Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Boon-Peng Puah | -- | 1255 | 2023-02-13 09:26:40 | | | |
| 2 | Camila Xu | Meta information modification | 1255 | 2023-02-13 09:43:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Puah, B.; Jalil, J.; Attiq, A.; Kamisah, Y. Lycopene Metabolism, Bioavailability and Immunomodulatory Effects. Encyclopedia. Available online: https://encyclopedia.pub/entry/41146 (accessed on 07 February 2026).
Puah B, Jalil J, Attiq A, Kamisah Y. Lycopene Metabolism, Bioavailability and Immunomodulatory Effects. Encyclopedia. Available at: https://encyclopedia.pub/entry/41146. Accessed February 07, 2026.
Puah, Boon-Peng, Juriyati Jalil, Ali Attiq, Yusof Kamisah. "Lycopene Metabolism, Bioavailability and Immunomodulatory Effects" Encyclopedia, https://encyclopedia.pub/entry/41146 (accessed February 07, 2026).
Puah, B., Jalil, J., Attiq, A., & Kamisah, Y. (2023, February 13). Lycopene Metabolism, Bioavailability and Immunomodulatory Effects. In Encyclopedia. https://encyclopedia.pub/entry/41146
Puah, Boon-Peng, et al. "Lycopene Metabolism, Bioavailability and Immunomodulatory Effects." Encyclopedia. Web. 13 February, 2023.
Copy Citation
Lycopene is a carotenoid found commonly in fruits and vegetables such as tomatoes, pink grapefruit and watermelons with non-provitamin A activity. It is the compound responsible for the red coloration of the fruits. It shares the same molecular mass and chemical formula with beta-carotene, but lycopene is an open-polyene chain which lacks the β-ionone ring structure found in beta-carotene.
lycopene
tomatoes
cancer
immune system
inflammation
1. Introduction
Lycopene is a carotenoid found commonly in fruits and vegetables such as tomatoes, pink grapefruit and watermelons with non-provitamin A activity. It is the compound responsible for the red coloration of the fruits. It shares the same molecular mass and chemical formula with beta-carotene, but lycopene is an open-polyene chain which lacks the β-ionone ring structure found in beta-carotene. Lycopene is a highly unsaturated hydrocarbon which is able to undergo cis-trans isomerization under induction by light, thermal or chemical reactions. Most of the lycopene found in nature, from existing plant are predominantly in trans-configuration, which is more thermodynamically stable than its cis-counterpart [11][12]. Years of research managed to identify some of the metabolites of lycopene found in human body, which could depict an idea of the metabolism of lycopene in human. Some of the known metabolites of lycopene which were detected in plasma of humans include apo-8′-lycopenal, apo-10′-lycopenal, apo-10′-lycopenoic acid and apo-10′-lycopenol [13]. Apo-8′-lycopenal and apo-10′-lycopenoic acid were the metabolites found to possess anti-cancer activity, despite being a degraded fragments of lycopene[14][15] (Figure 1). Such observation could reflect the potential of lycopene in anti-cancer as its bioactivity is considered robust as metabolic catabolism would not nullify its effect.
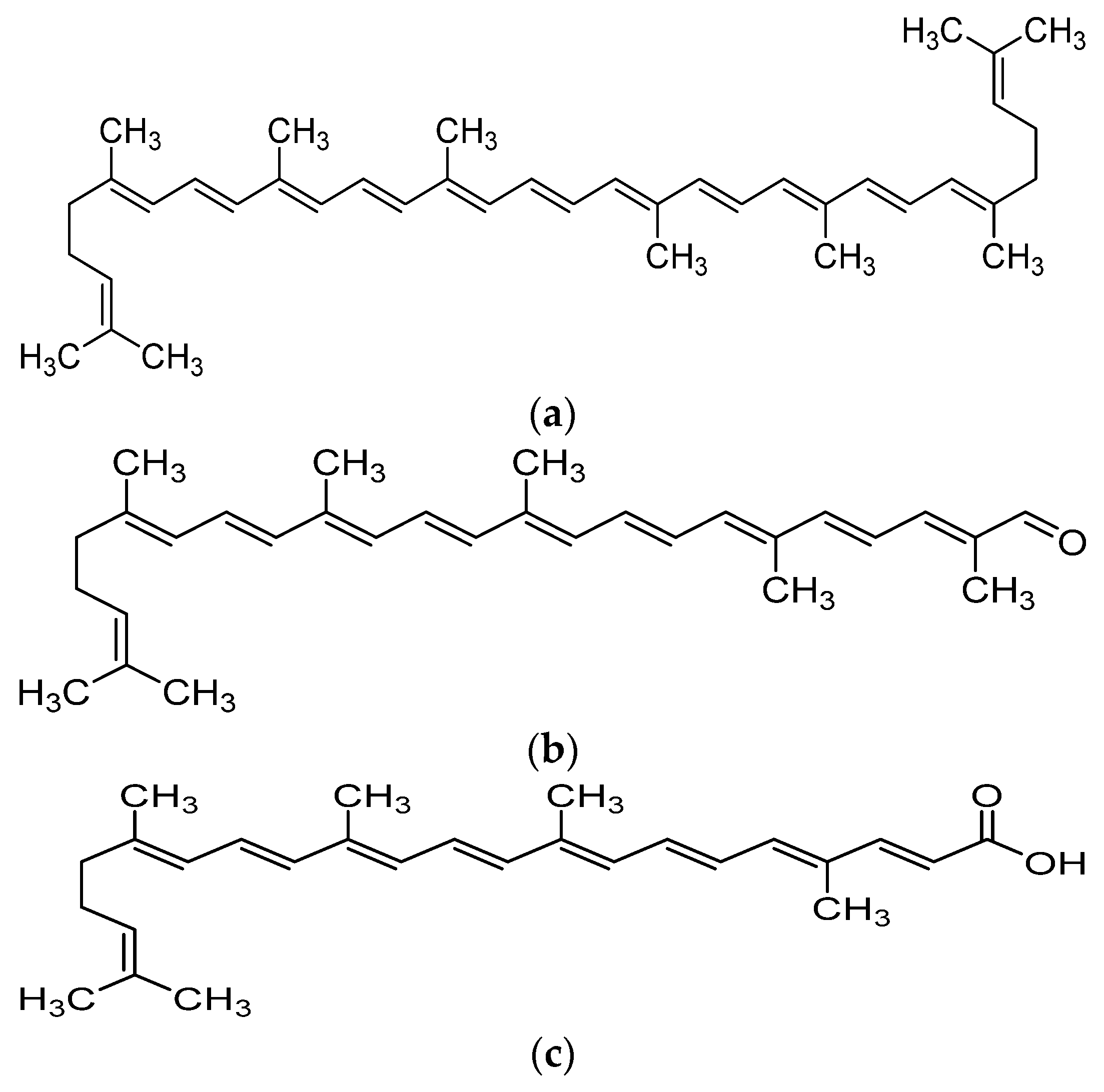
Figure 1. Chemical structure of lycopene and its metabolites with reported anti-cancer property. (a) Lycopene, (b) Apo-8′-lycopenal, (c) Apo-10′-lycopenoic acid.
Lycopene was known to be able to suppress cancerous cell proliferation, migration, invasion and adhesion activity in cell culture studies. Such suppression was often observed with changes of cancer-related gene expression and relief of oxidative stress. In general, lycopene could suppress the expression of MMP-2, MMP-7, MMP-9, Sp1, IGF-1R, VEGF while increasing E-cadherin stabilization, connexin 43, nm23-H1, TIMP-1 and TIMP-2 levels [15][16][17][18][19][20][21][22]. One of pathways involved in the anti-cancer property exhibited by lycopene was associated with its ability to regulate apoptosis-related protein and gene expression such as caspase-3, caspase-8, Bax levels and Bax:Bcl-2 and Bcl-xL among cancerous cells [23][24][25][26].
2. Metabolism and Bioavailability of Lycopene
The metabolism of lycopene is a complicated process, whereby it has to be released from the food matrix, emulsified and solubilized into micelles before absorption could occur as it is lipid soluble. Absorption of lycopene could occur either by passive diffusion or via SR-B1 transporter and CD36 surface membrane glycoprotein found in the small intestine. The absorption process is tightly regulated by intestine-specific homeobox (ISX) transcription factor and dependent on both intestinal β-carotene 15,15′-oxygenase (BCO1) and SR-B1 expression. After lycopene uptake by the small intestine, it will undergo isomerization from all-trans configuration to 5-cis lycopene and 13-cis lycopene, to be cleaved by carotene-9′,10′ monooxygenase (BCO2) to produce apo-10′-lycopenal. Apo-10′-lycopenal would then either be oxidized to apo-10′-lycopenoic acid or reduced to apo-10′-lycopenol. The metabolites mentioned above would later be packaged into chylomicrons and transported to the lymphatic system, liver and other peripheral tissues [27].
There are several factors which were found to be able to affect the bioavailability of lycopene from food. The release of lycopene from the plant itself is one of the determining factors of bioavailability while significant food processing also did find to improve the bioavailability of lycopene. It was proposed that the act of maceration could break down the plant cell walls and thus, leading to weakening of the bond between lycopene and the plant cell tissue matrix [28]. Some research suggested thermal processing could cause isomerization of naturally occurring all trans lycopene to cis lycopene, which is more easily oxidized and bioavailable towards humans [29]. Due to the fact that lycopene is highly lipophilic, consumption of lycopene with a certain amount of fats would greatly increase uptake rather than plain consumption as oil may improve absorption of lycopene by tissues [30]. However, it is recommended to avoid consumption of lycopene concurrently with high dietary fiber intake as several types of dietary fiber were found to be able to reduce the bioavailability of lycopene [31].
3. Immunomodulatory Effects of Lycopene
The earliest evidence came in 2004 when lycopene was able to modulate dendritic cell response by downregulation of CD80, CD86 and MHC II molecules expression, which are the common protein found on surface of dendritic cells. In vivo experiment further revealed that the effect of lycopene could be extended to decreased stimulation of T cells, accompanied by reduced expression of IL-2 and IL-12, the key stimulators of T cells. It was suggested that such effect was a result of MAPK/ERK signaling pathway inhibition (ERK1/2, p38, JNK) and reduced transcription of NF-κB. These evidences gave a direction whereby lycopene could suppress the maturation of murine dendritic cells and cell-mediated response under stimulation of LPS [98]. Mast cells are a type of granulocytes commonly known to be involved in allergic reaction and anaphylaxis. It plays a major role in inflammation as mast cell degranulation could release mediators or compounds which trigger an inflammatory response. Lycopene pretreatment with basophilic leukemia cell line suppressed mast cell degranulation but such activity was most probably not a direct result of lycopene cellular uptake as there was no correlation found between cellular carotenoids content and anti-degranulation activity. This suggests that the effect of lycopene in immune system modulation is not as simple as absorption and execution and it could be a result from a complicated network consisted of simultaneous activation of various immunomodulatory pathways [99]. In barrow and gilt finishing pigs, 0, 12.5, 25, 37.5, 50 mg/kg of lycopene administration followed by immunization using 1 mg BSA caused increased lymphocyte concentration and anti-BSA IgG, reduced neutrophil concentration and eosinophil while causing no change in basophil and monocyte [100]. Changes in lymphocyte concentration could be an indicator of activation of cell-mediated or humoral immune response and thus, lycopene was proposed to be able to influence immune response and the production of antigen-specific antibodies.
One study provided a lead whereby lycopene could activate the immune system, especially adaptive immunity in the process of eradicating cancerous cells. In this experiment, lycopene managed to enhance IFNβ, IFNγ, IRF1, IRF7, CXCL9, CXCL10 while suppressing IL-4, IL-10, DMNT3a, methylation of IRF1, IRF7 promoters and most importantly, the tumor volume. Increment of CD4+/CD8+ T cells ratio, percentage of IFNγ+/CD8+ T cell, percentage of perforin+/CD8+ T cell and percentage of granzyme B+/CD8+ T cell were reported, accompanied by no significant change in DNMT1 and DNMT3b [69]. Compiling these evidences depicted that lycopene could enhance activation and differentiation of T cells and T helper cells Th1/Th2 drift via suppression of IL-4 and upregulation of IFNγ, most probably by its ability to modulate cytokines, chemokines and interferons. Interferons had been known to be a central regulator for anti-tumor immunity whereby it had both anti-tumor and pro-tumor activity. In anti-tumor activity, interferons can increase antigenicity of tumor cells by upregulation of MH class I molecules and increase cytotoxic activity of both NK cells and cytotoxic T cells [101]. Suppression of DNMT3A could suppress DNA methylation of IRF1 and IRF7 promoters and thereby permitting transcription [102]. IRF1 was reported to be antioncogenic due to its ability to mediate apoptosis via induction of caspase-1, upregulation of caspase-8 and suppression of CDKs and survivin [103] while IRF7 was able to increase NK cell cytotoxic activity on cancerous cells via upregulation of IFNβ and inhibit bone metastasis [104].
References
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129, doi.:10.1016/j.jcrpr.2017.07.001.
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419, doi:10.3892/ijo.2018.4661.
- Mohan, G.; T. P., A.H.; A. J., J.; K. M., S.D.; Narayanasamy, A.; Vellingiri, B. Recent advances in radiotherapy and its associated side effects in cancer—A review. J. Basic Appl. Zool. 2019, 80, doi:10.1186/s41936-019-0083-5.
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive compounds: Natural defense against cancer? Biomolecules 2019, doi:10.3390/biom9120758.
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Roles of rutin in cardiac remodeling. J. Funct. Foods 2020, 64, 103606, doi:10.1016/j.jff.2019.103606.
- Gui, J.S.; Jalil, J.; Jubri, Z.; Kamisah, Y. Parkia speciosa empty pod extract exerts anti-inflammatory properties by modulating NFκB and MAPK pathways in cardiomyocytes exposed to tumor necrosis factor-α. Cytotechnology 2019, 71, 79–89, doi:10.1007/s10616-018-0267-8.
- Jalil, J.; Attiq, A.; Hui, C.C.; Yao, L.J.; Zakaria, N.A. Modulation of inflammatory pathways, medicinal uses and toxicities of uvaria species: Potential role in the prevention and treatment of inflammation. Inflammopharmacology 2020, 1–24, doi:10.1007/s10787-020-00734-2.
- Mohd Aluwi, M.F.F.; Rullah, K.; Haque, M.A.; Yamin, B.M.; Ahmad, W.; Amjad, M.W.; Leong, S.W.; Fahmizar, N.A.; Jalil, J.; Abas, F.; et al. Suppression of PGE2 production via disruption of MAPK Phosphorylation by unsymmetrical dicarbonyl curcumin derivatives. Med. Chem. Res. 2017, 26, 3323–3335, doi:10.1007/s00044-017-2025-4.
- Rowles, J.L., III; John, W.Erdman, J. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613.
- Mehta, N.; Patani, P.; Singhvi, I. A review on tomato lycopene. Int. J. Pharm. Sci. Res. 2018, 9, 916–923, doi:10.13040/IJPSR.0975-8232.9(3).916-23.
- Zechmeister, L.; LeRosen, A.L.; Went, F.W.; Pauling, L. Prolycopene, a naturally occuring stereoisomer of lycopene. Proc. Natl. Acad. Sci. 1941, 27, 468–474, doi:10.1073/pnas.27.10.468.
- Nguyen, M.L.; Schwartz, S.J. Lycopene: Chemical and biological properties. Food Technol. 1999, 53, 38–45.
- Arathi, B.P.; Sowmya, P.R.-R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Biofunctionality of carotenoid metabolites: An insight into qualitative and quantitative analysis. In Metabolomics Fundamentals and Applications; IntechOpen: London, UK, 2016. doi:10.5772/66210.
- Lian, F.; Smith, D.E.; Ernst, H.; Russell, R.M.; Wang, X.D. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 2007, 28, 1567–1574, doi:10.1093/carcin/bgm076.
- Yang, C.M.; Huang, S.M.; Liu, C.L.; Hu, M.L. Apo-8′-lycopenal induces expression of HO-1 and NQO-1 via the ERK/P38-Nrf2-ARE pathway in human HepG2 Cells. J. Agric. Food Chem. 2012, 60, 1576–1585, doi:10.1021/jf204451n.
- Huang, C.-S.; Shih, M.-K.; Chuang, C.-H.; Hu, M.-L. Lycopene inhibits cell migration and invasion and upregulates Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 Cells. J. Nutr. 2005, 135, 2119–2123, doi:10.1093/jn/135.9.2119.
- Huang, C.S.; Fan, Y.E.; Lin, C.Y.; Hu, M.L. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J. Nutr. Biochem. 2007, 18, 449–456, doi:10.1016/j.jnutbio.2006.08.007.
- Yang, C.M.; Hu, T.Y.; Hu, M.L. Antimetastatic effects and mechanisms of apo-8ʹ-lycopenal, an enzymatic metabolite of lycopene, against human hepatocarcinoma SK-Hep-1 Cells. Nutr. Cancer 2012, 64, 274–285, doi:10.1080/01635581.2012.643273.
- Jhou, B.Y.; Song, T.Y.; Lee, I.; Hu, M.L.; Yang, N.C. Lycopene inhibits metastasis of human liver adenocarcinoma SK-Hep-1 cells by downregulation of NADPH oxidase 4 protein expression. J. Agric. Food Chem. 2017, 65, 6893–6903, doi:10.1021/acs.jafc.7b03036.
- Koh, M.S.; Hwang, J.S.; Moon, A. Lycopene inhibits proliferation, invasion and migration of human breast cancer cells. Biomol. Ther. 2010, 18, 92–98, doi:10.4062/biomolther.2010.18.1.092.
- Lin, M.C.; Wang, F.Y.; Kuo, Y.H.; Tang, F.Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011, 59, 11304–11318, doi:10.1021/jf202433f.
- Huang, C.-S.; Liao, J.-W.; Hu, M.-L. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J. Nutr. 2008, 138, 538–543, doi:10.1093/jn/138.3.538.
- Takeshima, M.; Ono, M.; Higuchi, T.; Chen, C.; Hara, T.; Nakano, S. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 2014, 105, 252–257, doi:10.1111/cas.12349.
- Jeong, Y.; Lim, J.W.; Kim, H. Lycopene Inhibits reactive oxygen species-mediated Nf-Kb signaling and induces apoptosis in pancreatic cancer cells. Nutrients 2019, 11, doi:10.3390/nu11040762.
- Ip, B.C.; Hu, K.Q.; Liu, C.; Smith, D.E.; Obin, M.S.; Ausman, L.M.; Wang, X.D. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev. Res. 2013, 6, 1304–1316, doi:10.1158/1940-6207.CAPR-13-0178.
- Velmurugan, B.; Nagini, S. Combination chemoprevention of experimental gastric carcinogenesis by S-allylcysteine and lycopene: Modulatory effects on glutathione redox cycle antioxidants. J. Med. Food 2005, 8, 494–501, doi:10.1089/jmf.2005.8.494.
- Wang, X.D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214S–1222S, doi:10.3945/ajcn.111.032359.
- Srivastava, S.; Srivastava, A.K. Lycopene; Chemistry, Biosynthesis, Metabolism and Degradation under Various Abiotic Parameters. J. Food Sci. Technol. 2015, 41–53, doi:10.1007/s13197-012-0918-2.
- Stahl, W.; Sies, H. Uptake of lycopene and its geometrical isomers is greater from heat- processed than from unprocessed tomato juice in humans. J. Nutr. 1992, 122, 2161–2166, doi:10.1093/jn/122.11.2161.
- Gärtner, C.; Stahl, W.; Sies, H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr. 1997, 66, 116–122, doi:10.1093/ajcn/66.1.116.
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176, doi:10.1093/jn/129.12.2170.
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009, 425–430, doi:10.1158/1078-0432.CCR-08-0149.
- Giroux, V.; Rustgi, A.K. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat. Rev. Cancer 2017, 594–604, doi:10.1038/nrc.2017.68.
- Cordon-Cardo, C.; Prives, C. At the crossroads of inflammation and tumorigenesis. J. Exp. Med. 1999, 1367–1370, doi:10.1084/jem.190.10.1367.
- Maeda, H.; Akaike, T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biokhimiya 1998, 63, 1007–1019.
- Smyth, M.J.; Cretney, E.; Kershaw, M.H.; Hayakawa, Y. Cytokines in cancer immunity and immunotherapy. Immunol. Rev. 2004, 275–293, doi:10.1111/j.0105-2896.2004.00199.x.
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 1275–1288. doi:10.1111/j.1745-7254.2008.00889.x.
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 11553–11572, doi:10.1007/s13277-016-5098-7.
- Negus, R.P.M.; Stamp, G.W.H.; Relf, M.G.; Burke, F.; Malik, S.T.A.; Bernasconi, S.; Allavena, P.; Sozzani, S.; Mantovani, A.; Balkwill, F.R. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J. Clin. Investig. 1995, 95, 2391–2396, doi:10.1172/JCI117933.
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126, doi:10.4103/aam.aam_56_18.
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 1–10, doi:10.1016/j.immuni.2013.07.012.
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D.; Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 836–848, doi:10.1038/nri1961.
- Pio, R.; Corrales, L.; Lambris, J.D. The role of complement in tumor growth. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2014; Volume 772, pp. 229–262, doi:10.1007/978-1-4614-5915-6_11.
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943, doi:10.1038/onc.2008.267.
- Warrington, R.; Watson, W.; Kim, H.L.; Antonetti, F.R. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2011, 7, doi:10.1186/1710-1492-7-s1-s1.
- Harris, T.J.; Drake, C.G. Primer on tumor immunology and cancer immunotherapy. J. ImmunoTher. Cancer 2013, doi:10.1186/2051-1426-1-12.
- Minami, Y.; Kono, T.; Miyazaki, T.; Taniguchi, T. The IL-2 receptor complex: Its structure, function, and target genes. Annu. Rev. Immunol. 1993, 11, 245–268, doi:10.1146/annurev.iy.11.040193.001333.
- NORMAN, P. Immunobiology: The immune system in health and disease. J. Allergy Clin. Immunol. 1995, 96, 274–274, doi:10.1016/s0091-6749(95)70025-0.
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, doi:10.1016/j.jaci.2009.09.046.
- Sun, J.C.; Lanier, L.L. Natural killer cells remember: An evolutionary bridge between innate and adaptive immunity? Eur. J. Immunol. 2009, 39, 2059–2064, doi:10.1002/eji.200939435.
- Jiang, L.N.; Liu, Y. Bin; Li, B.H. Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression. Asian J. Androl. 2019, 21, 80–85, doi:10.4103/aja.aja_70_18.
- Li, C.C.; Liu, C.; Fu, M.; Hu, K.Q.; Aizawa, K.; Takahashi, S.; Hiroyuki, S.; Cheng, J.; von Lintig, J.; Wang, X.D. Tomato powder inhibits hepatic steatosis and inflammation potentially through restoring SIRT1 activity and adiponectin function independent of carotenoid cleavage enzymes in mice. Mol. Nutr. Food Res. 2018, 62, doi:10.1002/mnfr.201700738.
- Sun, X.; Jia, H.; Xu, Q.; Zhao, C.; Xu, C. Lycopene alleviates H2O2-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells: Via the NFE2L2 signaling pathway. Food Funct. 2019, 10, 6276–6285, doi:10.1039/c9fo01922g.
- Zhao, Q.; Yang, F.; Meng, L.; Chen, D.; Wang, M.; Lu, X.; Chen, D.; Jiang, Y.; Xing, N. Lycopene attenuates chronic prostatitis/chronic pelvic pain syndrome by inhibiting oxidative stress and inflammation via the interaction of NF-ΚB, MAPKs, and Nrf2 signaling pathways in rats. Andrology 2020, 8, 747–755, doi:10.1111/andr.12747.
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96, doi:10.4162/nrp.2017.11.2.90.
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective effects of nanoemulsions loaded with anti-inflammatory nutraceuticals against doxorubicin-induced cardiotoxicity. Nutrients 2018, 10, doi:10.3390/nu10091304.
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000, doi:10.1161/ATVBAHA.110.207449.
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018, doi:10.3389/fphar.2018.00976.
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 252–259, doi:10.1007/s10787-007-0013-x.
- Sengupta, A.; Ghosh, S.; Das, R.K.; Bhattacharjee, S.; Bhattacharya, S. Chemopreventive potential of diallylsulfide, lycopene and theaflavin during chemically induced colon carcinogenesis in rat colon through modulation of cyclooxygenase-2 and inducible nitric oxide synthase pathways. Eur. J. Cancer Prev. 2006, 15, 301–305, doi:10.1097/00008469-200608000-00005.
- Luo, C.; Wu, X.G. Lycopene enhances antioxidant enzyme activities and immunity function in N-Methyl-N′-Nitro-N-nitrosoguanidine-induced gastric cancer rats. Int. J. Mol. Sci. 2011, 12, 3340–3351, doi:10.3390/ijms12053340.
- Ip, B.C.; Liu, C.; Ausman, L.M.; Von Lintig, J.; Wang, X.D. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Chest 2014, 146, 1219–1227, doi:10.1158/1940-6207.CAPR-14-0154.
- Zhou, S.K.; Zhang, R.L.; Bi, T.N.; Lu, Y.; Jiang, L.X. Inhibitory effect of lycopene against the growth of human gastric cancer cells. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 184–190, doi:10.21010/ajtcam.v13i4.24.
- Gajowik, A.; Dobrzyńska, M.M. The evaluation of protective effect of lycopene against genotoxic influence of X-irradiation in human blood lymphocytes. Radiat. Environ. Biophys. 2017, 56, 413–422, doi:10.1007/s00411-017-0713-6.
- Wang, S.; Wu, Y.Y.; Wang, X.; Shen, P.; Jia, Q.; Yu, S.; Wang, Y.; Li, X.; Chen, W.; Wang, A.; et al. Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through P62-triggered autophagic keap1 degradation. Aging 2020, 12, 8167–8190, doi:10.18632/aging.103132.
- Lian, F.; Wang, X.D. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int. J. Cancer 2008, 123, 1262–1268, doi:10.1002/ijc.23696.
- Nagasawa, H.; Mitamura, T.; Sakamoto, S.; Yamamoto, K. Effects of lycopene on spontaneous mammary tumour development in SHN virgin mice. Anticancer Res. 1995, 15, 1173–1178.
- Sharoni, Y.; Giron, E.; Rise, M.; Levy, J. Effects of lycopene-enriched tomato oleoresin on 7,12-dimethyl-benz[a]anthracene-induced rat mammary tumors. Cancer Detect. Prev. 1997, 21, 118–123.
- Jiang, X.; Wu, H.; Zhao, W.; Ding, X.; You, Q.; Zhu, F.; Qian, M.; Yu, P. Lycopene improves the efficiency of anti-PD-1 therapy via activating IFN signaling of lung cancer cells. Cancer Cell Int. 2019, 19, doi:10.1186/s12935-019-0789-y.
- Astorg, P.; Gradelet, S.; Bergès, R.; Suschetet, M. Dietary lycopene decreases the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutr. Cancer 1997, 29, 60–68, doi:10.1080/01635589709514603.
- Passos Toledo, L.; Prates Ong, T.; Galvão Pinho, A.L.; Jordão, A.; Vanucchi, H.; Salvador Moreno, F. Inhibitory effects of lutein and lycopene on placental glutathione s-transferase-positive preneoplastic lesions and DNA strand breakage induced in wistar rats by the resistant hepatocyte model of hepatocarcinogenesis. Nutr. Cancer 2003, 47, 62–69, doi:10.1207/s15327914nc4701_8.
- Wang, Y.; Ausman, L.M.; Greenberg, A.S.; Russell, R.M.; Wang, X.D. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int. J. Cancer 2010, 126, 1788–1796, doi:10.1002/ijc24689.
- Velmurugan, B.; Bhuvaneswari, V.; Burra, U.K.; Nagini, S. Prevention of N-Methyl-N′-Nitro-N-nitrosoguanidine and saturated sodium chloride-induced gastric carcinogenesis in wistar rats by lycopene. Eur. J. Cancer Prev. 2002, 11, 19–26, doi:10.1097/00008469-200202000-00004.
- Ucci, M.; Di Tomo, P.; Tritschler, F.; Cordone, V.G.P.; Lanuti, P.; Bologna, G.; Di Silvestre, S.; Di Pietro, N.; Pipino, C.; Mandatori, D.; et al. Anti-inflammatory role of carotenoids in endothelial cells derived from umbilical cord of women affected by gestational diabetes mellitus. Oxid. Med. Cell. Longev. 2019, 2019, doi:10.1155/2019/8184656.
- Holzapfel, N.P.; Shokoohmand, A.; Wagner, F.; Landgraf, M.; Champ, S.; Holzapfel, B.M.; Clements, J.A.; Hutmacher, D.W.; Loessner, D. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am. J. Cancer Res. 2017, 7, 1322–1336.
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B.; et al. Lycopene protects against spontaneous ovarian cancer formation in laying hens. J. Cancer Prev. 2018, 23, 25–36, doi:10.15430/jcp.2018.23.1.25.
- Narisawa, T.; Fukaura, Y.; Hasebe, M.; Ito, M.; Aizawa, R.; Murakoshi, M.; Uemura, S.; Khachik, F.; Nishino, H. Inhibitory effects of natural carotenoids, α-carotene, β-carotene, lycopene and lutein, on colonic aberrant crypt foci formation in rats. Cancer Lett. 1996, 107, 137–142, doi:10.1016/0304-3835(96)04354-6.
- Narisawa, T.; Fukaura, Y.; Hasebe, M.; Nomura, S.; Oshima, S.; Sakamoto, H.; Inakuma, T.; Ishiguro, Y.; Takayasu, J.; Nishino, H. Prevention of N-methylnitrosourea-induced colon carcinogenesis in F344 rats by lycopene and tomato juice rich in lycopene. Jpn. J. Cancer Res. 1998, 89, 1003–1008, doi:10.1111/j.1349-7006.1998.tb00488.x.
- Valadez-Bustos, N.; Escamilla-Silva, E.M.; García-Vázquez, F.J.; Gallegos-Corona, M.A.; Amaya-Llano, S.L.; Ramos-Gómez, M. Oral Administration of Microencapsulated, B. Longum BAA-999 and lycopene modulates IGF-1/IGF-1R/IGFBP3 protein expressions in a colorectal murine model. Int. J. Mol. Sci. 2019, 20, doi:10.3390/ijms20174275.
- Kim, D.J.; Takasuka, N.; Kim, J.M.; Sekine, K.; Ota, T.; Asamoto, M.; Murakoshi, M.; Nishino, H.; Nir, Z.; Tsuda, H. Chemoprevention by lycopene of mouse lung neoplasia after combined initiation treatment with DEN, MNU and DMH. Cancer Lett. 1997, 120, 15–22, doi:10.1016/S0304-3835(97)00281-4.
- Watanabe, S.; Kitade, Y.; Masaki, T.; Nishioka, M.; Satoh, K.; Nishino, H. Effects of lycopene and sho-saiko-to on hepatocarcinogenesis in a rat model of spontaneous liver cancer. Nutr. Cancer 2001, 39, 96–101, doi:10.1207/S15327914nc391_13.
- Cohen, L.A.; Zhao, Z.; Pittman, B.; Khachik, F. Effect of dietary lycopene on N-methylnitrosourea-induced mammary tumorigenesis. Nutr. Cancer 1999, 34, 153–159, doi:10.1207/S15327914NC3402_5.
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of carotenoids and retino in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776, doi:10.1093/jnci/87.23.1767.
- Giovannucci, E. A prospective study of tomato products, lycopene, and prostate cancer risk. CancerSpectrum Knowl. Environ. 2002, 94, 391–398, doi:10.1093/jnci/94.5.391.
- Wu, K.; Erdman, J.W.; Schwartz, S.J.; Platz, E.A.; Leitzmann, M.; Clinton, S.K.; DeGroff, V.; Willett, W.C.; Giovannucci, E. Plasma and dietary carotenoids, and the risk of prostate cancer: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 260–269, doi:10.1158/1055-9965.EPI-03-0012.
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2001, 10, 861–868.
- Bowen, P.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; Kim, H.S.; Christov-Tzelkov, K.; Van Breemen, R. Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp. Biol. Med. 2002, 227, 886–893, doi:10.1177/153537020222701008.
- Paur, I.; Lilleby, W.; Bøhn, S.K.; Hulander, E.; Klein, W.; Vlatkovic, L.; Axcrona, K.; Bolstad, N.; Bjøro, T.; Laake, P.; et al. Tomato-based randomized controlled trial in prostate cancer patients: Effect on PSA. Clin. Nutr. 2017, 36, 672–679, doi:10.1016/j.clnu.2016.06.014.
- Chen, J.; Song, Y.; Zhang, L. Lycopene/tomato consumption and the risk of prostate cancer: A systematic review and meta-analysis of prospective studie. J. Nutr. Sci. Vitaminol. 2013, 59, 213–223, doi:10.3177/jnsv.59.213.
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and risk of prostate cancer. Medicine 2015, 94, e1260, doi:10.1097/MD.0000000000001260.
- Bae, J.M. Reinterpretation of the results of a pooled analysis of dietary carotenoid intake and breast cancer risk by using the interval collapsing method. Epidemiol. Health 2016, 38, e2016024, doi:10.4178/epih.e2016024.
- Yan, B.; Lu, M.S.; Wang, L.; Mo, X.F.; Luo, W.P.; Du, Y.F.; Zhang, C.X. Specific serum carotenoids are inversely associated with breast cancer risk among chinese women: A case-control study. Br. J. Nutr. 2016, 115, 129–137, doi:10.1017/S000711451500416X.
- Abar, L.; Vieira, A.R.; Aune, D.; Stevens, C.; Vingeliene, S.; Navarro Rosenblatt, D.A.; Chan, D.; Greenwood, D.C.; Norat, T. Blood concentrations of carotenoids and retinol and lung cancer risk: An update of the WCRF–AICR Systematic review of published prospective studies. Cancer Med. 2016, 5, 2069–2083, doi:10.1002/cam4.676.
- Hsing, A.W.; Comstock, G.W.; Abbey, H.; Polk, B.F. Serologic precursors of cancer. Retinol, carotenoids, and tocopherol and risk of prostate cancer. J. Natl. Cancer Inst. 1990, 82, 941–946, doi:10.1093/jnci/82.11.941.
- Vogt, T.M.; Mayne, S.T.; Graubard, B.I.; Swanson, C.A.; Sowell, A.L.; Schoenberg, J.B.; Swanson, G.M.; Greenberg, R.S.; Hoover, R.N.; Hayes, R.B.; et al. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US blacks and whites. Am. J. Epidemiol. 2002, 155, 1023–1032, doi:10.1093/aje/155.11.1023.
- Schuurman, A.G.; Goldbohm, R.A.; Brants, H.A.M.; Van Den Brandt, P.A. A Prospective cohort study on intake of retinol, vitamins C and E, and carotenoids and prostate cancer risk (Netherlands). Cancer Causes Control. 2002, 13, 573–582, doi:10.1023/A:1016332208339.
- Chan, J.M.; Weinberg, V.; Magbanua, M.J.; Sosa, E.; Simko, J.; Shinohara, K.; Federman, S.; Mattie, M.; Hughes-Fulford, M.; Haqq, C.; et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control 2011, 22, 141–150, doi:10.1007/s10552-010-9684-5.
- Kim, G.Y.; Kim, J.H.; Ahn, S.C.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Park, Y.M. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-ΚB. Immunology 2004, 113, 203–211, doi:10.1111/j.1365-2567.2004.01945.x.
- Manabe, Y.; Hirata, T.; Sugawara, T. Suppressive effects of carotenoids on the antigeninduced degranulation in RBL-2H3 rat basophilic leukemia cells. J. Oleo Sci. 2014, 63, 291–294, doi:10.5650/jos.ess13169.
- Fachinello, M.R.; Fernandes, N.L.M.; de Souto, E.R.; dos Santos, T.C.; da Costa, A.E.R.; Pozza, P.C. Lycopene affects the immune responses of finishing pigs. Ital. J. Anim. Sci. 2018, 17, 666–674, doi:10.1080/1828051X.2017.1401438.
- Zaidi, M.R. The interferon-gamma paradox in cancer. J. Interf. Cytokine Res. 2019, 39, 30–38, doi:10.1089/jir.2018.0087.
- Ren, W.; Gao, L.; Song, J. Structural basis of DNMT1 and DNMT3A-Mediated DNA methylation. Genes 2018, 9, 620, doi:10.3390/genes9120620.
- Alsamman, K.; El-Masry, O.S. Interferon regulatory factor 1 inactivation in human cancer. Biosci. Rep. 2018, 38, BSR20171672, doi:10.1042/BSR20171672.
- Yang, Z.; Chen, W.; Zhu, W.; Meng, H.; Chen, J.; Zhang, J. Overexpression of interferon regulatory factor 7 (IRF7) reduces bone metastasis of prostate cancer cells in mice. Oncol. Res. 2017, 25, 511–522, doi:10.3727/096504016X14756226781802.
- Makon-Sébastien, N.; Francis, F.; Eric, S.; Henri, V.P.; François, L.J.; Laurent, P.; Yves, B.; Serge, C. Lycopene modulates THP1 and Caco2 cells inflammatory state through transcriptional and nontranscriptional processes. Mediat. Inflamm. 2014, 2014, doi:10.1155/2014/507272.
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell. Mol. Biol. Lett. 2016, doi:10.1186/s11658-016-0031-z.
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 585–597, doi:10.1016/j.bbadis.2016.11.005.
- Zheng, Z.; Yin, Y.; Lu, R.; Jiang, Z. Lycopene ameliorated oxidative stress and inflammation in Type 2 diabetic rats. J. Food Sci. 2019, 84, 1194–1200, doi:10.1111/1750-3841.14505.
- Bignotto, L.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M.; Pinto, R.; Chaud, M.; De Carvalho, J.; Moreno, H.; Mota-Filipe, H. Anti-inflammatory effect of lycopene on carrageenan-induced paw oedema and hepatic ischaemia-reperfusion in the rat. Br. J. Nutr. 2009, 102, 126–133, doi:10.1017/S0007114508137886.
- Denniss, S.G.; Haffner, T.D.; Kroetsch, J.T.; Davidson, S.R.; Rush, J.W.E.; Hughson, R.L. Effect of short-term lycopene supplementation and postprandial dyslipidemia on plasma antioxidants and biomarkers of endothelial health in young, healthy individuals. Vasc. Health Risk Manag. 2008, 4, 213–222, doi:10.2147/vhrm.2008.04.01.213.
- Markovits, N.; Amotz, A. Ben; Levy, Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr. Med. Assoc. J. 2009, 11, 598–601.
- Petyaev, I.M.; Dovgalevsky, P.Y.; Klochkov, V.A.; Chalyk, N.E.; Pristensky, D.V.; Chernyshova, M.P.; Udumyan, R.; Kocharyan, T.; Kyle, N.H.; Lozbiakova, M.V.; et al. Effect of lycopene supplementation on cardiovascular parameters and markers of inflammation and oxidation in patients with coronary vascular disease. Food Sci. Nutr. 2018, 6, 1770–1777, doi:10.1002/fsn3.734.
- Li, W.W.; Wang, T.Y.; Cao, B.; Liu, B.; Rong, Y.M.; Wang, J.J.; Wei, F.; Wei, L.Q.; Chen, H.; Liu, Y.X. Synergistic protection of matrine and lycopene against lipopolysaccharide-induced acute lung injury in mice. Mol. Med. Rep. 2019, 20, 455–462, doi:10.3892/mmr.2019.10278.
- Veeramachaneni, S.; Ausman, L.M.; Choi, S.W.; Russell, R.M.; Wang, X.-D. High dose lycopene supplementation increases hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J. Nutr. 2008, 138, 1329–1335, doi:10.1093/jn/138.7.1329.
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care Off. Publ. Tufts Univ. 2002, 56–65, doi:10.1046/j.1523-5408.2002.00004.x.
- Mayne, S.T. Beta‐carotene, carotenoids, and disease prevention in humans. FASEB J. 1996, 10, 690–701, doi:10.1096/fasebj.10.7.8635686.
- Arab, L.; Steck-Scott, S.; Bowen, P. Participation of lycopene and beta-carotene in carcinogenesis: Defenders, aggressors, or passive bystanders? Epidemiol. Rev. 2001, 23, 211–230, doi:10.1093/oxfordjournals.epirev.a000803.
- The Alpha-Tocopherol, B.C.C.P.S.G. The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035, doi:10.1056/nejm199404143301501.
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and retinol efficacy trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559, doi:10.1093/jnci/88.21.1550.
- Männistö, S.; Smith-Warner, S.A.; Spiegelman, D.; Albanes, D.; Anderson, K.; Van Den Brandt, P.A.; Cerhan, J.R.; Colditz, G.; Feskanich, D.; Freudenheim, J.L.; et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 40–48, doi:10.1158/1055-9965.EPI-038-3.
- Touvier, M.; Kesse, E.; Clavel-Chapelon, F.; Boutron-Ruault, M.C. Dual association of β-carotene with risk of tobacco-related cancers in a cohort of french women. J. Natl. Cancer Inst. 2005, 97, 1338–1344, doi:10.1093/jnci/dji276.
- Goralczyk, R. Beta-carotene and lung cancer in smokers: Review of hypotheses and status of research. Nutr. Cancer 2009, 61, 767–774, doi:10.1080/01635580903285155.
- Michaud, D.S.; Feskanich, D.; Rimm, E.B.; Colditz, G.A.; Speizer, F.E.; Willett, W.C.; Giovannucci, E. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am. J. Clin. Nutr. 2000, 72, 990–997, doi:10.1093/ajcn/72.4.990.
- Rao, A.V.; Agarwal, S. Effect of diet and smoking on serum lycopene and lipid peroxidation. Nutr. Res. 1998, 18, 713–721, doi:10.1016/s0271-5317(98)00057-8.
- Heber, D. Colorful cancer prevention: α-carotene, lycopene, and lung cancer. Am. J. Clin. Nutr. 2000, 72, 901–902.
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Long-term use of β-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: Results from the vitamins and lifestyle (vital) study. Am. J. Epidemiol. 2009, 169, 815–828, doi:10.1093/aje/kwn409.
- Ford, N.A.; Smith, J.W.; Clinton, S.K.; Erdman, J.W. Tomato powder or lycopene reduces serum and testicular testosterone and enzymes controlling androgen and estrogen metabolism in mice lacking carotene-15,15’-monooxygenase. Exp. Biol. 2011, doi:10.1096/fasebj.25.1_supplement.975.6.
- Luca, L.M.D.; Darwiche, N.; Celli, G.; Kosa, K.; Jones, C.; Ross, S.; Chen, L. ‐C. Vitamin A in epithelial differentiation and skin carcinogenesis. Nutr. Rev. 1994, 52, S45–S52, doi:10.1111/j.1753-4887.1994.tb01386.x.
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic acid: A key regulator of lung development. Biomolecules 2020, 10, 152, doi:10.3390/biom10010152.
- National Research Council (US) Panel on Dosimetric Assumptions Affecting the Application of Radon Risk Estimates. Comparative Dosimetry of Radon in Mines and Homes; National Academy of Sciences: Washington, DC, USA, 1991; doi:10.17226/1799.
- Ben-Dor, A.; Nahum, A.; Danilenko, M.; Giat, Y.; Stahl, W.; Martin, H.D.; Emmerich, T.; Noy, N.; Levy, J.; Sharoni, Y. Effects of acyclo-retinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells. Arch. Biochem. Biophys. 2001, 391, 295–302, doi:10.1006/abbi.2001.2412.
- Stahl, W.; Von Laar, J.; Martin, H.D.; Emmerich, T.; Sies, H. Stimulation of gap junctional communication: Comparison of acyclo- retinoic acid and lycopene. Arch. Biochem. Biophys. 2000, 373, 271–274, doi:10.1006/abbi.1999.1510.
- Li, M.T.; Richter, F.; Chang, C.; Irwin, R.J.; Huang, H.F.S. Androgen and retinoic acid interaction in LNCaP cells, effects on cell proliferation and expression of retinoic acid receptors and epidermal growth factor receptor. BMC Cancer 2002, 2, doi:10.1186/1471-2407-2-16.
- Chen, M.C.; Hsu, S.L.; Lin, H.; Yang, T.Y. Retinoic acid and cancer treatment. BioMedicine 2014, 4, 1–6, doi:10.7603/s40681-014-0022-1.
- Alsafadi, S.; Even, C.; Falet, C.; Goubar, A.; Commo, F.; Scott, V.; Quidville, V.; Albiges, L.; Dieci, M.V.; Guegan, J.; et al. Retinoic acid receptor alpha amplifications and retinoic acid sensitivity in breast cancers. Clin. Breast Cancer 2013, 13, 401–408, doi:10.1016/j.clbc.2013.02.001.
- Wang, W.; Liu, S.; Jiang, C.; Wang, Y.; Zhu, H.; Wang, X.D. High expression of RARβ is a favorable factor in colorectal cancer. Dis. Markers 2019, 2019, doi:10.1155/2019/7138754.
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, doi:10.1242/dev.167502.
More
Information
Subjects:
Nutrition & Dietetics; Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
790
Revisions:
2 times
(View History)
Update Date:
13 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




