Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Atif Khurshid Wani | -- | 3048 | 2023-02-13 11:30:05 | | | |
| 2 | Jessie Wu | Meta information modification | 3048 | 2023-02-14 05:17:03 | | | | |
| 3 | Jessie Wu | + 3 word(s) | 3051 | 2023-02-14 05:18:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wani, A.K.; Akhtar, N.; Mir, T.U.G.; Singh, R.; Jha, P.K.; Mallik, S.K.; Sinha, S.; Tripathi, S.K.; Jain, A.; Jha, A.; et al. Hallmarks and Significance of Apoptosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/41155 (accessed on 08 February 2026).
Wani AK, Akhtar N, Mir TUG, Singh R, Jha PK, Mallik SK, et al. Hallmarks and Significance of Apoptosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/41155. Accessed February 08, 2026.
Wani, Atif Khurshid, Nahid Akhtar, Tahir Ul Gani Mir, Rattandeep Singh, Prakash Kumar Jha, Shyam Kumar Mallik, Shruti Sinha, Surya Kant Tripathi, Abha Jain, Aprajita Jha, et al. "Hallmarks and Significance of Apoptosis" Encyclopedia, https://encyclopedia.pub/entry/41155 (accessed February 08, 2026).
Wani, A.K., Akhtar, N., Mir, T.U.G., Singh, R., Jha, P.K., Mallik, S.K., Sinha, S., Tripathi, S.K., Jain, A., Jha, A., Devkota, H.P., & Prakash, A. (2023, February 13). Hallmarks and Significance of Apoptosis. In Encyclopedia. https://encyclopedia.pub/entry/41155
Wani, Atif Khurshid, et al. "Hallmarks and Significance of Apoptosis." Encyclopedia. Web. 13 February, 2023.
Copy Citation
Apoptosis is the elimination of functionally non-essential, neoplastic, and infected cells via the mitochondrial pathway or death receptor pathway. The process of apoptosis is highly regulated through membrane channels and apoptogenic proteins. Apoptosis maintains cellular balance within the human body through cell cycle progression. Loss of apoptosis control prolongs cancer cell survival and allows the accumulation of mutations that can promote angiogenesis, promote cell proliferation, disrupt differentiation, and increase invasiveness during tumor progression. The apoptotic pathway has been extensively studied as a potential drug target in cancer treatment.
apoptosis
drugs
cancer
1. Apoptosis: Molecular and Cellular Perspective
Apoptosis, programmed cell death, is a series of biochemical processes that are characterized by morphological changes and the death of the cells. The process is pivotal to the cell cycle processes of multicellular organisms that are represented by nuclear fragmentation, cell shrinking, mRNA decay, DNA fragmentation, and blebbing [1]. An adult human and average child (10–12 years old) loses about 50–70 billion and 20–30 billion cells/day, respectively. Apoptosis is an efficiently synchronized cell process that is important throughout the life cycle of an organism. The separation of toes and fingers is one of the common advantages known hitherto that is conferred by programmed cell death (PCD) at the embryonic stage. Apoptotic bodies are produced during the process of apoptosis which are utilized by the phagocytic cells that prevent their spread which otherwise can be damaging. It is generally accepted that the permeabilization of the outer mitochondrial membrane, a process controlled by the Bcl-2 family of proteins, marks the apoptotic cell’s point of no return. This is characterized by the release from the intermembrane space into the cytosol with key pro-apoptotic functions such as: cytochrome c, apoptosis-inducing factor, endonuclease G, etc. [2]. PCD starts with either an extrinsic pathway (signal from other cells) or an intrinsic pathway (feeble external signals) [3]. Both pathways activate caspase proteins that induce the cell death by degradation of cellular proteins. Even though apoptosis is a biologically important process but the uncontrolled apoptosis leads to atrophy (excessive apoptosis) or cancer (insufficient apoptosis/extreme cell proliferation) [4]. Caspase and Fas receptors are pro-apoptotic, while Bcl-2 family members are anti-apoptotic in action. The apoptotic signaling within a cell initiates as a stress response triggered by a viral infection, radiation, heat, hypoxia, or increase in calcium and fatty acid concentration within the cell [5]. Following an encounter with any of these stimuli, the cell undergoes ordered organelle degradation by the proteolytic activity of the caspases. This leads to the cell’s morphological changes, such as cytoskeleton breakdown, lamellipodia retraction, tight organelle packing, dense cytoplasm, pyknosis, discontinuous nuclear envelope, karyorrhexis, and cell shrinking. Before the disposing of an apoptotic cell, it undergoes a disassembly process which is a three-step process: 1. membrane blebbing, i.e., the formation of irregular membrane buds/blebs regulated by ROCK1 (Rho-associated coiled-coil containing protein kinase 1); 2. membrane protrusion formation; and 3. fragmentation, i.e., the breakdown of a cell into apoptotic bodies. The dead cells are finally removed via efferocytosis by the phagocytic cells as dying cells display a phagocytic molecule known as phosphatidylserine on the surface. The pathways related to cell death are inhibited by the negative regulators of the apoptotic process. The inhibition leads to the development of drug resistance and helps tumors in evading cell death. The ratio of Bax (pro-apoptotic protein) to Bcl-2 (anti-apoptotic protein) is a decisive factor in determining whether a cell dies or lives.
2. Apoptosis Activation: Mechanism
The apoptotic nature of the cell is efficiently regulated because it leads to cell death once the process begins [6]. The mitochondrial or intrinsic pathway (IP) and extrinsic pathway (EP) are so far the best understood molecular mechanisms of apoptosis. The IP activation is mediated by the signals generated inside the stressed cells and relies on mitochondria’s intermembrane space (IMS) for the release of proteins [7]. On the other hand, EP is activated by the ligand binding to the cell surface receptors forming a death-inducing signaling complex (DISC). Both IP and EP converge upon caspase-3 and caspase-7 activation. Before the precipitation of the cell death process by enzymes, apoptotic biochemical signals tightly regulate the release of apoptotic proteins. Figure 1 illustrates the intrinsic and extrinsic molecular activation of programmed cell death.
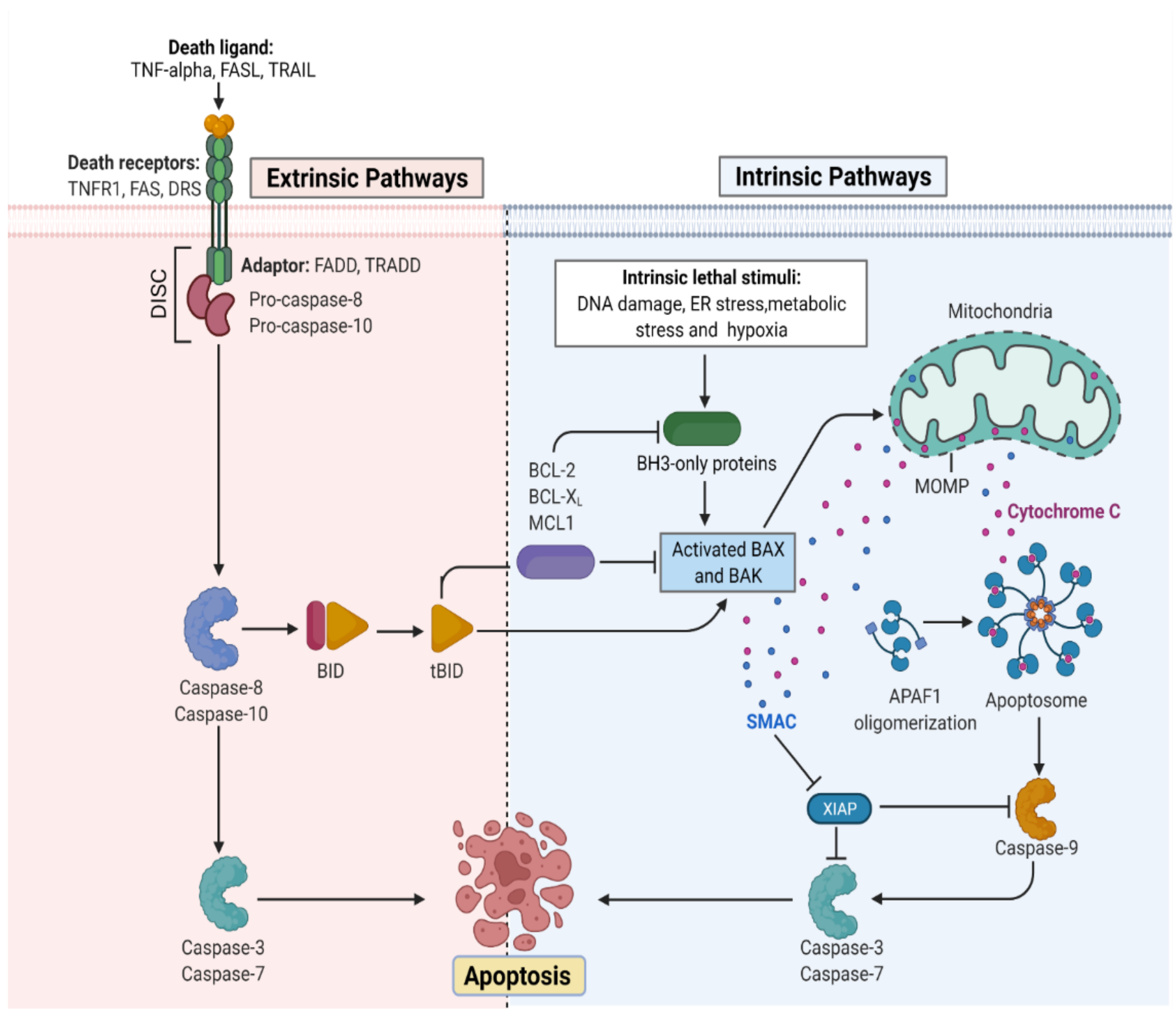
Figure 1. Representation of the molecular activation mechanism of intrinsic and extrinsic pathways of programmed cell death. Both intrinsic and extrinsic pathways depend on specific signals to initiate an energy-dependent cascade of molecular events, thereby activating initiator caspases which leads to the activation of executioner caspase-3.TNF (Tumor necrosis factor), FASL (FS-7-associated surface antigen ligand), TRAIL (Targeting TNF-related apoptosis-inducing ligand), TNFR1 (Tumor necrosis factor receptor 1), DRS (Death receptors), DISC (death-inducing signaling complex), BID (BH3 interacting-domain), MCL (myeloid leukemia cell differentiation protein), ER (Endoplasmic reticulum), BAX (Bcl-2-associated X protein), BAK (B-cell lymphoma 2 killer), SMAC (Second mitochondria-derived activator of caspase), XIAP (X-linked inhibitor of apoptosis protein), MOMP (mitochondrial outer membrane permeabilization), and APAF (Apoptotic protease activating factor). (Adapted from “Apoptosis Extrinsic and Intrinsic Pathways” by BioRender.com. (https://app.biorender.com/biorender-templates by BioRender.com, access on 30 November 2022).
2.1. Mitochondria Pathway of Cellular Apoptosis
Mitochondria is important for multicellular organisms because it allows aerobic respiration which is essential for cell survival. This forms the bedrock for the intrinsic apoptotic pathway. Apoptotic proteins target mitochondria that may cause its swelling through the formation of pores in the membrane or may increase mitochondrial membrane permeability allowing the apoptotic effectors to leak [8]. Because of the sensitivity related to IP, tumors arise more through IP than EP. Bcl-2 proteins are the driving forces of IP, and each of its members have homology domains (BH1-BH4) with Bcl-2 that may be one or more [9]. Apoptosis is initiated by the pro-apoptotic proteins such as BH3 having one BH domain [10]. The key effector proteins that are committed to apoptosis include the Bcl-2-interacting mediator (BIM) that is encoded by Bcl2L11, p53 upregulated modulator (PUM) of apoptosis encoded by Bcl-2 binding component 3 protein (BBC3)BH3-interacting death agonist (BID) that is encoded by BID, and other effector molecules that help in the activation of pro-apoptotic proteins or the pore-forming proteins which includes Bcl-2-associated-X-protein (BAX) and Bcl-2-antagonist killer (BAK) [11]. The activation of BAK and BAX at the surface of mitochondria induces an allosteric alteration that enables their oligomerization and results in the formation of macropores in the membrane causing mitochondrial outer membrane permeabilization (MOMP). MOMP ensures the apoptogenic protein release from the IMS. The released proteins activate the caspases in the cytoplasm directly or indirectly. The direct mode of action is studied in the cytochrome c which sticks to the protein scaffold, i.e., apoptotic protease activating factor-1 (APAF1), to transform it into an apoptosome. The indirect mode of action is exemplified in the case of the second-mitochondria-derived activator of caspases (SMAC) that are known to neutralize the caspase-inhibitory proteins [12]. A series of biochemical events lead to the activation of caspase-9, an initiation caspase, which is followed by the activation of caspase-3, -6, and -7, executioner caspases. Although caspase activation is ubiquitous in cell death, it is a principal event for the process of apoptosis because the interception in caspase activation does not rescue dying cells following MOMP under normal physiological conditions. Thus, MOMP is the crucial point where a cell commits irreversibly to undergoing cell death. The cells with restricted MOMP potential are tumorigenic owing to the actions of post-MOMP proteins such as DNases that can mutate the DNA leading to a neoplastic transformation [13]. Besides being the driving force in the mitochondrial protein release, BAK and/or BAX also helps in releasing the mtDNA into the cytoplasm and thereby activating pro-inflammatory signals in the absence of caspases [14]. After MOMP, a process known as met-induced mitochondrial protein (MIMP) is thought to take place, during which components of the mitochondrial matrix are released into the cytoplasm [15]. When Bcl-2 family members tightly regulate MOMP and cause a rapid spillage of mitochondrial IMS proteins, MIMP exhibits unique characteristics in both kinetic and molecular regulation. The slower release of mitochondrial factors, rapid mitochondrial Ca2+ uptake, and complete independence from Bcl-2 proteins serve as proof of this. It is interesting that the antiapoptotic Bcl-2 members also prevent cell death by inhibiting IP3-mediated Ca2+ transfer from the ER to mitochondria [16].
2.2. Death Receptor Pathway of Cellular Apoptosis
There are two molecular mechanistic models put forth to understand the extrinsic pathway of apoptosis. These include the TNF-induced (tumor necrosis factor) model and Fas-Fas ligand-mediated model, both of which are associated with extrinsic signals involving TNF-receptor. TNF-α, a cytokine synthesized by macrophages, is a main extrinsic apoptotic mediator [17]. Human cells possess two TNF receptors, namely TNFR-1 and TNFR-2. The binding of TNF-α to TNFR-1 results in the initiation of a biochemical pathway that ultimately causes caspase activation through the membrane proteins [18]. The death domain associated with the TNF receptor known as TNFR1-associated death domain protein (TRADD) and death domain associated with Fas, FAS-associated death domain protein (FADD), can bind to TRAF-2 and thus inhibit the caspase-8 activation. Indirectly, this attachment most likely activates the transcription factors involved in the inflammatory response and cell survival [19]. The signaling cascade via TNFR-1 may also initiate PCD without caspase.
The first apoptosis signal (Fas), also referred to as CD95 or APO-1, a TNF transmembrane protein, binds to the Fas ligand. The association of Fas with FasL leads to the formation of DISC, which is a complex of caspase-8, caspase-10, and FADD. In type I cells (branched cells with multiple cytoplasmic plates), the caspase-8 which is preprocessed directly activates other caspase family members which initiates the apoptosis execution [20]. On the other hand, in type II cells (progenitors of type I), Fas-DISC triggers a feedback system that coils into the release of increased pro-apoptotic factors from mitochondria, thereby increasing the degree of caspase-8 activation [21].
3. Hallmarks of Apoptosis
3.1. Morphological Indicators
The change in cellular morphology during PCD is one of the defining features that characterizes the process of apoptosis. The microscopic examination in the initial stages of apoptosis is symbolized by nuclear material condensation and margination of chromatin along the nuclear envelope [22]. This is followed by nuclear fragmentation and intricate folding of the nuclear membrane. The early stage apoptosis on the surface of cells is characterized by apoptotic cell detachment from neighboring cells, formation of cytoplasmic blebs, and loss of microvilli. As apoptosis advances, the cellular cytoplasm undergoes a peculiar condensation that leads to the compaction of cell organelles and then forms the apoptotic bodies which are membrane-bound. Finally, these are consumed by macrophages or nearby parenchymal cells.
3.2. Biochemical Indicators
The evaluation of biochemical indicators of PCD have certain limitations given the fact that cell death in tissues is asynchronous in nature; therefore, only a fraction of the cells undergoes PCD at a particular point in time. In addition, the cells are instantly phagocytosed once they enter the apoptotic process [23]. To overcome the hindrances that make biochemical characterization difficult, the model system has been expanded, which depends on the hormonal enrichment of cells. One common model system is the immature thymocyte in which glucocorticoid hormones act as apoptotic activators. One of the biochemical events studied by using the thymocyte model system is the genome degradation process in apoptosis [24]. Conradt et al. studied the degradation of DNA through programmed cell death, which occurs via the cleavage of genomes at the linker region in Caenorhabditis elegans [25]. Some of the studies demonstrated that PCD is mediated through the glucocorticoid receptor pathway (GRP) (Table 1). The studies were based on the fact that endogenous and exogenous glucocorticoids activate endonuclease activity, the antagonist nature of glucocorticoid can block the apoptotic response partially, a functional GR is required for the apoptosis activation, and non-glucocorticoid hormones were unable to evoke endonuclease activity.
4. Significance of Apoptosis
Apoptosis acts in association with the process of mitogenesis and cellular differentiation to organize cellular function which ultimately orchestrates physiological functioning. Initially, PCD plays a significant role at the time of intrauterine development. Figure 2 illustrates the significance of the normal and abnormal apoptotic process. It shapes the sculpture and organizes the interdigital webs of toes and fingers [26]. Moreover, apoptosis is a fundamental process for the usual overturn of intestinal cells, regression of tail in tadpoles via thyroxine dependency, insect cell metamorphosis, eye lens development in chicks, prostate epithelium regression, mammary gland, thymus gland, and adrenal gland. Thus, the role of apoptosis is quite ubiquitous in human biology. PCD is also pivotal as a fetal abnormality determinant. One of the studies reported that the wild-type p53 embryo of mouse aborts promptly following the induction of teratogenesis with radiation, but p53-null embryos do not. The biological systems such as the nervous system and immune system develop in response to the cellular overproduction and then followed by PCD of the cells that are unable to develop productive synaptic connections or functional antigen specificities [27]. In a normal adult human being, about 10 billion cells undergo apoptosis to maintain the cellular balance within the body. Thus, regular homeostasis is not only a passive process but highly regulated through PCD. However, with aging, the apoptotic reaction to DNA damages lessens and is weakly controlled, thereby paving the way for degenerative diseases. The induced sensitivity in apoptotic responses also contributes to cancer susceptibility.
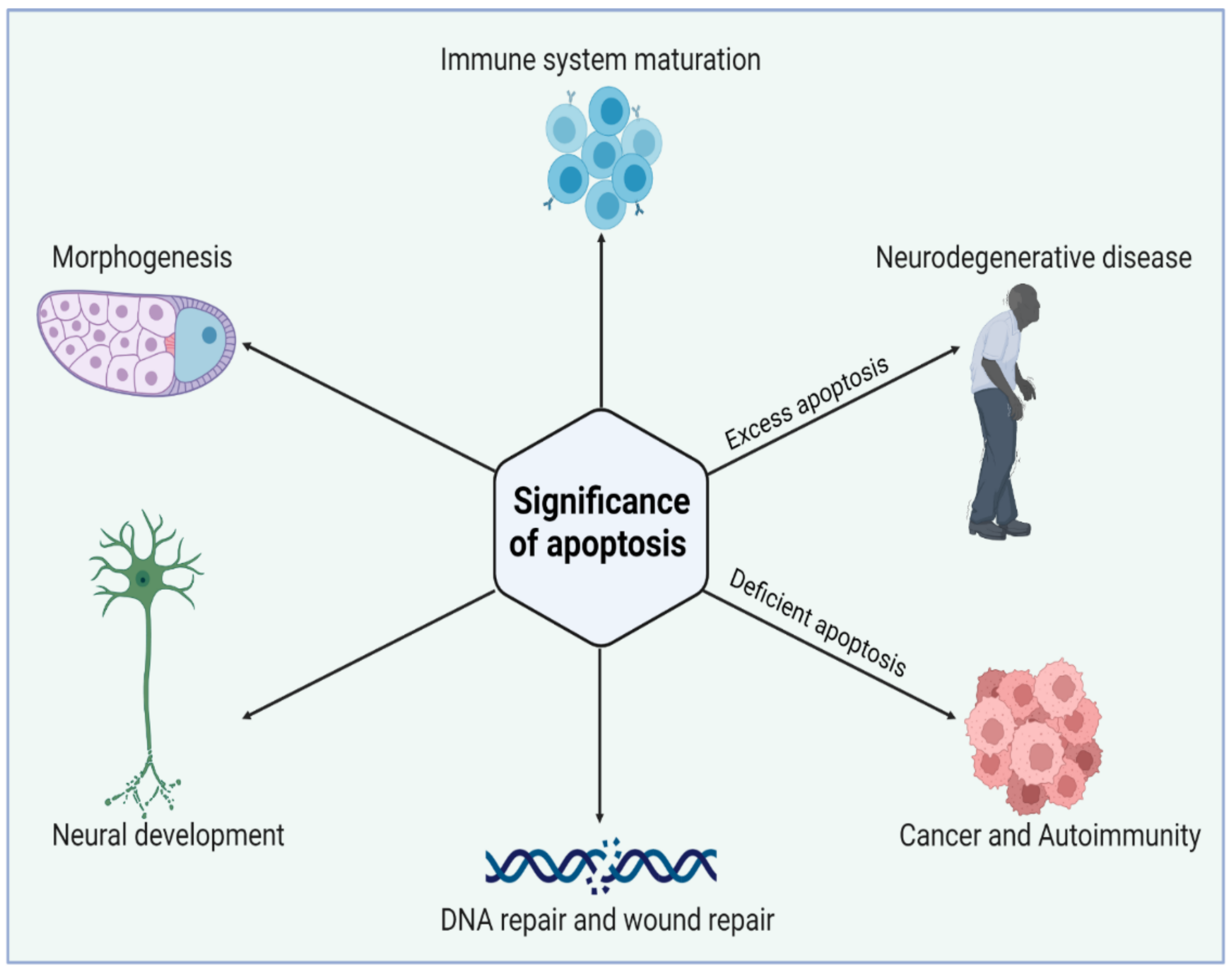
Figure 2. Biological significance of apoptosis in maintaining tissue homeostasis during cell life. Apoptosis has an essential role in the elimination of unwanted cells during early development. Excess or deficient apoptosis leads to several diseased conditions. Figure is created with BioRender.com (https://app.biorender.com, access on 22 November 2022).
4.1. Apoptosis: A Disease Paragon
Apoptosis combats infections by helping with the removal of pathogenic niches inside the cell and manages cancer prevention by eliminating the nascent neoplastic cells in developed organisms. However, aberrant PCD has been reported to add to the pathologies associated with long-standing degenerative diseases and acute injuries [4]. Both intrinsic and extrinsic apoptotic pathways play a substantial role in immune response activation and thereby help in the fight against pathogens and cancer. The pathogens specific to the intracellular environment when released by the dying cells are consumed by the neutrophils and macrophages that are in the vicinity, which subsequently leads to chemokine and cytokine secretions. Dying cells release antigens, pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs), which are identified and consumed by the specific dendritic cells and allows antigen-presenting cells (APCs) to prime T-lymphocytes, which enables them to recognize and damage the other infected cells, besides helping B-cell differentiation into antibody-producing plasma cells [28].
4.2. Apoptosis: A Cancer Cell Hallmark
Finding new anticancer treatments that target the apoptotic pathway is an intriguing strategy. Numerous mutations are found in intrinsic and extrinsic pathways in cancer, which enable the cells to avoid apoptosis. An all-encompassing cancer treatment would be possible if an apoptotic pathway could be targeted and activated [29]. Apoptosis is best characterized and studied in synchrony with cancer. Bcl-2, an anti-apoptotic protein family, was discovered after its overexpression and translocation in B cell follicular lymphomas. The copy number (CN) amplifications acquired somatically of a specific gene marker (loci) encoding Bcl-XL and MCL-1 (pro-survival proteins) are found in 3% and 10% of human cancers [30]. The unavailability of BIM (Bcl-2-interacting mediator), a pro-apoptotic protein, and the availability of overexpressed Bcl-2 were reported to increase c-myc oncogene-mediated lymphoma development which strongly indicates that apoptosis evasion promotes cancer. It is pertinent to mention that defective PCD does not alone aid in developing tumorigenesis, as only 5% of mice overexpressing Bcl-2 over eighteen months developed lymphoma, rather only the PCD inhibition aids in tumor development, which allows cells to live that would normally die. The reports suggest that the anti-cancer agents induce the IP for apoptosis, whereas direct activation of EP may not be needed to kill the cells downstream of anti-neoplastic agents [29]. However, the upregulation of TRAIL and FAS receptors mediated by p53, a tumor suppressor, may stimulate the malignant cell to the death receptor ligands. The ligands are efficiently expressed by cytotoxic lymphocytes that are activated and as a result, the ensuing DR-induced killing contributes to the in vivo cancer therapy. The studies on cell lines and mice for gene knockouts have shown the induction of apoptosis by anti-cancer agents in a BH-3-protein-dependent manner [31]. The PCD induction by dexamethasone, a glucocorticoid, depends on BIM. BIM is known to be suitable for tumor cell killing via oncogenic kinase inhibitors such as EGRF mutant, BCR-ABL, or B-RAF mutant. In HeLa cells, apoptosis is stopped by specific cellular proteins that are inhibitory in action and act by targeting retinoblastoma-associated tumor-suppressing proteins [32]. These proteins are known to regulate the cell cycle under normal conditions, but when attached to a protein of an inhibitory nature, these are rendered inactive. Human papillomavirus (HPV), forming a cervical tumor, expresses HPV-E6 and HPV-E7 inhibitory proteins. HPV-E6 inactivates p53 which regulates the cell cycle and acts as a tumor suppressor. On the other hand, HPV-E7 attaches to the tumor-suppressing proteins of retinoblastoma and reduces the cell division control ability. These two proteins with inhibitory action are somewhat responsible for the immortality of HeLa cells. As a result of advancements in cellular and molecular biology, immunology, and genomics leading to the discovery of numerous novel oncogenes, tumor suppressor genes, and immunologic and therapeutic targets, significant progress has been made with more new therapeutic drugs, including chemotherapy, targeted therapy, and immunotherapy, being approved for cancer therapy. The effectiveness and clinical applications of therapeutic drugs are nevertheless constrained by drug-induced toxicities and drug resistance. To increase efficacy and lessen toxicity, novel anticancer agents must be discovered and developed immediately.
4.3. Apoptosis: Role in Degenerative Diseases
The defects in controlled cell death may also lead to excess apoptosis which results in degenerative disorders, tissue damage, and hematological diseases. It is noteworthy that neurons depending on mitochondrial respiration go through PCD in diseases such as Parkinson’s and Alzheimer’s [33]. Interestingly, scientists have established that there exists an inverse epidemiological comorbidity relationship between cancer and neurodegenerative diseases. HIV progression is precisely an outcome of uncontrolled and excess apoptosis. This is because of the defects in the molecular signaling pathways that control the activity of Bcl-2 proteins [34]. The overexpression of BIM apoptotic proteins or their reduced proteolysis results in cell death, which ultimately results in a series of pathologies owing to the BIM activity site. Oxygen supply disruption, nutrient provision loss, and cellular waste removal inability lead to tissue injury and promote cell death during blood vessel blockage through embolism or thrombosis in an ischemic event.
References
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328.
- Keeble, J.A.; Gilmore, A.P. Apoptosis Commitment—Translating Survival Signals into Decisions on Mitochondria. Cell Res. 2007, 17, 976–984.
- Wallach, D.; Kang, T.-B. Programmed Cell Death in Immune Defense: Knowledge and Presumptions. Immunity 2018, 49, 19–32.
- Favaloro, B.; Allocati, N.; Graziano, V.; Di Ilio, C.; De Laurenzi, V. Role of Apoptosis in Disease. Aging 2012, 4, 330–349.
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. Int. J. Cell Biol. 2010, 2010, e214074.
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-Apoptosis and Cell Survival: A Review. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011, 1813, 238–259.
- Hongmei, Z. Extrinsic and Intrinsic Apoptosis Signal Pathway Review. Apoptosis Med. 2012.
- Webster, K.A. Mitochondrial Membrane Permeabilization and Cell Death during Myocardial Infarction: Roles of Calcium and Reactive Oxygen Species. Future Cardiol. 2012, 8, 863–884.
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 Family Proteins: Changing Partners in the Dance towards Death. Cell Death Differ. 2018, 25, 65–80.
- Lomonosova, E.; Chinnadurai, G. BH3-Only Proteins in Apoptosis and beyond: An Overview. Oncogene 2008, 27, S2–S19.
- Tzifi, F.; Economopoulou, C.; Gourgiotis, D.; Ardavanis, A.; Papageorgiou, S.; Scorilas, A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2011, 2012, e524308.
- Bratton, S.B.; Salvesen, G.S. Regulation of the Apaf-1–Caspase-9 Apoptosome. J. Cell Sci. 2010, 123, 3209–3214.
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193.
- Vringer, E.; Tait, S.W.G. Mitochondria and Inflammation: Cell Death Heats Up. Front. Cell Dev. Biol. 2019, 7, 100.
- Wang, F.; Ogasawara, M.A.; Huang, P. Small Mitochondria-Targeting Molecules as Anti-Cancer Agents. Mol. Aspects Med. 2010, 31, 75–92.
- Marchi, S.; Pinton, P. Mitochondria in the Line of Fire. Cell Death Differ. 2022, 29, 1301–1303.
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103.
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91.
- Xie, P. TRAF Molecules in Cell Signaling and in Human Diseases. J. Mol. Signal. 2013, 8, 7.
- MacFarlane, M.; Merrison, W.; Dinsdale, D.; Cohen, G.M. Active Caspases and Cleaved Cytokeratins Are Sequestered into Cytoplasmic Inclusions in Trail-Induced Apoptosis. J. Cell Biol. 2000, 148, 1239–1254.
- Tummers, B.; Green, D.R. Caspase-8; Regulating Life and Death. Immunol. Rev. 2017, 277, 76–89.
- Toné, S.; Sugimoto, K.; Tanda, K.; Suda, T.; Uehira, K.; Kanouchi, H.; Samejima, K.; Minatogawa, Y.; Earnshaw, W.C. Three Distinct Stages of Apoptotic Nuclear Condensation Revealed by Time-Lapse Imaging, Biochemical and Electron Microscopy Analysis of Cell-Free Apoptosis. Exp. Cell Res. 2007, 313, 3635–3644.
- Sherstnev, V.V.; Yurasov, V.V.; Storozheva, Z.I.; Gruden, M.A.; Yakovleva, N.E. Biochemical Markers of Apoptosis in Different Parts of the Brain during Learning. Neurosci. Behav. Physiol. 2006, 36, 915–919.
- Abaza, A.; Vasavada, A.M.; Sadhu, A.; Valencia, C.; Fatima, H.; Nwankwo, I.; Anam, M.; Maharjan, S.; Amjad, Z.; Khan, S. A Systematic Review of Apoptosis in Correlation With Cancer: Should Apoptosis Be the Ultimate Target for Cancer Treatment? Cureus 2022, 14, e28496.
- Conradt, B.; Wu, Y.-C.; Xue, D. Programmed Cell Death During Caenorhabditis Elegans Development. Genetics 2016, 203, 1533–1562.
- Gilbert, S.F. Cell Death and the Formation of Digits and Joints, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000.
- Yang, X.; Zhou, J.; He, J.; Liu, J.; Wang, H.; Liu, Y.; Jiang, T.; Zhang, Q.; Fu, X.; Xu, Y. An Immune System-Modified Rat Model for Human Stem Cell Transplantation Research. Stem Cell Rep. 2018, 11, 514–521.
- Sangiuliano, B.; Pérez, N.M.; Moreira, D.F.; Belizário, J.E. Cell Death-Associated Molecular-Pattern Molecules: Inflammatory Signaling and Control. Mediat. Inflamm. 2014, 2014, 821043.
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448.
- Young, A.I.; Timpson, P.; Gallego-Ortega, D.; Ormandy, C.J.; Oakes, S.R. Myeloid Cell Leukemia 1 (MCL-1), an Unexpected Modulator of Protein Kinase Signaling during Invasion. Cell Adhes. Migr. 2017, 12, 513–523.
- Vo, T.-T.; Letai, A. BH3-Only Proteins and Their Effects on Cancer. Adv. Exp. Med. Biol. 2010, 687, 49–63.
- Bourgo, R.J.; Braden, W.A.; Wells, S.I.; Knudsen, E.S. Activation of the Retinoblastoma Tumor Suppressor Mediates Cell Cycle Inhibition and Cell Death in Specific Cervical Cancer Cell Lines. Mol. Carcinog. 2009, 48, 45–55.
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in Neuroplasticity and Neurological Disorders. Neuron 2008, 60, 748–766.
- Chandrasekar, A.P.; Cummins, N.W.; Badley, A.D. The Role of the BCL-2 Family of Proteins in HIV-1 Pathogenesis and Persistence. Clin. Microbiol. Rev. 2019, 33, e00107-19.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
6.9K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
3 times
(View History)
Update Date:
14 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




