3+ source: Fe(NO3)3, c (Fe3+) = 25 mg/L Temperature: 70 °C Initial c (H2O2) = 2 g/L Initial PH = 3 |
Fenton oxidation | COD, TOC, drugs | [11] |
| 6 | Spain | Temperature: 20 °C Catalyst: c (Fe-BTC) = 0.6 g/L Initial c (H2O2) = 0.75 g/L Initial PH = 3 |
Fenton oxidation combined with photochemical catalysis | COD, TOC, drugs | [11] |
| 7 | China | The treatment plan: fine grid-ultrafiltration-catalytic wet oxidation Catalyst: carbonized red soil |
Catalytic wet oxidation | cephalexin, TOC | [12] |
| 8 | Spain | Temperature: 120–150 °C c (catalyst) = 1 g/L |
Catalytic wet oxidation | COD, TOC, drugs | [11] |
| 9 | Colombia | Material: cylindrical, plastic Size: L × W × H = 20 × 2.7 × 0.3 cm Working volume: 1 L Cathode and aluminum: iron and aluminum, respectively connected to a DC power supply. |
Electrocoagulation | COD, BOD, phenols, phosphates, TSS, naproxen | [13] |
| 10 | China | Working volume: 120 mL Anode and cathode: IrO2/RuO2 grids (effective size 2.5 × 2 × 0.1 cm) and graphite felt (effective size 2.5 × 2 × 1.2 cm), respectively, with a spacing of 2 cm. |
Electro-peroxone | TOC, COD, NH3-N, ciprofloxacin | [14] |
| 11 | China | Anode and cathode: platinum plate (3 × 3 cm) and graphite felt (effective area 42 cm2) Sacrificial anode: iron electrode (2 × 14 cm) connected to a DC power supply. |
Electro-peroxone combined with Sacrificial iron anode | TOC; ciprofloxacin | [5] |
| 12 | India | Type: horizontal subsurface flow Material: galvanized sheets Size: L × W × H = 1.2 × 0.6 × 0.6 m Plant: Australis phragmites Flow rate: 20 Ld−1. The outlet of CWs was connected to a tubesettler |
CWs combined with tubesettlers | paracetamol, ibuprofen, carbamazepine, lorazepam, erythromycin, ciprofloxacin, and simvastatin | [15] |
| 13 | Saudi Arabia | Size: L × W × H = 1 × 0.7 × 0.6 m Substrate: gravel and sand, Plant: Phragmites australis CWs performance was evaluated with respect to pre-monsoon, monsoon, and post-monsoon seasons |
CWs combined with tubesettlers | paracetamol, ibuprofen, carbamazepine, lorazepam, ciprofloxacin, sulfamethoxazole, and Fluvastatin. |
[16] |
| 14 | Saudi Arabia | Material: galvanized sheets Size: L × W × H = 1.5 × 0.65 × 0.5 m Substrate: sand plant: Phragmites Australis. |
CWs combined with tubesettlers | paracetamol, ketoprofen, carbamazepine, lorazepam, sulfamethoxazole, ciprofloxacin, and Fluvastatin | [6] |
| 15 | Thailand | Type: vertical flow Size: L × W × H = 1.5 × 0.6 × 0.6 m Substrate: sand and gravel plant: Scirpus validus |
CWs | paracetamol | [17] |
2. Traditional Treatment
The ASP and MBRs have a long history of technological advancement [3][18]. The two techniques have similar mechanisms of biological treatment. After mixing activated sludge with wastewater, a large volume of bacteria can biodegrade various pollutants via bio-respiration. This is followed by sludge–water separation to generate the effluent [3]. The ASP has played a key role in hospital wastewater treatment in the past, but its large volume of excess sludge production during treatment and final disposal makes it tedious and costly with respect to sustainable applications. Undoubtedly, MBRs enhance the separation of sludge and water. An MBR separates sludge from water using a filter membrane. The treatment is effective, and the occupancy area is small. The literature on traditional hospital wastewater treatment processes is presented in Table 1.
The combined process associated with the ASP can be used to eliminate pharmaceuticals and other contaminants, and such combinations include ASP followed by the use of a biofilter [8] or dosing activated carbon [19]. Mir-tutusaus et al. [7] reported an average removal rate of 83% for 22 pharmaceuticals when the ASP was combined with H2O2. In some studies, the removal rate of quinolones such as norfloxacin, ofloxacin, and ciprofloxacin by an MBR exceeded 90% [9][10][20][21][22]. An MBR’s filter membrane is often a microfiltration or ultrafiltration membrane. If the MBR device does not effectively treat bacteria or viruses, the addition of a nanofiltration membrane (1–2 nm) or reverse osmosis membrane (0.1–0.7 nm) is necessary. Most bacteria and viruses (including SARS-CoV-2) have a diameter larger than these parameters, and thus the filtering ability is sufficient to eliminate these pathogenic microorganisms [4][23].
As time passes, the filtration performance of the filter membrane will deteriorate, resulting in membrane fouling. At this point, the cleaning or replacement of the membrane is required [24]; otherwise, filtration efficiency will be reduced. Furthermore, models based on mathematics, artificial neural networks, random forests, and other technologies can forecast membrane fouling. Emphasis should be placed on the implementation of these technologies [25].
3. Advanced Technologies
The literature on the treatment of hospital wastewater by advanced technology is shown in Table 1. Fenton oxidation, photocatalysis, electrocoagulation, and the electro-peroxone process are effective for the removal of both organic matter and drugs from wastewater.
Fenton oxidation is the oxidation of contaminants by hydroxyl radicals (•OH) generated by Fenton reagents (Fe2+ and H2O2), and the process is suited to the treatment of industrial wastewater and landfill leachate [26]. According to a scientific paper, •OH attacks the molecular structure of trace contaminants in three distinct ways: (1) H-abstraction, (2) single-electron transfer, and (3) electrophilic addition (hydroxylation) [27]. In recent years, Fenton oxidation has been increasingly used, but its use had mainly been small scale [11]. The electro-peroxone process and Fenton oxidation both rely on the great oxidation ability of •OH to eliminate contaminants from wastewater. The electro-peroxone process utilizes electricity as a catalyst to enhance ozone oxidation, resulting in a more effective treatment than ozone and significantly reduced battery usage [28][29]. In many cases, the highest contaminant-treatment efficiency can be achieved by adjusting just a few parameters (ozone flow rate, initial solution pH, applied current, etc.) [30]. Catalytic wet oxidation is appropriate for treating wastewater with a high organic load (approximately 10 to 100 g/L COD) [31]. At 150 °C, Segura et al. [11] utilized catalytic wet oxidation to eliminate 98% of COD and 90% of total pharmaceuticals from hospital wastewater.
In addition, the combination of multiple advanced technologies can improve treatment outcomes. Kashani et al. [5] added an iron electrode (as a sacrificial electrode) to treat hospital wastewater based on an electro-peroxone device and treated it under optimal conditions (initial PH = 3, ozone 33.1 mg/L, applied current 0.18 A) for 40 min. Resultantly, ciprofloxacin was eliminated, while the TOC removal rate surpassed 70%. Indeed, multiple kinds of electro-peroxone, electro-Fenton, ozone oxidation, and electrocoagulation processes coexist in this system, of which each possesses a remarkably strong oxidizing capacity. In addition, this combination confers a disinfecting action that can eliminate the majority of organic matter, pharmaceuticals, and pathogens in hospital wastewater [14][32][33]. This is a promising technique for the treatment of hospital wastewater. Fenton oxidation requires H2O2 and higher temperatures, whereas photocatalysis, electrocoagulation, and the electro-peroxone process require a great amount of electrical energy [5]. These advanced technologies can effectively treat hospital wastewater, but due to the expenses and technical difficulties involved in their use, they have not become popular and have not been implemented in large-scale operations. Furthermore, the combined process may have negative impacts that reduce removal effectiveness [34]. Segura et al. [11] enhanced Fenton oxidation at 70 °C to remove 70% and 50% of COD and TOC in hospital wastewater, respectively. The removal rate of 78 kinds of drugs reached 99.8% in a photocatalytic coupling Fenton oxidation technique, but it was discovered that photocatalysis could hinder Fenton oxidation’s capacity to remove COD and TOC. The latter COD removal rate declined to 30% under the same conditions, while the TOC removal rate was only 5%. Currently, the mechanism of action is still unclear.
4. Constructed Wetlands
4.1. Mechanism of Pollutant Removal by Components of CWs
A CW is a green, sustainable wastewater treatment technology with striking features such as ecologically restorative functions, low operational costs, and low energy consumption, and it has been widely used globally for various wastewater treatments, especially in recent years [1][6][15][16]. In CWs, the substrate, plants, and microbial community all collaborate to eliminate contaminants from wastewater. Figure 2 depicts the mechanism of contaminant elimination by each component.
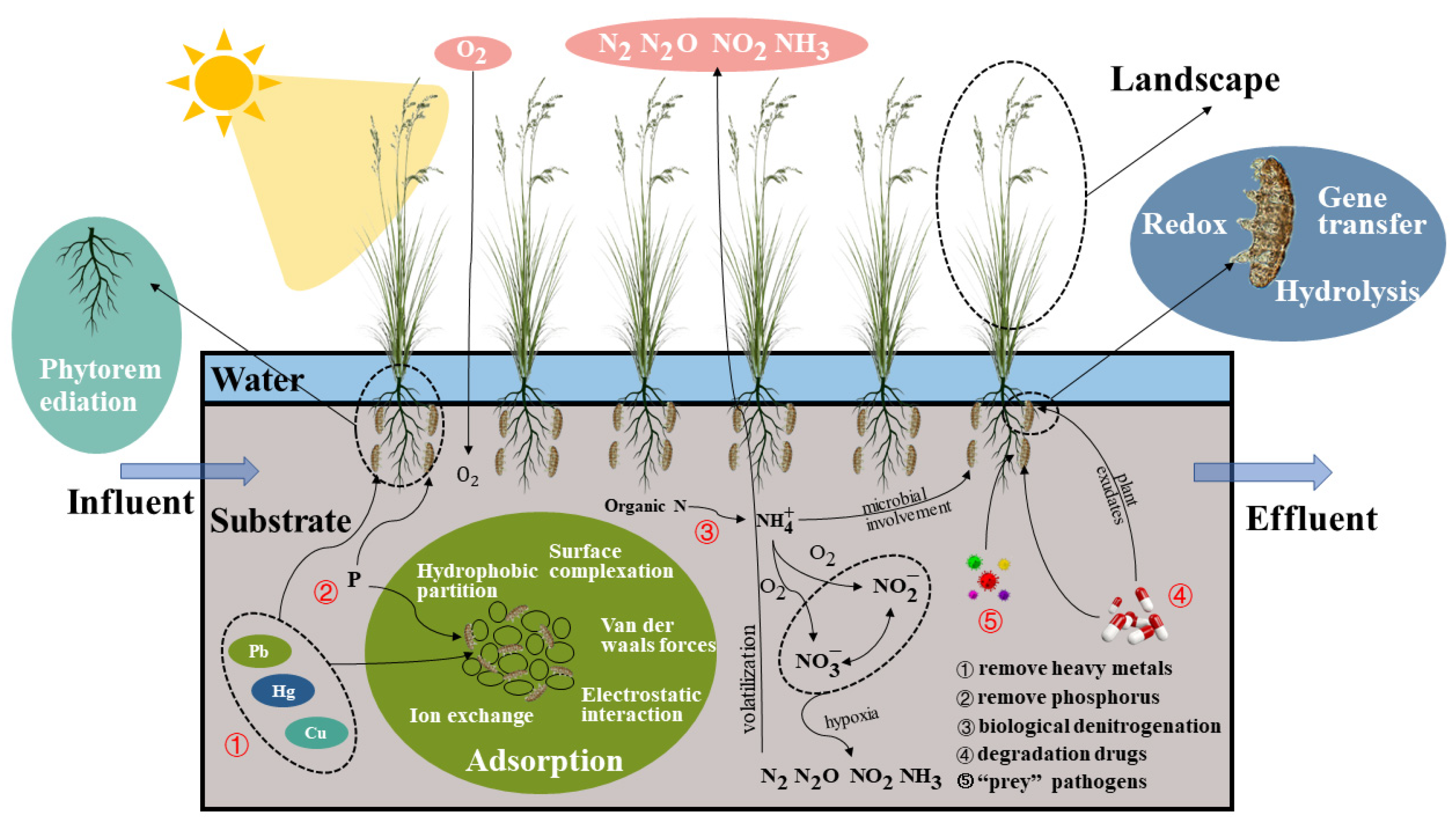
Figure 2. Mechanism of pollutant removal by components of CWs.
Substrates
For many years, gravel, sand, and soil have been common substrates for CWs [35]. These substrates provide a habitat for microbes. Through van der Waals interactions, surface complexation, hydrophobic partitioning, electrostatic interactions, and ion exchange, the substrate adsorbs contaminants [36].
A CW’s substrate plays a key role with respect to pollutant removal, and thus seeking alternative/novel substrates represents important CWs research and development. It is necessary to choose a substrate that exerts a strong removal effect towards antibiotics, resistance genes, and other pollutants in order to treat hospital wastewater. Zeolites have an exceptional capacity to eliminate antibiotics and resistance genes [37]. Lightly expanded clay aggregate (LECA) is a novel substrate that contains alkaline oxides and carbonates. It has good P removal activity, conductivity, and high mechanical strength; can provide improved plant rooting and biofilm growth support [38]; and through its use pharmaceutically active chemicals (carbamazepine, diclofenac, and ibuprofen) and nutrients are efficiently removed from hospital effluent [39]. A consensus has been reached regarding the characteristics of the sustainable development of CW systems; one such characteristic is the availability of a broad selection of substrates—based on the concept of waste utilization—with which to select some of the so-called waste materials. Alum sludge generated in water treatment plants will ultimately be landfilled or burned. However, alum sludge, as a potential substrate of a CWs due to its outstanding absorption performance, is of tremendous importance from a waste utilization perspective [40]. In addition, the coupling of alum sludge-based CWs with the ASP offers a greater capacity for P adsorption and can serve as a habitat for microbes, thereby increasing the biomass of the aeration tank, microbial activity, ammonia nitrogen load, and hydraulic load [41]. In addition to alum sludge, other waste such as broken bricks and coal ash can also be utilized as a substrate, and these types of waste are more successful with respect to eliminating antibiotics and P.
With regard to CWs, substrate clogging is a thorny problem. Biological and abiotic factors contribute to the clogging of substrates. The excessive growth of biofilm and extracellular polymers on the substrate constitute the biological reason, whereas the accumulation of organic matter and suspended matter constitute the abiotic cause [42]. There are several technical methods used to alleviate this condition. Aside from the replacement of the clogging substrate, some technical measures, including the change of the operation mode, such as the anti-sized arrangement of the substrate [43]; the use of composite CWs [44]; intermittent operation [45]; and tidal flow CWs [46][47], are usually employed. Additionally, the occurrence of substrate clogging can be predicted, for which a certain mathematical model needs to be established [48].
Plants
Common reeds, Scirpus validus, rushes, cattails, etc., are the typical plant species present in CWs [49]. These plants consist of two parts: one is the stem and leaves above ground, which can be considered to be a landscape contributor, and the other is the rhizosphere below ground, which offers a living environment for bacteria and can eliminate antibiotics and pathogens [50]. For example, Scirpus validus can eliminate paracetamol [17].
Dires et al. [51] compared the nitrogen and P removal capabilities of planted (sugar cane) and non-planted CWs and discovered that planted wetlands had a greater capacity to eliminate nitrogen and P, which was probably due to the stimulation of the plant rhizosphere to produce more microbes. Through phytoremediation [52], plants remove contaminants such as antibiotics, heavy metals, and pathogens. This involves plant adsorbents, root exudation, and microbial degradation. The influence of a plant adsorbent is negligible in comparison to that of root exudates and microbial degradation [53][54][55]. The roots accumulate the most pollutants among plant tissues [56], but the pollutants may migrate upward; thus, the potential risk of plant harvesting (antibiotic enrichment) should be seriously considered [57][58].
Microbes
Different plant rhizospheres have varying densities of microbes. Chen et al. [55] utilized denaturing gradient gel electrophoresis to measure the microbial density in the rhizospheres of various plant species. The ranking of microbe density is as follows: Canna indica, Cyperus flabelliformis, Hymenocallis littoralis, and Iris. Tectorum, in descending order. Even in the rhizosphere of the same plant, the density of microbes may vary, which may be correlated with the availability of nutrients, pH, temperature, and the humidity of the plant’s living environment [59].
Microbes destroy pollutants via redox reactions, gene transfer, hydrolysis, etc. [60]. Under aerobic and anaerobic conditions, ammonification, nitrification, and denitrification bacteria can eliminate nitrogen from wastewater [61]. This alternative aerobic and anaerobic environment is afforded by CWs’ distinctive structure. Antibiotics can also be degraded by microbes, such as ammonia-oxidizing microorganisms, which can eliminate antibiotics [62]. Curvularia can effectively eradicate erythromycin [63]. Microbes in CW environments can “prey” on pathogens. Wand et al. [64] created CWs by planting a mixture of rushes and reeds and employing coarse sand as a substrate. The primary elimination process for E. coli is the ability of leelovibrio and protozoa to “prey” on the bacteria. In the investigation conducted by Proakis et al. [65], a similar “prey” mechanism was also observed in rotifers. There are few reports on the microbial degradation of pathogens in CWs, and thus further research is required.
The Interaction among Substrates, Plants, and Microbes
When treating wastewater, CWs rely on the synergy of the substrate, plants, and microbes, wherein the substrate is the most important part, as it provides a habitat for bacteria and plants and plays a crucial role in the process of eliminating pollutants.
By producing particular molecules that mediate the link between roots and microbes, plant roots “choose” the microbial community that is beneficial to their survival [66], which affects the microbe density and diversity in the roots [55]. The term “choose” may refer to the habitat in which the plant thrives. If the plant does not acquire the necessary microbial community, its growth will be stunted, and it may even die [67]. Nitrogen-fixing bacteria, such as Bacillus and Paenibacillus species, enable plants to uptake nitrogen [61]. Certain root system bacteria affect the uptake of orthophosphate by plants [68]. Iron is a critical trace element for chlorophyll synthesis. Certain volatile organic compounds (VOCs) generated by rhizobia “signal” to plants to increase iron absorption by acidifying plant roots and boosting iron reductase activity [69]. Nonetheless, plants may also pose a hazard to the survival of microbes. For instance, the alkaloids released by Nuphar lutea inhibit the action of microbes, and even the phenolic compounds generated by certain plants are toxic to microbes [70].
References
- Parida, V.K.; Sikarwar, D.; Majumder, A.; Gupta, A.K. An assessment of hospital wastewater and biomedical waste generation, existing legislations, risk assessment, treatment processes, and scenario during COVID-19. J. Environ. Manag. 2022, 308, 114609.
- Rodríguez-Llorente, D.; Hernández, E.; Gutiérrez-Sánchez, P.; Navarro, P.; Ismael Águeda, V.; Álvarez-Torrellas, S.; García, J.; Larriba, M. Extraction of pharmaceuticals from hospital wastewater with eutectic solvents and terpenoids: Computational, experimental, and simulation studies. Chem. Eng. J. 2023, 451, 138544.
- Dai, H.; Sun, Y.; Wan, D.; Abbasi, H.N.; Guo, Z.; Geng, H.; Wang, X.; Chen, Y. Simultaneous denitrification and phosphorus removal: A review on the functional strains and activated sludge processes. Sci. Total Environ. 2022, 835, 155409.
- Zhao, Y.; Qiu, Y.B.; Mamrol, N.; Ren, L.F.; Li, X.; Shao, J.H.; Yang, X.; van der Bruggen, B. Membrane bioreactors for hospital wastewater treatment: Recent advancements in membranes and processes. Front. Chem. Sci. Eng. 2022, 16, 634–660.
- Kashani, M.R.K.; Kiani, R.; Hassani, A.; Kadier, A.; Madihi-Bidgoli, S.; Lin, K.Y.A.; Ghanbari, F. Electro-peroxone application for ciprofloxacin degradation in aqueous solution using sacrificial iron anode: A new hybrid process. Sep. Purif. Technol. 2022, 292, 12.
- Alsubih, M.; El Morabet, R.; Khan, R.A.; Khan, N.A.; Khan, A.R.; Khan, S.; Mubarak, N.M.; Dehghani, M.H.; Singh, L. Field performance investigation for constructed wetland clubbed with tubesettler for hospital wastewater treatment. J. Water Process. Eng. 2022, 49, 10.
- Mir-Tutusaus, J.A.; Jaen-Gil, A.; Barcelo, D.; Buttiglieri, G.; Gonzalez-Olmos, R.; Rodriguez-Mozaz, S.; Caminal, G.; Sarra, M. Prospects on coupling UV/H2O2 with activated sludge or a fungal treatment for the removal of pharmaceutically active compounds in real hospital wastewater. Sci. Total Environ. 2021, 773, 12.
- Pirsaheb, M.; Mohamadisorkali, H.; Hossaini, H.; Hossini, H.; Makhdoumi, P. The hybrid system successfully to consisting of activated sludge and biofilter process from hospital wastewater: Ecotoxicological study. J. Environ. Manag. 2020, 276, 11.
- Vo, T.K.; Bui, X.T.; Chen, S.S.; Nguyen, P.D.; Cao, N.D.; Vo, T.D.; Nguyen, T.T.; Nguyen, T.B. Hospital wastewater treatment by sponge membrane bioreactor coupled with ozonation process. Chemosphere 2019, 230, 377–383.
- Nguyen, T.T.; Bui, X.T.; Dang, B.T.; Ngo, H.H.; Jahng, D.; Fujioka, T.; Chen, S.S.; Dinh, Q.T.; Nguyen, C.N.; Nguyen, P.T.V. Effect of ciprofloxacin dosages on the performance of sponge membrane bioreactor treating hospital wastewater. Bioresour. Technol. 2019, 273, 573–580.
- Segura, Y.; Cruz del Álamo, A.; Munoz, M.; Álvarez-Torrellas, S.; García, J.; Casas, J.A.; De Pedro, Z.M.; Martínez, F. A comparative study among catalytic wet air oxidation, Fenton, and Photo-Fenton technologies for the on-site treatment of hospital wastewater. J. Environ. Manag. 2021, 290, 112624.
- Yu, L.; Wang, L.; Liu, Y.K.; Sun, C.L.; Zhao, Y.; Hou, Z.J.; Peng, H.B.; Wang, S.Z.; Wei, H.Z. Pyrolyzed carbon derived from red soil as an efficient catalyst for cephalexin removal. Chemosphere 2021, 277, 10.
- Yánes, A.; Pinedo-Hernández, J.; Marrugo-Negrete, J. Continuous flow electrocoagulation as a hospital wastewater treatment. Port. Electrochim. Acta 2021, 39, 403–413.
- Yu, Y.; Xiong, Z.; Huang, B.; Wang, X.; Du, Y.; He, C.; Liu, Y.; Yao, G.; Lai, B. Synchronous removal of pharmaceutical contaminants and inactivation of pathogenic microorganisms in real hospital wastewater by electro-peroxone process. Environ. Int. 2022, 168, 107453.
- Khan, R.A.; Khan, N.A.; El Morabet, R.; Alsubih, M.; Khan, A.R.; Khan, S.; Mubashir, M.; Balakrishnan, D.; Khoo, K.S. Comparison of constructed wetland performance coupled with aeration and tubesettler for pharmaceutical compound removal from hospital wastewater. Environ. Res. 2023, 216, 10.
- Alsubih, M.; El Morabet, R.; Khan, R.A.; Khan, N.A.; Khan, A.R.; Khan, S.; Mushtaque, N.; Hussain, A.; Yousefi, M. Performance evaluation of constructed wetland for removal of pharmaceutical compounds from hospital wastewater: Seasonal perspective. Arab J. Chem. 2022, 15, 13.
- Vo, H.N.P.; Koottatep, T.; Chapagain, S.K.; Panuvatvanich, A.; Polprasert, C.; Nguyen, T.M.H.; Chaiwong, C.; Nguyen, N.L. Removal and monitoring acetaminophen-contaminated hospital wastewater by vertical flow constructed wetland and peroxidase enzymes. J. Environ. Manag. 2019, 250, 9.
- Tang, K.; Xie, J.W.; Pan, Y.W.; Zou, X.Y.; Sun, F.Q.; Yu, Y.B.; Xu, R.; Jiang, W.H.; Chen, C.J. The optimization and regulation of energy consumption for MBR process: A critical review. J. Environ. Chem. Eng. 2022, 10, 10.
- Campinas, M.; Viegas, R.M.C.; Almeida, C.M.M.; Martins, A.; Silva, C.; Mesquita, E.; Silva, S.; Coelho, M.R.; Benoleil, M.J.; Rosa, M.J.; et al. Powdered activated carbon full-scale addition to the activated sludge reactor of a municipal wastewater treatment plant: Pharmaceutical compounds control and overall impact on the process. J. Water Process. Eng. 2022, 49, 13.
- Lan, Y.D.; Groenen-Serrano, K.; Coetsier, C.; Causserand, C. Nanofiltration performances after membrane bioreactor for hospital wastewater treatment: Fouling mechanisms and the quantitative link between stable fluxes and the water matrix. Water Res. 2018, 146, 77–87.
- Lan, Y.D.; Groenen-Serrano, K.; Coetsier, C.; Causserand, C. Fouling control using critical, threshold and limiting fluxes concepts for cross-flow NF of a complex matrix: Membrane BioReactor effluent. J. Membr. Sci. 2017, 524, 288–298.
- Nguyen, T.T.; Bui, X.T.; Luu, V.P.; Nguyen, P.D.; Guo, W.S.; Ngo, H.H. Removal of antibiotics in sponge membrane bioreactors treating hospital wastewater: Comparison between hollow fiber and flat sheet membrane systems. Bioresour. Technol. 2017, 240, 42–49.
- Kumari, A.; Maurya, N.S.; Tiwari, B. Hospital wastewater treatment scenario around the globe. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 549–570.
- Asif, M.B.; Ren, B.; Li, C.; He, K.; Zhang, X.; Zhang, Z. Understanding the role of in-situ ozonation in Fe(II)-dosed membrane bioreactor (MBR) for membrane fouling mitigation. J. Membr. Sci. 2021, 633, 119400.
- Zhang, B.; Kotsalis, G.; Khan, J.; Xiong, Z.; Igou, T.; Lan, G.; Chen, Y. Backwash sequence optimization of a pilot-scale ultrafiltration membrane system using data-driven modeling for parameter forecasting. J. Membr. Sci. 2020, 612, 118464.
- Pacheco-Álvarez, M.; Picos Benítez, R.; Rodríguez-Narváez, O.M.; Brillas, E.; Peralta-Hernández, J.M. A critical review on paracetamol removal from different aqueous matrices by Fenton and Fenton-based processes, and their combined methods. Chemosphere 2022, 303, 134883.
- Chen, Q.; Lu, F.; Zhang, H.; He, P. Where should Fenton go for the degradation of refractory organic contaminants in wastewater? Water Res. 2023, 229, 119479.
- Ma, Y.; Zhan, J.; Wang, H.; Wang, Y. Study on abatement of acetamiprid by electro-peroxone process. Environ. Eng. 2021, 39, 107.
- Cui, X.; Lin, Z.; Wang, H.; Yu, G.; Wang, Y. Effective degradation of ibuprofen by flow-through electro-peroxone process. China Environ. Sci. 2019, 39, 1619–1626.
- Zheng, H.S.; Guo, W.Q.; Wu, Q.L.; Ren, N.Q.; Chang, J.S. Electro-peroxone pretreatment for enhanced simulated hospital wastewater treatment and antibiotic resistance genes reduction. Environ. Int. 2018, 115, 70–78.
- Kim, K.-H.; Ihm, S.-K. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review. J. Hazard. Mater. 2011, 186, 16–34.
- Guo, Y.; Zhao, E.Z.; Wang, J.; Zhang, X.Y.; Huang, H.O.; Yu, G.; Wang, Y.J. Comparison of emerging contaminant abatement by conventional ozonation, catalytic ozonation, O3/H2O2 and electro-peroxone processes. J. Hazard. Mater. 2020, 389, 8.
- Bayarri, B.; Cruz-Alcalde, A.; López-Vinent, N.; Micó, M.M.; Sans, C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J. Hazard. Mater. 2021, 415, 125658.
- Nidheesh, P.V.; Trellu, C.; Vargas, H.O.; Mousset, E.; Ganiyu, S.O.; Oturan, M.A. Electro-Fenton process in combination with other advanced oxidation processes: Challenges and opportunities. Curr. Opin. Electrochem. 2023, 37, 7.
- Yang, C.; Zhang, X.L.; Tang, Y.Q.; Jiang, Y.; Xie, S.Q.; Zhang, Y.L.; Qin, Y.J. Selection and optimization of the substrate in constructed wetland: A review. J. Water Process. Eng. 2022, 49, 13.
- Fu, J.M.; Zhao, Y.Q.; Yao, Q.; Addo-Bankas, O.; Ji, B.; Yuan, Y.J.; Wei, T.; Esteve-Nunez, A. A review on antibiotics removal: Leveraging the combination of grey and green techniques. Sci. Total Environ. 2022, 838, 16.
- Liu, Y.; Liu, X.H.; Lu, S.Y.; Zhao, B.; Wang, Z.; Xi, B.D.; Guo, W. Adsorption and biodegradation of sulfamethoxazole and ofloxacin on zeolite: Influence of particle diameter and redox potential. Chem. Eng. J. 2020, 384, 13.
- Mlih, R.; Bydalek, F.; Klumpp, E.; Yaghi, N.; Bol, R.; Wenk, J. Light-expanded clay aggregate (LECA) as a substrate in constructed wetlands ? A review. Ecol. Eng. 2020, 148, 15.
- Karthik, R.M.; Philip, L. Sorption of pharmaceutical compounds and nutrients by various porous low cost adsorbents. J. Environ. Chem. Eng. 2021, 9, 16.
- Zhou, M.; Cao, J.S.; Lu, Y.H.; Zhu, L.S.; Li, C.; Wang, Y.T.; Hao, L.S.; Luo, J.Y.; Ren, H.Q. The performance and mechanism of iron-modified aluminum sludge substrate tidal flow constructed wetlands for simultaneous nitrogen and phosphorus removal in the effluent of wastewater treatment plants. Sci. Total Environ. 2022, 847, 12.
- Liu, R.; Zhao, Y. Coupling Process of Alum Sludge-based Constructed Wetland and Activated Sludge Process (GBR) for Enhancing Nutrients Removal. China Water Wastewater 2018, 34, 7–13.
- Zhong, H.; Hu, N.; Wang, Q.H.; Chen, Y.C.; Huang, L. How to select substrate for alleviating clogging in the subsurface flow constructed wetland? Sci. Total Environ. 2022, 828, 12.
- Zhao, Y.Q.; Sun, G.; Allen, S.J. Anti-sized reed bed system for animal wastewater treatment: A comparative study. Water Res. 2004, 38, 2907–2917.
- Huang, F.; Chen, D.; Wu, J.; Xu, D.; Wu, Z.; He, F. Optimization of configuration and process for effectively mitigating substrate clogging in integrated vertical-flow constructed wetland. China Water Wastewater 2017, 33, 31–36.
- Hua, G.F.; Kong, J.; Ji, Y.Y.; Li, M. Influence of clogging and resting processes on flow patterns in vertical flow constructed wetlands. Sci. Total Environ. 2018, 621, 1142–1150.
- Liao, Y.; Wan, Z.; Cao, X.; Jiang, L.; Feng, L.; Zheng, H.; Ji, F. The importance of rest phase and pollutant removal mechanism of tidal flow constructed wetlands (TFCW) in rural grey water treatment. Chemosphere 2023, 311, 137010.
- Suthar, S.; Chand, N.; Singh, V. Fate and toxicity of triclosan in tidal flow constructed wetlands amended with cow dung biochar. Chemosphere 2023, 311, 136875.
- Nivala, J.; Knowles, P.; Dotro, G.; Garcia, J.; Wallace, S. Clogging in subsurface-flow treatment wetlands: Measurement, modeling and management. Water Res. 2012, 46, 1625–1640.
- Jan, V.; Wei, T.; Zhao, Y.; Ulo, M.; Florent, C.; Liu, R.; Zhou, M. Counting the roles of plants in constructed wetlands for wastewater treatment. China Water Wastewater 2021, 37, 25–30.
- Wang, J.X.; Man, Y.; Ruan, W.F.; Tam, N.F.Y.; Tao, R.; Yin, L.; Yang, Y.; Dai, Y.; Tai, Y.P. The effect of rhizosphere and the plant species on the degradation of sulfonamides in model constructed wetlands treating synthetic domestic wastewater. Chemosphere 2022, 288, 10.
- Dires, S.; Birhanu, T.; Ambelu, A. Use of broken brick to enhance the removal of nutrients in subsurface flow constructed wetlands receiving hospital wastewater. Water Sci. Technol. 2019, 79, 156–164.
- Agarwal, P.; Rani, R. Strategic management of contaminated water bodies: Omics, genome-editing and other recent advances in phytoremediation. Environ. Technol. Innov. 2022, 27, 102463.
- Chen, J.; Ying, G.G.; Wei, X.D.; Liu, Y.S.; Liu, S.S.; Hu, L.X.; He, L.Y.; Chen, Z.F.; Chen, F.R.; Yang, Y.Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Effect of flow configuration and plant species. Sci. Total Environ. 2016, 571, 974–982.
- Chandrasena, G.I.; Shirdashtzadeh, M.; Li, Y.L.; Deletic, A.; Hathaway, J.M.; McCarthy, D.T. Retention and survival of E. coli in stormwater biofilters: Role of vegetation, rhizosphere microorganisms and antimicrobial filter media. Ecol. Eng. 2017, 102, 166–177.
- Chen, Z.J.; Tian, Y.H.; Zhang, Y.; Song, B.R.; Li, H.C.; Chen, Z.H. Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol. Eng. 2016, 92, 243–250.
- Cui, E.P.; Cui, B.J.; Fan, X.Y.; Li, S.J.; Gao, F. Ryegrass (Lolium multiflorum L.) and Indian mustard (Brassica juncea L.) intercropping can improve the phytoremediation of antibiotics and an- tibiotic resistance genes but not heavy metals. Sci. Total Environ. 2021, 784, 11.
- Huang, W.; Liu, X.; Tang, H.; Wang, Y.; Chen, J. Progress on application of phytoremediation in antibiotic pollution control. Ecol. Sci. 2022, 41, 222–229.
- Yu, X.; Chen, J.; Liu, X.; Sun, Y.; He, H. The mechanism of uptake and translocation of antibiotics by pak choi (Brassica rapa subsp. chinensis). Sci. Total Environ. 2022, 810, 151748.
- Xiong, Q.Q.; Hu, J.L.; Wei, H.Y.; Zhang, H.C.; Zhu, J.Y. Relationship between plant roots, rhizosphere microorganisms, and nitrogen and its special focus on rice. Agriculture 2021, 11, 234.
- Zhan, H.; Zhou, Q. Research progress on treatment technology of tetracycline antibiotics pollution in the environment. J. Environ. Eng. Technol. 2021, 11, 571–581.
- Lu, J.X.; Guo, Z.Z.; Kang, Y.; Fan, J.L.; Zhang, J. Recent advances in the enhanced nitrogen removal by oxygen-increasing technology in constructed wetlands. Ecotoxicol. Environ. Saf. 2020, 205, 7.
- Li, S.; Peng, L.; Yang, C.; Song, S.; Xu, Y. Cometabolic biodegradation of antibiotics by ammonia oxidizing microorganisms during wastewater treatment processes. J. Environ. Manag. 2022, 305, 114336.
- Ren, J.J.; Deng, L.J.; Niu, D.Z.; Wang, Z.Z.; Fan, B.; Taoli, H.H.; Li, Z.J.; Zhang, J.; Li, C.Y. Isolation and identification of a novel erythromycin-degrading fungus, Curvularia sp. RJJ-5, and its degradation pathway. FEMS Microbiol. Lett. 2021, 368, 8.
- Wand, H.; Vacca, G.; Kuschk, P.; Krüger, M.; Kästner, M. Removal of bacteria by filtration in planted and non-planted sand columns. Water Res. 2007, 41, 159–167.
- Proakis, E. Pathogen removal in constructed wetlands focusing on biological predation and marine recreational water quality. In Proceedings of the WEFTEC 2003, Leeds, UK, January 2003; pp. 310–332.
- Wang, X.L.; Wang, M.X.; Xie, X.G.; Guo, S.Y.; Zhou, Y.; Zhang, X.B.; Yu, N.; Wang, E.T. An amplification-selection model for quantified rhizosphere microbiota assembly. Sci. Bull. 2020, 65, 983–986.
- Zhou, Q.; Huang, A. Recent progress on modulation of microbiota by plant root metabolites. Plant Physiol. J. 2020, 56, 2288–2295.
- Castrillo, G.; Teixeira, P.; Paredes, S.H.; Law, T.F.; de Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518.
- Orozco-Mosqueda, M.D.; Macias-Rodriguez, L.I.; Santoyo, G.; Farias-Rodriguez, R.; Valencia-Cantero, E. Medicago truncatula increases its iron-uptake mechanisms in response to volatile organic compounds produced by Sinorhizobium meliloti. Folia Microbiol. 2013, 58, 579–585.
- Barco, A.; Borin, M. Treatment performances of floating wetlands: A decade of studies in North Italy. Ecol. Eng. 2020, 158, 13.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
14 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




