
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kevin Yang Wu | -- | 3692 | 2023-02-12 22:56:30 | | | |
| 2 | Jessie Wu | Meta information modification | 3692 | 2023-02-13 06:31:59 | | | | |
| 3 | Jessie Wu | Meta information modification | 3692 | 2023-02-13 07:14:29 | | | | |
| 4 | Jessie Wu | + 5 word(s) | 3697 | 2023-02-13 07:21:09 | | | | |
| 5 | Jessie Wu | + 3 word(s) | 3700 | 2023-02-13 07:22:04 | | | | |
| 6 | Jessie Wu | + 1 word(s) | 3701 | 2023-02-13 07:36:50 | | |
Video Upload Options
Sjögren’s syndrome is a chronic and insidious auto-immune disease characterized by lymphocyte infiltration of exocrine glands. The patients typically present with ocular surface diseases related to dry eye and other systemic manifestations. However, due to the high prevalence of dry eye disease and the lack of objective and clinically reliable diagnostic tools, discriminating Sjögren’s syndrome dry eye (SSDE) from non-Sjögren’s syndrome dry eye (NSSDE) remains a challenge for clinicians. Diagnosing Sjögren’s syndrome is important to improve the quality of life of patients through timely referral for systemic workups, as SS is associated with serious systemic complications such as lymphoma and other autoimmune diseases.
1. Introduction
Sjögren's syndrome (SS) is a chronic autoimmune disease characterized by inflammation of exocrine glands, most commonly the lacrimal and salivary glands. This leads to dry eyes and dry mouth, the classical symptoms of the disease [1]. The disease was first described by Swedish ophthalmologist Dr. Henrik Sjögren in 1933.
1.1. Classification of Sjögren's Syndrome
SS can be classified as primary Sjögren's syndrome (pSS) when it occurs without another autoimmune disease or secondary Sjögren's syndrome (sSS) when it is associated with another autoimmune disease such as rheumatoid arthritis or systemic lupus erythematosus [2]. This research focuses only on pSS.
1.2. Ophthalmic Manifestations of Sjögren's Syndrome
The main ocular symptoms of Sjögren's syndrome (SS) are dry eye symptoms, including foreign body sensations, burning sensations, eyestrain, photophobia, blurry vision, and red eyes. These symptoms are often made worse by prolonged visual effort or environmental extremes [3]. Physical examination findings may show conjunctival hyperemia, decreased meniscal height, and a tear break-up time of less than 10 seconds. Ocular staining may reveal dead epithelium on the conjunctiva, and fluorescein may reveal punctate epithelial erosions on the cornea [3].
1.3. Glandular Manifestations
Xerostomia, or dry mouth, is a hallmark sign of SS and may lead to difficulties with eating, swallowing, or speaking. The salivary glands may become enlarged, which is usually due to sialadenitis caused by lymphocytic infiltration. However, malignancy such as MALT lymphoma must be ruled out [4]. While malignancy in the lacrimal glands is less common than in the salivary glands, it can still occur [5].
1.4. Extra-Glandular/Systemic Manifestations
Arthralgia is the most common extraglandular manifestation of SS. Other systemic manifestations include vasculitis and xeroderma, bronchiolitis, interstitial lung diseases, tubulointerstitial nephritis, liver and pancreas involvement, sensory neuropathy, myelitis, meningitis, and a range of hematological manifestations such as anemia, leucopenia, thrombocytopenia, and hypergammaglobulinemia [3][6][7]. SS is also associated with a higher risk of Hashimoto's thyroiditis, cardiovascular disease, depression, and lymphoma.
2. Pathogenesis
The pathogenesis of primary Sjogren's syndrome (pSS) is a complex process with multiple contributing factors (Figure 1). It is believed to involve the interaction between genetic susceptibility and environmental exposures, leading to the development of an autoimmune T-cells mediated reaction. This reaction leads to the destruction of the lacrimal glands and a decrease in aqueous tear production, partly due to an increase in programmed cell death. This, in turn, affects the functional lacrimal unit, initiating a vicious cycle of dry eye disease (DED).
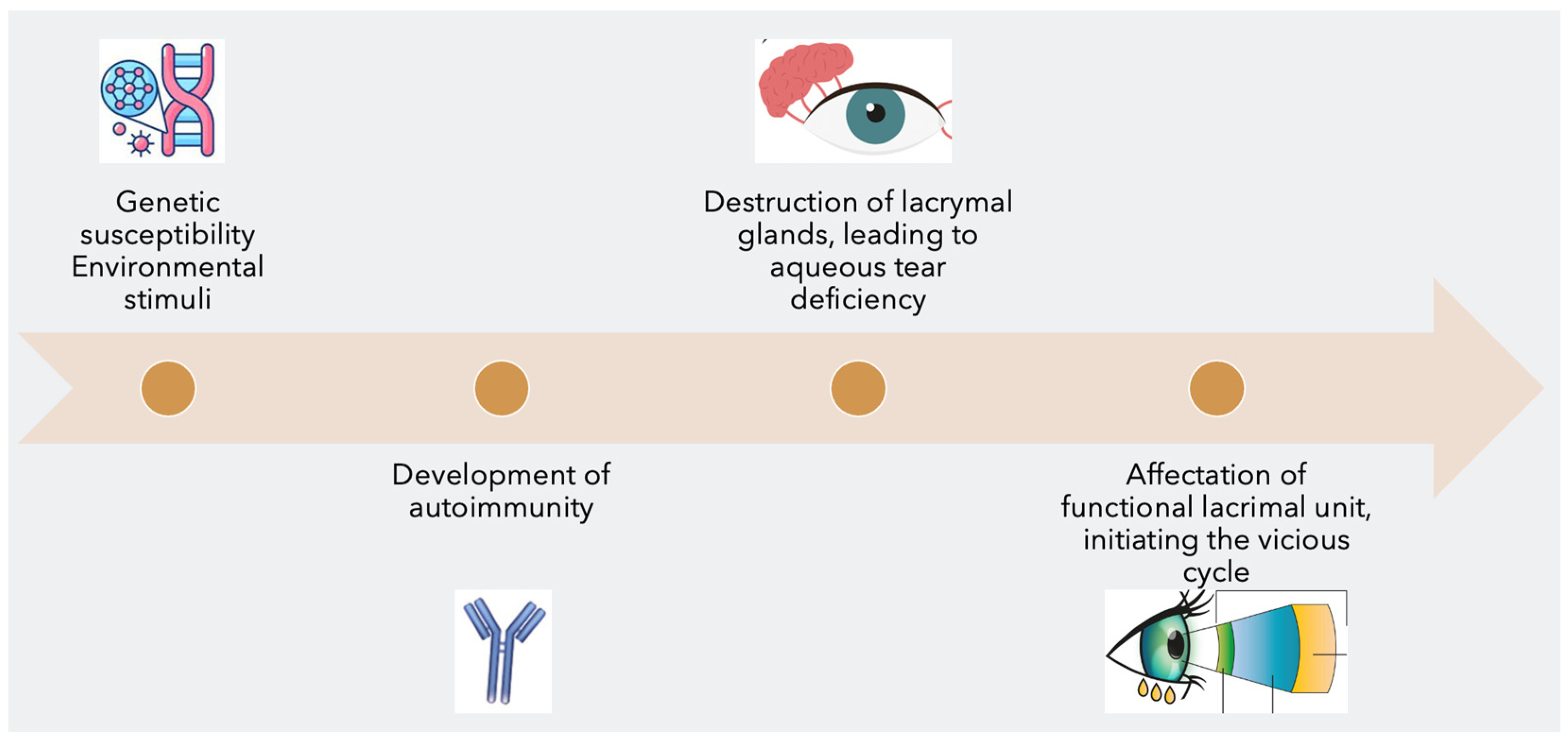
Figure 1. The 4 steps in the pathogenesis of Sjögren’s syndrome dry eye
2.1. Genetic Predisposition
Studies have widely underlined the crucial role of genetic predisposition in the development of pSS. The genes within and outside the major histocompatibility complex (MHC), such as HLA class II alleles, IRF5, TNIP1, BLK, STAT4, IL12A, CXCR5, and GTF2I, have been linked to an increased risk of pSS. The presence of auto-antibodies, such as anti-Ro/SSA and anti-La/SSB, is a hallmark of pSS diagnosis and is strongly linked to patients with DRB103 and DQB102 alleles. Epigenetic factors, including DNA methylation, histone acetylation, non-coding RNAs, and gene recombination, may also influence gene expression [8][9][10][11].
2.2. Environmental Triggers
pSS disease activation in genetically predisposed patients may be triggered by viral infections that affect epithelial cells in exocrine glands. The Ebstein-Barr virus (EBV) is a human herpesvirus-4 (HH-4) associated with infectious mononucleosis, neoplastic disorders, and other autoimmune diseases. It often infects and lies latent in the salivary glands, and induces autoimmunity through multiple proposed mechanisms, including molecular mimicry and cross reactivity between the viral EBNA-2 protein and Ro-60 antigen, as well as EBER-1 and EBER-2 viral proteins and La antigens. Increased prevalence and titers of serologic EBV were found in patients with pSS compared to healthy individuals, supporting the role of active EBV infection in promoting lymphocytic autoreactivity in pSS. Other viruses, such as hepatitis C virus (HCV), human immunodeficiency virus (HIV), human T-lymphotropic virus type 1 (HTLV-1), and coxsackievirus, may also play a role in the development of Sjögren’s syndrome. Hormonal changes, such as menopause, and stressful events, such as major surgery or trauma, may also trigger the development of pSS [12] [13][14][15][16][17][18][19][20][21][22][23][24].
2.3. Immune Response
The immune response in pSS can be divided into two pathways, the innate immune response or the adaptive immune system. Studies have described the molecular pathways involved in disease initiation and progression, involving toll-like receptor (TLR) agonists and important molecular partners in the immune response. These pathways are involved in disease initiation, progression, and histopathological changes [18][19][20][21][22][23].
The pathogenesis of primary Sjogren's syndrome (pSS) is believed to involve alterations in the innate immune response, which is the body's rapid initial defense against foreign microorganisms. Environmental triggers, such as viruses, activate the innate immune system by expressing pathogen-associated molecular patterns (PAMPs) that bind to pattern recognition receptors (PRRs) on immune cells and professional antigen-presenting cells. TLRs, a subclass of PRRs, are overexpressed in serum and exocrine glands in pSS and lead to an increase in type 1 interferon (IFN) activity, which is involved in the activation of the immune system. IFNαsubtypes and IFNβ, the most studied type 1 IFNs in rheumatic diseases, are produced mainly by plasmacytoid dendritic cells (pDCs). They are associated with an increase pSS disease activity, and higher autoantibody levels. Pro-inflammatory cytokines, such as IL-6, IL-21, and IL-23, are involved in activating B and T cells and promoting inflammation. Salivary gland epithelial cells also play a key role in the pathogenesis of pSS by enhancing the innate immune system and acting as a target for autoimmunity.
The alterations in the adaptive immune response play a major role in the pathogenesis of primary Sjogren's Syndrome (pSS). The activation of BAFF (B-cell Activating Factor of the TNF family) leads to the survival of plasma cells and hypergammaglobulinemia, which increases the quantity of plasma cells that contain autoreactive cells. B-cells are attracted to salivary gland epithelial cells (SGEC) due to enhanced expression of CXCL3 and CXCL4, which causes cell infiltration and glandular tissue inflammation. Germinal Center-like (GC-like) structures are formed due to the presence of T follicular helper (Tfh) cells and B cells, which positively correlate with focus scores, cell infiltration status, and autoantibody levels. CD4+ T cells can differentiate into Th1 and Th2 subtypes and the Th17 cells seem to be involved in the disease process by producing cytokines such as IL-17 and TNF-α. The T regulatory cells (Treg) cells may also be involved in pSS pathogenesis, but their role is not yet fully understood. The SGEC activate their apoptotic pathway, leading to the release of ribonucleoprotein complexes that trigger autoimmunity by recruiting pDCs within exocrine glands. The autoantibodies anti-Ro/SSA and anti-La/SSB are present in approximately 50-70% and 25-40% of patients with pSS, respectively, and represent the hallmark of this disease. Recent studies have suggested two distinct entities in anti-Ro/SSA autoantibodies, anti-Ro52 and anti-Ro60 antibodies, which have anti-proliferative and pro-apoptotic properties, and regulate type 1 IFN gene expression respectively.
2.4. Destruction of Lacrymal Glands and Aqueous Tear Deficiency
The destruction of lacrimal glands in Sjogren's Syndrome (SS) is caused by autoimmunity that infiltrates the glands and causes lymphocytic infiltration and periductal foci. This leads to glandular atrophy and a predominance of lymphocyte B cells, which can organize into tertiary lymphoid tissue with a germinal center, leading to hyposecretion and continuous B-cell activation. This can increase the risk of lymphoproliferative diseases and cause aqueous tear deficiency and hyperosmolarity of the tear film. This hyperosmolarity activates pro-inflammatory cascades, which increase specific cytokines for SS.
2.5. Affectation of Functional Lacrimal Unit and Vicious Cycle of SSDE
The pro-inflammatory cytokines caused by ocular surface hyperosmolarity affect substantially the entire functional lacrimal unit. It is noteworthy that all the constituents of the functional lacrimal unit, as well as all three major components of the tear film (lipid, aqueous, and mucin layers), are affected at this stage [25][26]:
- Meibomian gland dysfunction and atrophy reduce the lipid layer of the tear film, leading to increased evaporation.
- Apoptosis of goblet cells decreases the mucin layer of the tear film, leading to de-creased wettability.
- Degeneration of epithelial cells leads to a loss of microvilli, also contributing to decreased wettability.
- Neurogenic inflammation of the lacrimal gland further contributes to aqueous tear deficiency.
- Disturbances of corneal nerves lead to decreased corneal sensation and blinking reflex.
These events lead to an unstable ocular surface tear film, which contributes back to ocular surface hyperosmolarity, maintaining the vicious cycle of chronic ocular surface inflammation [27]. Despite SSDE starting with an aqueous tear deficiency, the clinical manifestations of SSDE are similar to those of NSSDE, as the entire functional lacrimal unit eventually gets compromised. This makes the clinical distinction between the two difficult.
3. Current Diagnostic Challenges
Sjögren's Syndrome affects 0.06% of the world's population, with women making up over 90% of those affected. It is estimated that 10% of patients with dry eye disease (DED) suffer from SS, but two-thirds of these patients remain undiagnosed with a median diagnostic delay of 10 years [28]. Some factors contributing to the underdiagnosis of SS include:
- Difficulty for clinicians to identify patients with SS due to the high prevalence of dry eye and dry mouth [28].
- The broad spectrum of non-specific clinical manifestation and insidious onset of SS [29].
- Asymptomatic or mildly symptomatic patients with SSDE despite signs of significant ocular inflammation, because of the neurotrophic aspect of ocular surface disease [29].
- Lack of reliable and effective screening tools to determine which DED patients should be worked up for SS [30].
- Ophthalmologists often underestimate the importance of SS and therefore refer few patients with DED for SS workup [31].
3.1. Importance of SS Diagnosis
Untreated SSDE can lead to vision-threatening complications such as neurotrophic keratitis and corneal thinning, ulceration, melting, and perforation [3][32]. SS is also associated with an increased risk of type III hypersensitivity reactions, severe inflammation of other ocular structures such as scleritis, uveitis, optic neuropathy, and retinal vasculitis [25][33][34][35][36].
Although SS tends to have a mild and benign course [24], around a third of patients with SS will develop systemic complications, affecting their quality of life, increasing morbidity and mortality, and even increasing the risk of lymphoma development [3][28]. Early detection of SS can prevent complications and allow for clinical surveillance for the development of serious systemic manifestations. It can also influence future ocular surgical decision-making, as refractive surgery is an absolute contraindication [37][38] and blepharoplasty is a relative contraindication in the presence of SSDE [39].
3.2. Conventional Diagnostic Tools and Criteria
Diagnosing primary Sjögren's syndrome (pSS) can be challenging, as there is currently no reliable and effective diagnostic tool available. The American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria for pSS established in 2016 is the most commonly used system [40]. This system involves calculating a diagnostic score based on several criteria:
- Labial salivary gland with focal lymphocytic sialadenitis and a focus score of 1 or more foci per 4 mm2. (3 points)
- Presence of auto-antibodies, including anti-Ro or anti-La. (3 points)
- Ocular staining score (OSS) of 5 or higher or a van Bijsterveld score (vBS) of 4 or higher in at least one eye. (1 point)
- Schirmer’s test 5 mm/5 min or lower in at least one eye. (1 point)
- Unstimulated whole saliva flow rate of 0.1 mL/min or lower. (1 point)
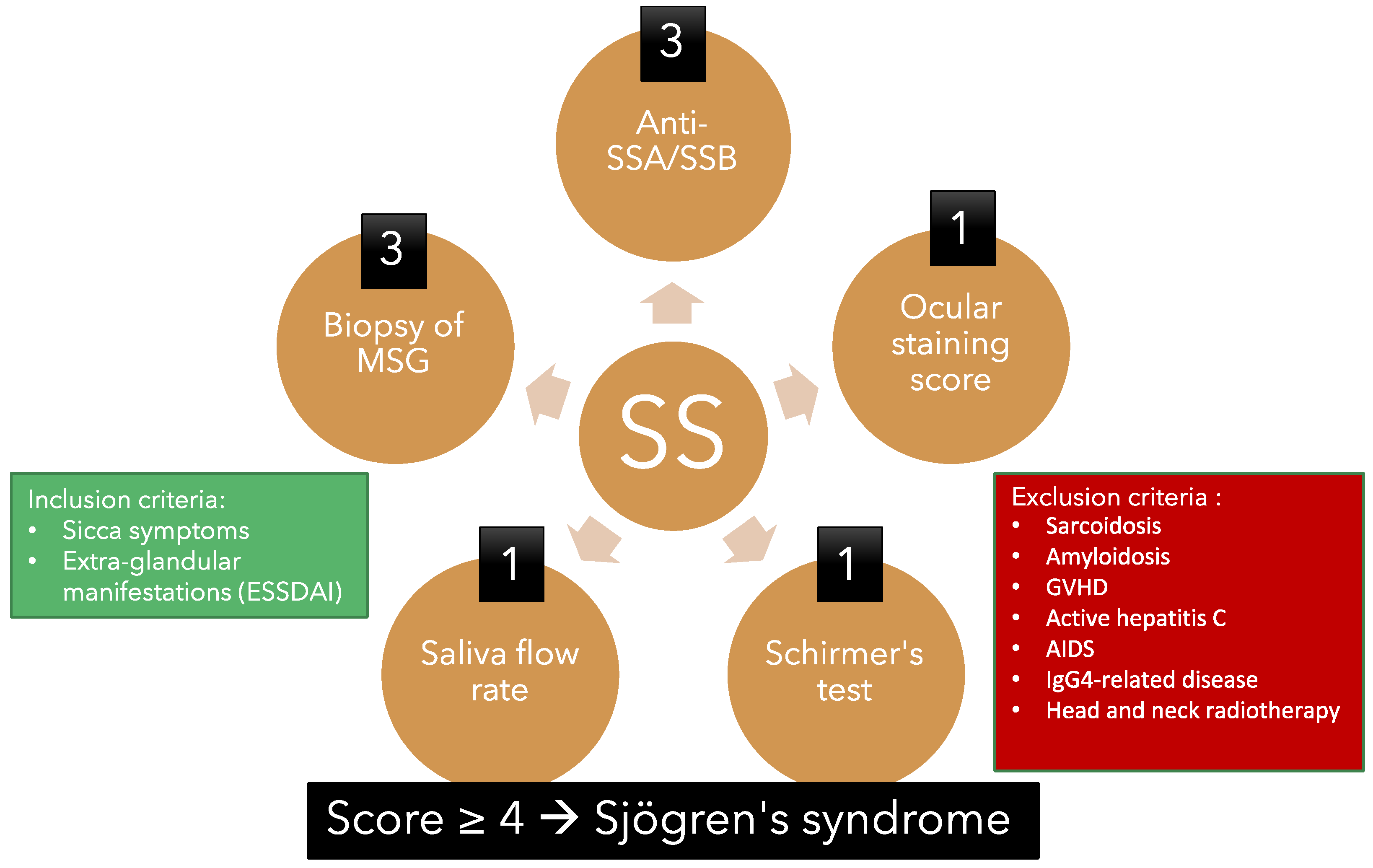
Figure 2. Overview of the American College of Rheumatology/European League Against Rheu-matism criteria for primary Sjögren’s syndrome 2016. This classification criteria applies to an individual who meets one of the inclusion criteria and does not have any of the exclusion criteria. A score of 4 or above confirms the diagnosis of pSS.
However, the ACR/EULAR criteria have limitations. Firstly, the diagnostic markers such as anti-RO/SSA and anti-La/SSB antibodies are insensitive and cannot be used to screen for pSS. Secondly, clinical tests like the Schirmer's test and the saliva flow rate are rarely performed due to the time required and lack of specificity to pSS. Thirdly, the subjective nature of the ocular examination systems, such as the Van Bijsterveld Score and Ocular Staining Score, make them unreliable and not commonly used by ophthalmologists. Finally, a minor salivary gland biopsy is not always sensitive to detect the disease at its onset and may carry the risk of complications.
These limitations highlight the need for a more sensitive, reliable, and practical diagnostic tool for early pSS diagnosis that is cost-effective.
3.3. Other Diagnostic Tools
3.3.1. Serum Testing and Biomarkers
Recent advancements in the diagnostic armamentarium of Sjögren’s Syndrome (SS) have shown promising results in the utilization of serum biomarkers. A 2021 study involving 74 female patients with primary SS revealed a correlation between serum Vitamin D 25(OH)D3 levels and SS signs, but not symptoms [41]. This was the first study to establish a link between vitamin D and pSS, which is noteworthy given that the disease primarily affects women in their later years and with lower vitamin D levels. Vitamin D is also an immunomodulator in other structures and its deficiency has been positively correlated with the severity of Dry Eye Disease (DED) signs. Additionally, tests for pulmonary disease such as a non-invasive induced sputum analysis for B-cell activating factor (BAFF), interleukin-6 (IL-6), and interleukin-8 (IL-8) [42] have revealed higher levels in pSS patients. BAFF is a known modulator of B-cell hyperactivation which is included in the definition of SS and its detection by enzyme-linked immunosorbent assay (ELISA) is less invasive than a salivary gland biopsy.
In addition, a 2021 study used serum biomarkers to differentiate between SS, rheumatoid arthritis (RA), and systemic lupus erythremous (SLE). Serum concentrations of 63 markers were measured in 95 patients and the concentrations of BDNF and I-TAC/CXCL11 were found to significantly discriminate between SS and RA. Further, concentrations of sCD163, Fractalkine/CX3CL1, MCP-1/CCL2, and TNFa could differentiate pSS from SLE. The combination of low concentrations of BDNF and Fractalkine/CX3CL1 was highly specific for pSS (specificity 96.2%; positive predictive value 80%) in comparison to RA and SLE. These markers are involved in networks of signaling pathways and their interaction implies the role of Nf-kb and IL-17 signaling pathways. It is also noteworthy that Nf-kB I expression is increased in pSS patients’ salivary gland cells, and that IL-17 is found in Fractalkine/CX3CL1, which is a chemokine involved specifically in chemotactic activity on monocytes and T-lymphocytes, and the extravasation of leukocytes towards the inflammation site. The I-TAC/CXCL11 is an IFN-gamma-inducible chemokine, which attracts activated T-lymphocytes, natural killer cells, and macrophages to the inflammation site [43].
These recent findings demonstrate how serum biomarkers can be used to improve the current diagnostic armamentarium of SS and to differentiate between SS, RA, and SLE. While further investigation is needed to clearly establish these biomarkers as diagnostic criteria for SS, the potential benefits of their implementation are significant, as they provide a more accessible and less invasive alternative to salivary gland biopsy.
3.3.2. Tear Film Analysis
Tear film analysis is an important tool for diagnosing dry eye disease (DED). Tear osmolarity is the most commonly used measure, with a threshold of 308 mmol/L being universally accepted as an indicator of DED. However, lower thresholds have been shown to be associated with mild DED as well, with a 98.4% positive predictive value for a threshold of 305 mmol/L [44]. Tear ferning, which involves the collection and drying of a tear sample on a glass slide, can also be used to distinguish between dry eye and non-dry eye patients, although studies may vary in terms of the sensitivity and specificity of this method, although sensitivity and specificity may vary among studies [45]. Advanced imaging techniques, such as in vivo confocal microscopy, meibography, and MRI/CT/US scans of the lacrimal gland, can also be used to assess the integrity of the cornea, meibomian glands, and lacrimal gland. In vivo confocal microscopy can be used to measure the density and tortuosity of corneal nerves and to assess the number of inflammatory cells [46]. Meibography can be used to measure meibomian gland integrity, shape, and loss, with a greater MG impairment being seen in SSDE than NSSDE [29]. Radiomics, the mathematical analysis of radiology images, can be used to quantitatively differentiate between the textural features of pSS and healthy lacrimal glands (102). MRI sialography is the gold standard for staging the disease, and is used to identify the modified MRI signal from the diseased tissue [47]. Overall, tear film analysis, advanced imaging, and radiomics offer clinicians valuable tools for assessing and diagnosing DED.
3.3.3. Advanced Imaging
In vivo confocal microscopy (IVCM) is a non-invasive technique used to evaluate various components of the cornea including the epithelium, sub-basal nerves, stroma, and endothelium. The corneal nerves are important for epithelial integrity, maintaining corneal sensation and wound healing. Additionally, IVCM can assess the shape and integrity of the meibomian and lacrimal glands [48]. In a 2022 retrospective study of 71 patients, it was found that those with Sjögren's Syndrome (SS) had reduced corneal sensitivity, reduced density of the corneal subbasal plexus, increased tortuosity of the nerves, and an increased number of inflammatory cells compared to the healthy control group and MGD group [46]. Furthermore, the average number of subbasal neuromas was higher in SS patients, particularly those with small fiber neuropathy.
Meibography is a valuable imaging tool used to assess the Meibomian glands (MGs), which are located posteriorly on the upper and lower eyelids. Through adaptive transillumination and interferometry, images of the MGs can be taken and compared to clinical scales to measure their integrity, shape, and loss. A 2018 study by Kang et al. showed that Sjogren syndrome-related dry eye (SSDE) had a greater MG impairment than non-Sjogren syndrome-related dry eye (NSSDE) [74]. In 2021, a study on 108 female patients further demonstrated the correlation between the duration of pSSDED and meibomian gland dropout (r = 0.776, p < 0.001) [100]. Meibography is a useful diagnostic tool for clinicians as it provides accurate diagnosis, even if it does not differentiate between SSDE and NSSDE.
Magnetic resonance imaging (MRI), computerized tomography (CT) and ultrasound (US) can be used to image the lacrimal gland and assess its shape and function. Ultrasonography is commonly used for diagnostic purposes and to monitor anatomical changes, as well as response to therapy and any activity in patients with primary Sjögren’s Syndrome (pSS) [49]. MRI is the gold standard for staging this disease and a study in 2022 showed that the mathematical analysis of radiology images (radiomics) could differentiate the textural features of pSS from healthy lacrimal glands [47]. Both lobes of the lacrimal gland are affected in pSS, and coronal T-1 weighed images with fat saturation can detect the modified MRI signal from the diseased tissue.
4. On the Horizon—Novel Diagnostic Modalities
Tear film analysis is a promising diagnostic modality for Sjogren's Syndrome (SS). Over 1500 proteins, including gel-forming mucins MUC5AC, matrix metalloproteinases (MMPs), and thrombospondin (TSP-1), can be identified in the tear film through mass spectrometry. A lower expression of MUC5AC and higher expression of MMP-9 have been associated with dry eye disease, including SS. The ratio of TSP-1 to MMP-9 has been proven to accurately distinguish between SS dry eye and non-SS dry eye, as a lower ratio signals a SS condition [50]. Other proteins, such as salivary protein-1 (SP-1), LACTO, LYS-C, and LIPOC-1, have been associated with SS dry eye disease. Additionally, autophagy-related proteins 5 and 7 (ATG5, ATG7), the LC3B protein, and the autophagic cell biomarkers, ULK1 and Beclin-1, have been identified at higher levels in SS eyes versus non-SS dry eyes, and the concentration of epidermal fatty acid binding protein (E-FABP) is lower in SS eyes than healthy eyes [51][52][53][54][55]. Although these biomarkers offer potential avenues for diagnostic testing for pSS, further research is needed for their validation [45]. These biomarkers could help screen potential DED and pSS patients before symptoms arise and provide prognosis, diagnosis, and follow-up of these patients. The use of tear proteomics as a non-invasive, highly specific diagnostic tool for SS could be a game-changer. In addition to being a reliable diagnostic modality, it could help reduce the time and cost of diagnosis, as well as improve patient management and outcome.
The salivary proteomics level offers a non-invasive testing avenue to diagnose pSS. A 2020 study identified 1013 proteins in whole saliva, 219 in plasma, and 3166 in salivary gland tissue, with 40 proteins in saliva differing between pSS and non-pSS patients. These proteins included those involved in inflammatory processes, such as elastase, calreticulin, and tripartite motif-containing protein 29, which were upregulated, and those involved in salivary regulation, which were reduced [56], yielding 97% accuracy in diagnosis. Additionally, salivary proinflammatory cytokines, such as IL-6 and TNF-alpha, are more elevated in SS patients, though not specific to the disease. Other salivary biomarkers being studied include B-2 microglobulin, lactoferrin, neutrophil gelatinase-associated lipocalin, soluble sialic acid-binding immunoglobulin-like lectin, autoantibodies, calprotectin, carbonic anhydrase VI, and adiponectin [57]. Of these, B2m, autoantibodies, carbonic anhydrase VI, and adiponectin have been shown to differ between pSS and non-pSS patients, with serum anti-Ro/La already being a diagnostic criterion for pSS, and salivary autoantibodies showing potential as a marker. Lactoferrin, neutrophil gelatinase-associated lipocalin, and soluble sialic acid-binding immunoglobulin-like lectin are all found at elevated levels in pSS, but also in other conditions, thus not being specific to the disease. Further research is needed to confirm their utility as clinically useful biomarkers.
5. Conclusions
Sjögren's syndrome (SS) is a chronic autoimmune disorder characterized by inflammation of exocrine glands, primarily those involved in producing tears and saliva, leading to dry eye and other non-specific symptoms. Diagnosis of SS is a challenge, as the established criteria of clinical tests, blood tests, and tear film parameters are either impractical in clinical settings or unreliable. Early diagnosis is essential for improved patient care and adequate monitoring of ophthalmic and systemic manifestations. Novel diagnostic modalities, such as proteomics and exosomal biomarkers, may provide more accurate and timely diagnosis. The translation of innovative biomarkers from bench to bedside requires extensive clinical trials, but presents a promising prospect for improving the quality of life of those suffering from SS.
References
- Bombardieri, M.; Argyropoulou, O.D.; Ferro, F.; Coleby, R.; Pontarini, E.; Governato, G.; Lucchesi, D.; Fulvio, G.; Tzioufas, A.G.; Baldini, C. One Year in Review 2020: Pathogenesis of Primary Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 126), 3–9.
- Stefanski, A.-L.; Tomiak, C.; Pleyer, U.; Dietrich, T.; Rüdiger Burmester, G.; Dörner, T. The Diagnosis and Treatment of Sjögren’s Syndrome. Dtsch. Ärztebl. Int. 2017, 114, 354–361.
- Akpek, E.K.; Mathews, P.; Hahn, S.; Hessen, M.; Kim, J.; Grader-Beck, T.; Birnbaum, J.; Baer, A.N. Ocular and Systemic Morbidity in a Longitudinal Cohort of Sjögren’s Syndrome. Ophthalmology 2015, 122, 56–61.
- van der Reijden, W.A.; Vissink, A.; Veerman, E.C.I.; Amerongen, A.V.N. Treatment of Oral Dryness Related Complaints (Xerostomia) in Sjogren’s Syndrome. Ann. Rheum. Dis. 1999, 58, 465–474.
- Parkin, B.; Chew, J.B.; White, V.A.; Garcia-Briones, G.; Chhanabhai, M.; Rootman, J. Lymphocytic Infiltration and Enlargement of the Lacrimal Glands: A New Subtype of Primary Sjögren’s Syndrome? Ophthalmology 2005, 112, 2040–2047.
- Chung, A.; Wilgus, M.L.; Fishbein, G.; Lynch, J.P. Pulmonary and Bronchiolar Involvement in Sjogren’s Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 235–254.
- François, H.; Mariette, X. Renal Involvement in Primary Sjögren Syndrome. Nat. Rev. Nephrol. 2016, 12, 82–93.
- Gottenberg, J.-E.; Busson, M.; Loiseau, P.; Dourche, M.; Cohen-Solal, J.; Lepage, V.; Charron, D.; Miceli, C.; Sibilia, J.; Mariette, X. Association of Transforming Growth Factor β1 and Tumor Necrosis Factor α Polymorphisms with Anti-SSB/La Antibody Secretion in Patients with Primary Sjögren’s Syndrome. Arthritis Rheum. 2004, 50, 570–580.
- Cruz-Tapias, P.; Rojas-Villarraga, A.; Maier-Moore, S.; Anaya, J.-M. HLA and Sjögren’s Syndrome Susceptibility. A Meta-Analysis of Worldwide Studies. Autoimmun. Rev. 2012, 11, 281–287.
- Song, I.-W.; Chen, H.-C.; Lin, Y.-F.; Yang, J.-H.; Chang, C.-C.; Chou, C.-T.; Lee, M.-T.M.; Chou, Y.-C.; Chen, C.-H.; Chen, Y.-T.; et al. Identification of Susceptibility Gene Associated with Female Primary Sjögren’s Syndrome in Han Chinese by Genome-Wide Association Study. Hum. Genet. 2016, 135, 1287–1294.
- Imgenberg-Kreuz, J.; Rasmussen, A.; Sivils, K.; Nordmark, G. Genetics and Epigenetics in Primary Sjögren’s Syndrome. Rheumatology 2021, 60, 2085–2098.
- Maślińska, M. The Role of Epstein–Barr Virus Infection in Primary Sjögren’s Syndrome. Curr. Opin. Rheumatol. 2019, 31, 475–483.
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.-M. Molecular Mimicry and Autoimmunity. J. Autoimmun. 2018, 95, 100–123.
- Nakamura, H.; Kawakami, A. What Is the Evidence for Sjögren’s Syndrome Being Triggered by Viral Infection? Subplot: Infections That Cause Clinical Features of Sjögren’s Syndrome. Curr. Opin. Rheumatol. 2016, 28, 390–397.
- Ghrenassia, E.; Martis, N.; Boyer, J.; Burel-Vandenbos, F.; Mekinian, A.; Coppo, P. The Diffuse Infiltrative Lymphocytosis Syndrome (DILS). A Comprehensive Review. J. Autoimmun. 2015, 59, 19–25.
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Opthalmol. Vis. Sci. 2018, 59, DES192.
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease. Ophthalmology 2017, 124, S4–S13.
- Deshmukh, U.S.; Nandula, S.R.; Thimmalapura, P.-R.; Scindia, Y.M.; Bagavant, H. Activation of Innate Immune Responses through Toll-like Receptor 3 Causes a Rapid Loss of Salivary Gland Function: TLR3 Engagement and Salivary Gland Function. J. Oral Pathol. Med. 2008, 38, 42–47.
- Nandula, S.-R.; Scindia, Y.; Dey, P.; Bagavant, H.; Deshmukh, U. Activation of Innate Immunity Accelerates Sialoadenitis in a Mouse Model for Sjögren’s Syndrome-like Disease: Innate Immunity and Sjögren’s Syndrome. Oral Dis. 2011, 17, 801–807.
- Zhou, J.; Jin, J.-O.; Du, J.; Yu, Q. Innate Immune Signaling Induces IL-7 Production, Early Inflammatory Responses, and Sjögren’s-Like Dacryoadenitis in C57BL/6 Mice. Investig. Opthalmol. Vis. Sci. 2015, 56, 7831.
- Hu, P.; Ming, B.; Wu, X.; Cai, S.; Tang, J.; Dong, Y.; Zhou, T.; Tan, Z.; Zhong, J.; Zheng, F.; et al. Intratracheal Poly(I:C) Exposure Accelerates the Immunological Disorder of Salivary Glands in Sjogren’s-Like NOD/ShiLtJ Mice. Front. Med. 2021, 8, 645816.
- Ohyama, Y.; Carroll, V.A.; Deshmukh, U.; Gaskin, F.; Brown, M.G.; Fu, S.M. Severe Focal Sialadenitis and Dacryoadenitis in NZM2328 Mice Induced by MCMV: A Novel Model for Human Sjögren’s Syndrome. J. Immunol. 2006, 177, 7391–7397.
- Weller, M.L.; Gardener, M.R.; Bogus, Z.C.; Smith, M.A.; Astorri, E.; Michael, D.G.; Michael, D.A.; Zheng, C.; Burbelo, P.D.; Lai, Z.; et al. Hepatitis Delta Virus Detected in Salivary Glands of Sjögren’s Syndrome Patients and Recapitulates a Sjögren’s Syndrome-Like Phenotype in Vivo. Pathog. Immun. 2016, 1, 12.
- Sjögren’s Syndrome|Harrison’s Principles of Internal Medicine, 21e|AccessPharmacy|McGraw Hill Medical. Available online: https://accesspharmacy.mhmedical.com/content.aspx?bookid=3095§ionid=263362529 (accessed on 8 January 2023).
- Pflugfelder, S.C. Tear Dysfunction and the Cornea: LXVII Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2011, 152, 900–909.
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry Eye Disease: An Immune-Mediated Ocular Surface Disorder. Arch. Ophthalmol. 2012, 130, 90–100.
- Lemp, M.A.; Baudouin, C.; Baum, J.; Dogru, M.; Foulks, G.N.; Kinoshita, S.; Laibson, P.; McCulley, J.P.; Murube, J.; Pflugfelder, S.C.; et al. The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 5, 75–92.
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of Primary Sjögren’s Syndrome: A Systematic Review and Meta-Analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989.
- Kang, Y.S.; Lee, H.S.; Li, Y.; Choi, W.; Yoon, K.C. Manifestation of Meibomian Gland Dysfunction in Patients with Sjögren’s Syndrome, Non-Sjögren’s Dry Eye, and Non-Dry Eye Controls. Int. Ophthalmol. 2018, 38, 1161–1167.
- Caban, M.; Omulecki, W.; Latecka-Krajewska, B. Dry Eye in Sjögren’s Syndrome—Characteristics and Therapy. Eur. J. Ophthalmol. 2022, 32, 3174–3184.
- Bunya, V.Y.; Fernandez, K.B.; Ying, G.-S.; Massaro-Giordano, M.; Macchi, I.; Sulewski, M.E.; Hammersmith, K.M.; Nagra, P.K.; Rapuano, C.J.; Orlin, S.E. Survey of Ophthalmologists Regarding Practice Patterns for Dry Eye and Sjogren Syndrome. Eye Contact Lens 2018, 44 (Suppl. 2), S196–S201.
- Vivino, F.B.; Minerva, P.; Huang, C.H.; Orlin, S.E. Corneal Melt as the Initial Presentation of Primary Sjögren’s Syndrome. J. Rheumatol. 2001, 28, 379–382.
- Braithwaite, T.; Adderley, N.J.; Subramanian, A.; Galloway, J.; Kempen, J.H.; Gokhale, K.; Cope, A.P.; Dick, A.D.; Nirantharakumar, K.; Denniston, A.K. Epidemiology of Scleritis in the United Kingdom From 1997 to 2018: Population-Based Analysis of 11 Million Patients and Association Between Scleritis and Infectious and Immune-Mediated Inflammatory Disease. Arthritis Rheumatol. 2021, 73, 1267–1276.
- Rosenbaum, J.T.; Bennett, R.M. Chronic Anterior and Posterior Uveitis and Primary Sjögren’s Syndrome. Am. J. Ophthalmol. 1987, 104, 346–352.
- Sun, J.-Y.; Liu, Z.; Zhao, P.; Liu, T. Optic Neuritis as an Initial Presentation of Primary Sjögren Syndrome: A Case Report and Literature Review. Medicine 2016, 95, e5194.
- Foulks, G.N.; Forstot, S.L.; Donshik, P.C.; Forstot, J.Z.; Goldstein, M.H.; Lemp, M.A.; Nelson, J.D.; Nichols, K.K.; Pflugfelder, S.C.; Tanzer, J.M.; et al. Clinical Guidelines for Management of Dry Eye Associated with Sjögren Disease. Ocul. Surf. 2015, 13, 118–132.
- Di Pascuale, M.A.; Liu, T.-S.; Trattler, W.; Tseng, S.C.G. Lipid Tear Deficiency in Persistent Dry Eye after Laser in Situ Keratomileusis and Treatment Results of New Eye-Warming Device. J. Cataract Refract. Surg. 2005, 31, 1741–1749.
- Albietz, J.M.; Lenton, L.M.; McLennan, S.G. Dry Eye after LASIK: Comparison of Outcomes for Asian and Caucasian Eyes. Clin. Exp. Optom. 2005, 88, 89–96.
- Saadat, D.; Dresner, S.C. Safety of Blepharoplasty in Patients with Preoperative Dry Eyes. Arch. Facial Plast. Surg. 2004, 6, 101–104.
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45.
- Athanassiou, P.; Mavragani, C.; Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M. Vitamin D Deficiency in Primary Sjögren’s Syndrome: Association with Clinical Manifestations and Immune Activation Markers. Mediterr. J. Rheumatol. 2022, 33, 106.
- Nilsson, A.; Tufvesson, E.; Hesselstrand, R.; Olsson, P.; Wollmer, P.; Mandl, T. Increased B-Cell Activating Factor, Interleukin-6, and Interleukin-8 in Induced Sputum from Primary Sjögren’s Syndrome Patients. Scand. J. Rheumatol. 2019, 48, 149–156.
- Padern, G.; Duflos, C.; Ferreira, R.; Assou, S.; Guilpain, P.; Maria, A.T.J.; Goulabchand, R.; Galea, P.; Jurtela, M.; Jorgensen, C.; et al. Identification of a Novel Serum Proteomic Signature for Primary Sjögren’s Syndrome. Front. Immunol. 2021, 12, 631539.
- Versura, P.; Profazio, V.; Campos, E.C. Performance of Tear Osmolarity Compared to Previous Diagnostic Tests for Dry Eye Diseases. Curr. Eye Res. 2010, 35, 553–564.
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403.
- Luzu, J.; Labbé, A.; Réaux-Le Goazigo, A.; Rabut, G.; Liang, H.; Dupas, B.; Blautain, B.; Sène, D.; Baudouin, C. In Vivo Confocal Microscopic Study of Corneal Innervation in Sjögren’s Syndrome with or without Small Fiber Neuropathy. Ocul. Surf. 2022, 25, 155–162.
- Muntean, D.D.; Bădărînză, M.; Ștefan, P.A.; Lenghel, M.L.; Rusu, G.M.; Csutak, C.; Coroian, P.A.; Lupean, R.A.; Fodor, D. The Diagnostic Value of MRI-Based Radiomic Analysis of Lacrimal Glands in Patients with Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 10051.
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47.
- Schäfer, V.S.; Schmidt, W.A. Ultraschalldiagnostik beim Sjögren-Syndrom. Z. Rheumatol. 2017, 76, 589–594.
- Masli, S.; Akpek, E.K. Reduced Tear Thrombospondin-1/Matrix Metalloproteinase-9 Ratio Can Aid in Detecting Sjögren’s Syndrome Etiology in Patients with Dry Eye. Clin. Transl. Sci. 2022, 15, 1999–2009.
- Tan, X.; Chen, Y.; Foulsham, W.; Amouzegar, A.; Inomata, T.; Liu, Y.; Chauhan, S.K.; Dana, R. The Immunoregulatory Role of Corneal Epithelium-Derived Thrombospondin-1 in Dry Eye Disease. Ocul. Surf. 2018, 16, 470–477.
- Bunya, V.Y.; Ying, G.-S.; Maguire, M.G.; Kuklinski, E.; Lin, M.C.; Peskin, E.; Asbell, P.A.; the DREAM Study Research Group. Prevalence of Novel Candidate Sjogren Syndrome Autoantibodies in the Dry Eye Assessment and Management (DREAM) Study. Cornea 2018, 37, 1425–1430.
- Versura, P.; Giannaccare, G.; Vukatana, G.; Mulè, R.; Malavolta, N.; Campos, E.C. Predictive Role of Tear Protein Expression in the Early Diagnosis of Sjögren’s Syndrome. Ann. Clin. Biochem. Int. J. Lab. Med. 2018, 55, 561–570.
- Liu, Z.; Chen, D.; Chen, X.; Bian, F.; Gao, N.; Li, J.; Pflugfelder, S.C.; Li, D.-Q. Autophagy Activation Protects Ocular Surface from Inflammation in a Dry Eye Model In Vitro. Int. J. Mol. Sci. 2020, 21, 8966.
- Shinzawa, M.; Dogru, M.; Den, S.; Ichijima, T.; Higa, K.; Kojima, T.; Seta, N.; Nomura, T.; Tsubota, K.; Shimazaki, J. Epidermal Fatty Acid-Binding Protein: A Novel Marker in the Diagnosis of Dry Eye Disease in Sjögren Syndrome. Int. J. Mol. Sci. 2018, 19, 3463.
- Sembler-Møller, M.L.; Belstrøm, D.; Locht, H.; Pedersen, A.M.L. Proteomics of Saliva, Plasma, and Salivary Gland Tissue in Sjögren’s Syndrome and Non-Sjögren Patients Identify Novel Biomarker Candidates. J. Proteom. 2020, 225, 103877.
- Jung, J.-Y.; Kim, J.-W.; Kim, H.-A.; Suh, C.-H. Salivary Biomarkers in Patients with Sjögren’s Syndrome—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 12903.




