Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dongjin Pan | -- | 1780 | 2023-02-12 10:57:16 | | | |
| 2 | Catherine Yang | Meta information modification | 1780 | 2023-02-13 02:47:56 | | | | |
| 3 | Dongjin Pan | + 1 word(s) | 1781 | 2023-02-13 03:59:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pan, D.; Yang, Y.; Nong, A.; Tang, Z.; Li, Q.X. Roles of GRP78 in Regulating Lipid Metabolism. Encyclopedia. Available online: https://encyclopedia.pub/entry/41127 (accessed on 15 January 2026).
Pan D, Yang Y, Nong A, Tang Z, Li QX. Roles of GRP78 in Regulating Lipid Metabolism. Encyclopedia. Available at: https://encyclopedia.pub/entry/41127. Accessed January 15, 2026.
Pan, Dongjin, Yunzhu Yang, Aihua Nong, Zhenzhou Tang, Qing X. Li. "Roles of GRP78 in Regulating Lipid Metabolism" Encyclopedia, https://encyclopedia.pub/entry/41127 (accessed January 15, 2026).
Pan, D., Yang, Y., Nong, A., Tang, Z., & Li, Q.X. (2023, February 12). Roles of GRP78 in Regulating Lipid Metabolism. In Encyclopedia. https://encyclopedia.pub/entry/41127
Pan, Dongjin, et al. "Roles of GRP78 in Regulating Lipid Metabolism." Encyclopedia. Web. 12 February, 2023.
Copy Citation
Glucose-regulated protein 78 (GRP78), a molecular chaperone, is overexpressed in patients suffering from obesity, fatty liver, hyperlipidemia and diabetes. GRP78, therefore, can be not only a biomarker to predict the progression and prognosis of obesity and metabolic diseases but also a potential therapeutic target for anti-obesity treatment.
GRP78
molecular target
action mechanism
metabolic disorder
1. GRP78 Promotes Adipogenesis and Lipogenesis
In a high energy diet state, adipogenesis contributes to the increased adipose tissue mass of obesity. On the contrary, adipogenesis failure makes it unable to differentiate into enough mature adipocytes which can absorb, utilize, and store excessive energy, thereby inducing diabetes, etc. [1]. Inhibition of adipogenesis can effectively prevent obesity. Peroxisome proliferator activated receptor γ (PPARγ), a nuclear transcription factor, plays an important role in adipogenesis. Biological functions and roles of GRP78 in weight control and energy restriction are through regulating mitochondrial autophagy and adenosine monophosphate activated protein kinase – PPARγ coactivator 1α – sirtuin1 (AMPK-PGC1α-SIRT1) signal pathway (Figure 1 Pathway 1). The molecular targets of PPARγ include CCAAT/enhancer-binding protein α (C/EBPα), perilipin (PLIN), hormone sensitive lipase (HSL) and glucose transporter type 4 (GLUT4), which participate in adipogenesis, fat mobilization, lipid metabolism and glucose metabolism, respectively [2][3] (Figure 1 Pathway 2 & 3). As compared with wild-type, PPARγ expression in GRP78 knockout mouse embryo fibroblasts is reduced [4]. GRP78 overexpression can promote the expression of PPARγ. GRP78 is a homolog of heat shock protein family A (Hsp70) member 12A (HSPA12A). Studies have found that HSPA12A regulates transcription of PPARγ through a positive feedback loop, sustaining overexpression of PPARγ and maintaining the phenotype of adipocytes after differentiation (Figure 1 Pathway 4) [5].
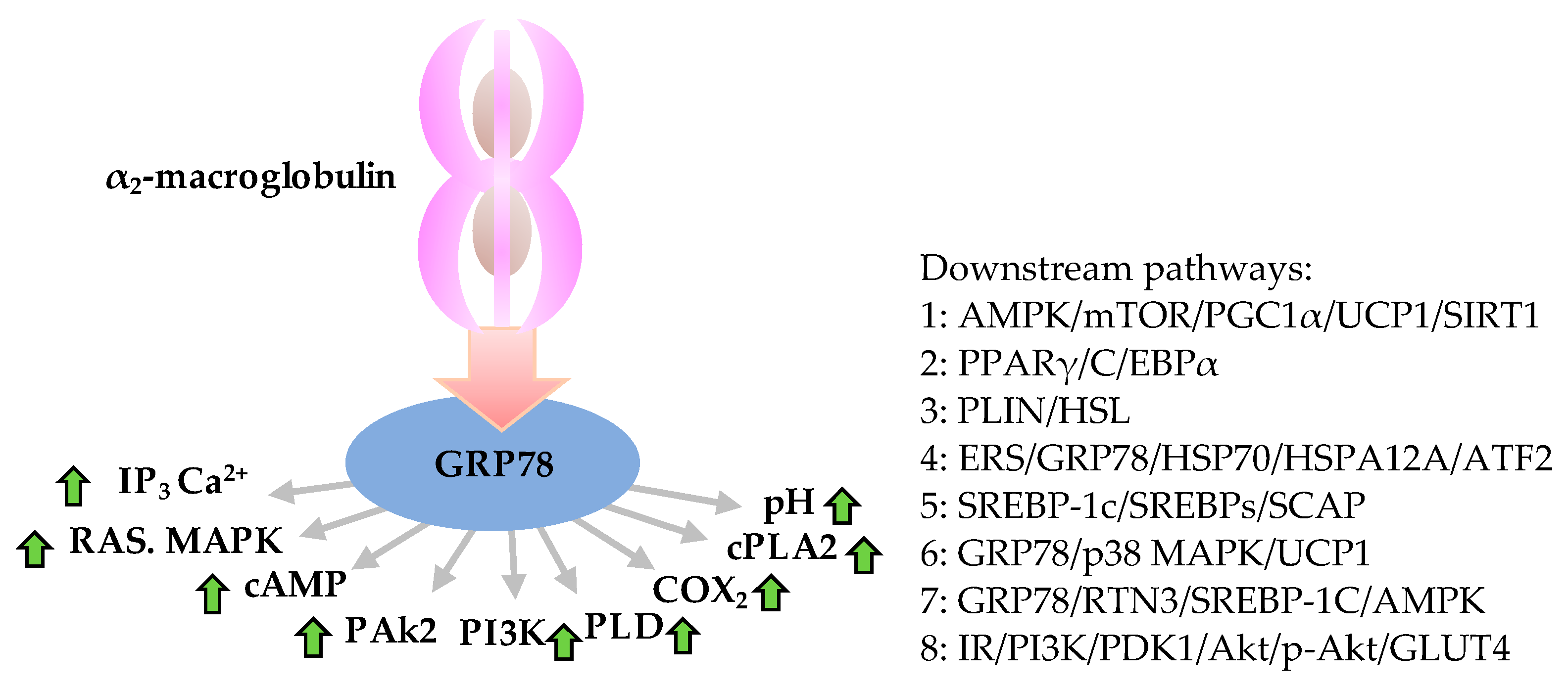
Figure 1. Anti-obesity role of GRP78 and downstream molecular functions associated with obesity. Roles and downstream pathways of GRP78 relevant to obesity initiated by binding of α2-macroglobulin-GRP78 that triggers the expression of IP3, RAS and MAPK, PAk2, PI3K, PLD, COX2, and cPLA2 and increases concentrations of cAMP, Ca2+ and pH value (left), which can activate the downstream pathways (right). Green arrows indicate up-expression. AMPK, adenosine monophosphate (AMP) activated protein kinase; Akt, an ubiquitous serine/threonine kinase, also known as protein kinase B (PKB) or RAS-alpha; ATF2, activating transcription factor-2; cAMP, cyclic adenosine monophosphate; C/EBPα, CCAAT/enhancer-binding protein α (which CCAAT is a distinct pattern of nucleotides with GGCCAATCT consensus sequence that occurs upstream by 60–100 bases to the initial transcription site); COX2, cicloxigenases; cPLA2, cytosolic phospholipase A 2; ERS, endoplasmic reticulum stress; GLUT4, glucose transporter type 4; GRP78, 78 kDa glucose-regulated protein; HSP70, heat shock protein 70 family; HSL, hormone sensitive lipase; HSPA12A, heat shock protein family a (hsp70) member 12A; IP3, inositol triphosphate; IR, insulin resistance; mTOR, mammalian target of rapamycin; PAk2, p21-activated protein kinase; PGC1α, PPARγ coactivator 1α; PDK1, pyruvate dehydrogenase kinase 1; PLD, phospholipase D; PI3K, phosphoinositide 3-kinases; PPARγ, peroxisome proliferator activated receptor γ; PLIN, perilipin; p38 MAPK, p38 mitogen-activated protein kinases; p-Akt, phosphorylated Akt; RAS, renin-angiotensin system; RTN3, reticular protein 3; SIRT1, sirtuin1; SREBP-1c, sterol regulatory element binding protein-1c; SREBPs, sterol regulatory element binding protein; SCAP, SREBP cleavage-activating protein; TG, triglyceride fatty acid; UCP1, uncoupling protein 1.
Shown by drug affinity responsive target stability and surface plasmon resonance results, epigallocatechin gallate (EGCG), dihydromyricetin (DHM) and berberine probably induce white fat tissue browning but prevent adipogenesis or obesity via GRP78 [6]. The dissociation constants of DHM and EGCG binding GRP78 were 22 µM and 6 µM, respectively, with significant anti-obesity activity with a half maximum effective concentration (EC50) of 400 µM and 75 µM, respectively [7].
GRP78 plays an important role in adipogenesis, lipogenesis, metabolic homeostasis, fetal and postnatal growth, and development of mice [4]. Knockout GRP78 in mouse embryonic fibroblasts, 3T3-L1 cells and adipocyte tissue showed adipogenesis and lipogenesis problems. The aP2-cre-mediated GRP78 deletion leads to reduction of lipoatrophy in gonadal and subcutaneous white adipose tissue and brown adipose tissue up to ∼90%, severe growth retardation, bony defects and grossly expanded endoplasmic reticulum (ER) in white adipose tissue. However, plasma triglyceride levels, and plasma glucose and insulin levels are reduced by 40-60% as compared to wild-type mice, suggesting an improvement of the insulin sensitivity in GRP78 knockout mice. The results indicated that GRP78 is essential for adipogenesis in vivo. Unexpectedly, the mutant mice showed early postnatal death and unique distinction from previously characterized lipodystrophic mouse models.
Sterol regulatory element binding protein-1c (SREBP-1c) is a transcription factor that critically regulates lipid metabolism. Insulin induced cleavage and activation of SREBP-1c, which is the cause of ectopic fat deposition in the liver. SREBP-1c directly binds GRP78, which remains in the ER without transcription activity. Nevertheless, dissociation of SREBP-1c and GRP78 promotes the transport of SREBPs-SCAP complex to Golgi, where SREBP-1c is cleaved, and then active SREBP-1c is transferred into the nucleus to initiate expression of genes involved in triglyceride and cholesterol synthesis (Figure 1 Pathway 5). Therefore, triglyceride and cholesterol levels are significantly increased [8][9][10]. Through hepatic overexpression of GRP78 in ob/ob mice using adenovirus vector, it was found that GRP78 overexpression inhibits SREBP-1c cleavage and the expression of SREBP-1c target genes lowers liver triglycerides and cholesterol levels and improves insulin sensitivity [8].
Intermittent fasting can induce pregnancy zone protein (PZP) production and release in the liver, followed by the translocation of GRP78 to cell surface of brown adipose tissue (BAT). The binding between PZP and GRP78 upregulates UCP1 expression through the p38 mitogen-activated protein kinases–activating transcription factor-2 (p38 MAPK-ATF2) signaling pathway and promotes the thermogenic metabolism of BAT. These results indicated that GRP78 is an indispensable regulator of PZP-induced thermogenesis [11]. (Figure 1 Pathway 6).
2. GRP78 Promotes De Novo Formation of Lipid Droplets
Lipid droplets are the main cellular sites for triglycerides and other lipids storage [12]. Lipid droplet fusion and growth is closely regulated by lipid droplet coated proteins adapted for cellular energy needs. Proteomic studies have found that HSP70 proteins including GRP78 are major structural proteins of lipid droplet [13]. Expressed GRP78 is translocated to lipid droplets in rat adipocytes upon heat stimulation. This process occurs neither in a temperature-dependent nor time-dependent manner, but occurs suddenly in 30-40 min, and rapidly reaches a stable state within 1 h at 40 ℃ heat shock.
Although GRP78 is co-localized with phospholipids on the surface of lipid droplets, co-immunoprecipitation experiments did not show direct interactions between GRP78 and phospholipids. Alkaline treatment indicated association of GRP78 with the surface of the droplets through non-hydrophobic interactions. Therefore, it is speculated that GRP78 may non-covalently associate with monolayer microdomains of lipid droplets in a manner like its interaction with lipid bilayer moieties composed of specific fatty acids. As an acute cell-specific response to heat stimulation, the accumulation of GRP78 on adipocytes lipid droplets may be involved in the stabilizing of droplet monolayer phospholipid, transferring or chaperoning denatured proteins to the lipid droplets for subsequent refolding.
Reticular protein 3 (RTN3) plays a key role in regulating triglyceride synthesis, storage, and lipid droplet fusion. Studies have found that RTN3 enhances SREBP-1C and AMPK activity through its interactions with GRP78 and leading to obesity and hyperlipidemia [14] (Figure 1 Pathway 7). SREBP-1c and AMPK are downstream of GRP78. Berbamine inhibits GRP78 and also induces AMPK activation, which regulates the mammalian target of the rapamycin/ SREBP-1c (mTOR/SREBP-1c) axis and the nuclear factor E2-related factor 2 (Nrf2)/antioxidant response element (Nrf2/ARE) pathway to allay lipid accumulation and oxidative stress in steatotic HepG2 cells. [15]
3. GRP78 Negatively Regulates Mitochondrial Biosynthesis and Energy Balance
A decrease in mitochondrial numbers and dysfunction can lead to obesity. GRP78 regulates mitophagy, mitochondrial biogenesis and energy balance [16][17]. Mitophagy is inhibited by GRP78 down expression, which is a vital way to activate browning and prevent obesity [18][19][20]. Further research found potential mitophagy mediation by GRP78 through the AMPK/mTOR signaling pathway, which leads to that energy intake exceeding expenditure and obesity. It was also found that GRP78 overexpression in the mitochondria triggers PINK1/IP3R mediated neuroprotective mitophagy [21].
4. GRP78 Causes Insulin Resistance
Insulin resistance is considered an underlying etiology of metabolic syndrome and cardiovascular disease associated with obesity, such as type 2 diabetes. Both human and animal experiments have shown positive correlation between GRP78 levels and insulin resistance [22][23] and effective improvement on insulin sensitivity and glycemic control via GRP78 down-regulation [24].
Further studies showed that downregulating GRP78 could activate the insulin signaling pathway and improve insulin sensitivity through phosphorylation modification of protein kinase B (Akt) [25]. GRP78 down-regulates Akt expression and phosphorylation but does not directly affect upstream pyruvate dehydrogenase kinase 1 (PDK1) activity. PDK1 is a critical activator of protein kinase B (PKB, also known as Akt), which is a serine/threonine-specific protein kinase. Co-immunoprecipitated GRP78 and p-Akt (Ser473) immune-complex contains non-phosphorylated Akt (Ser473 and Thr308) (Figure 1 Pathway 8). In-situ proximity ligation analysis showed co-location of GRP78 with Akt in cell membrane after ER stress induction and increase in phosphorylation of Akt Ser473 but decrease (i.e., inhibition) of Thr308. siRNA-mediated GRP78 knockdown enhances phosphorylation at Ser473 by 3.6-fold, but no impact at Thr308 [25].
Human jejunal mucosa secretes GRP78 in vitro, and bariatric surgery improves insulin resistance and type 2 diabetes through reducing intestinal GRP78 secretion. Plasma GRP78 levels in insulin resistance patients are higher than in healthy people and those who returned to normal physiology after duodenal jejunal bypass surgery, and plasma GRP78 level is negatively correlated with insulin sensitivity but positively correlated with body mass index (BMI) [26].
A high-calorie diet can increase plasma GRP78 levels and induce insulin resistance. GRP78 stimulates the accumulation of lipid droplets, inhibits Akt Ser473 phosphorylation and glucose uptake in both immortal liver cells and peripheral blood plasma cells. However, a converse phenomenon occurs when GRP78 serum levels decrease or insulin resistant patients undergo duodenal jejunal bypass surgery. Intestinal secretion of GRP78 may be the cause of insulin resistance, and duodenal jejunal bypass surgery may reduce GRP78 secretion and improve insulin sensitivity by shortening food transportation or reducing lipid stimulus released from endocrine cells [26]. GRP78 is essential for proinsulin synthesis, and up-regulation of GRP78 can increase insulin secretion in response to hyperglycemia, while down-regulation of GRP78 can decrease insulin secretion and lead to significantly low levels of insulin [27].
5. GRP78 Can Eliminate Liver Lipotoxicity and then Improve Liver Steatosis
ER stress plays an important role in hepatic steatosis and insulin resistance in obese mice models [28]. GPR78 is involved in the pathogenesis of nonalcoholic fatty liver disease and is associated with hepatic steatosis, insulin resistance, inflammation, and apoptosis [29][30]. GRP78 plays a key role in maintaining lipid balance in liver, and GRP78 overexpression can reduce the hydrolysis of SREBP-1c induced by ER stress and liver steatosis [28]. It was also reported that GRP78 overexpression in HepG2 cells prevents ER stress and cytotoxicity induced by palmitic acid, and further studies found that GRP78 may reduce lipid peroxidation and damage induced by oxidative stress [31]. Some studies suggest that GRP78 overexpression in the liver can reduce the expression of SREBP-1c, reduce ectopic triglyceride deposition in liver, and enhance insulin sensitivity of ob/ob mice [8].
References
- Camacho A, Segoviano-Ramírez JC, Sánchez-Garcia A, de Jesus Herrera-de la Rosa J, García-Juarez J, Hernandez-Puente CA, Calvo-Anguiano G, Maltos-Uro SR, Olguin A, Gojon-Romanillos G, Gojon-Zorrilla G, Ortiz-Lopez R. Tyrphostin AG17 in-hibits adipocyte differentiation in vivo and in vitro. Lipids Health Dis. 2018, 17(1):128. doi:10.1186/s12944-018-0784-7.
- Perera RJ, Marcusson EG, Koo S, Kang X, Kim Y, White N, Dean NM. Identification of novel PPARgamma target genes in primary human adipocytes. Gene 2006, 369:90-99. doi:10.1016/j.gene.2005.10.021.
- Nakachi Y, Yagi K, Nikaido I, Bono H, Tonouchi M, Schönbach C, Okazaki Y. Identification of novel PPARgamma target genes by integrated analysis of ChIP-on-chip and microarray expression data during adipocyte differentiation. Biochem. Biophys. Res. Commun. 2008, 372(2):362-366. doi:10.1016/j.bbrc.2008.05.037.
- Zhu G, Ye R, Jung DY, Barron E, Friedline RH, Benoit VM, Hinton DR, Kim JK, Lee AS. GRP78 plays an essential role in adipogenesis and postnatal growth in mice. FASEB J. 2013, 27(3): 955-964.
- Zhang X, Chen X, Qi T, Kong Q, Cheng H, Cao X, Li Y, Li C, Liu L, Ding Z. HSPA12A is required for adipocyte differentiation and diet-induced obesity through a positive feedback regulation with PPARγ. Cell Death Differ. 2019, 26(11):2253-2267. doi:10.1038/s41418-019-0300-2.
- Samadi P, Sarvarian P, Gholipour E, Asenjan KS, Aghebati-Maleki L, Motavalli R, Hojjat-Farsangi M, Yousefi M. Berberine: A novel therapeutic strategy for cancer. IUBMB Life. 2020, 72(10):2065-2079. doi: 10.1002/iub.2350.
- Sun B, Tan D, Pan D, Baker MR, Liang Z, Wang Z, Lei J, Liu S, Hu CY, Li QX. Dihydromyricetin imbues antiadipogenic effects on 3T3-L1 cells via direct interactions with 78-kDa glucose-regulated protein. J Nutr. 2021, 151(7):1717-1725. doi:10.1093/jn/nxab057.
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009, 119(5):1201-2015. doi:10.1172/JCI37007.
- Colgan SM, Hashimi AA, Austin RC. Endoplasmic reticulum stress and lipid dysregulation. Expert Rev Mol Med. 2011, 13:e4. doi:10.1017/S1462399410001742.
- Lhoták S, Sood S, Brimble E, Carlisle RE, Colgan SM, Mazzetti A, Dickhout JG, Ingram AJ, Austin RC. ER stress contributes to renal proximal tubule injury by increasing SREBP-2-mediated lipid accumulation and apoptotic cell death. Am J Physiol Renal Physiol. 2012, 303(2):F266-278. doi:10.1152/ajprenal.00482.2011.
- Lin J, Jiang X, Dong M, Liu X, Shen Q, Huang Y, Zhang H, Ye R, Zhou H, Yan C, Yuan S, Wu X, Chen L, Wang Y, He M, Tao Y, Zhang Z, Jin W. Hepatokine pregnancy zone protein governs the diet-induced thermogenesis through activating brown adipose tissue. Adv Sci. 2021, 8(21):e2101991. doi: 10.1002/advs.202101991.
- Smolič T, Zorec R, Vardjan N. Pathophysiology of Lipid Droplets in Neuroglia. Antioxidants 2021, 11(1):22. doi:10.3390/antiox11010022.
- Wang W, Wei S, Li L, Su X, Du C, Li F, Geng B, Liu P, Xu G. Proteomic analysis of murine testes lipid droplets. Sci Rep. 2015, 5:12070. doi:10.1038/srep12070.
- Xiang R, Fan LL, Huang H, Chen YQ, He W, Guo S, Li JJ, Jin JY, Du R, Yan R, Xia K. Increased reticulon 3 (RTN3) leads to obesity and hypertriglyceridemia by interacting with heat shock protein family A (Hsp70) member 5 (HSPA5). Circulation 2018, 138(17):1828-1838. doi:10.1161/circulationaha.117.030718.
- Sharma A, Anand SK, Singh N, Dwivedi UN, Kakkar P. Berbamine induced AMPK activation regulates mTOR/SREBP-1c axis and Nrf2/ARE pathway to allay lipid accumulation and oxidative stress in steatotic HepG2 cells. Eur J Pharmacol. 2020, 882:173244. doi: 10.1016/j.ejphar.2020.173244.
- Prasad M, Pawlak KJ, Burak WE, Perry EE, Marshall B, Whittal RM, Bose HS. Mitochondrial metabolic regulation by GRP78. Sci Adv. 2017, 3(2):e1602038. doi:10.1126/sciadv.1602038.
- Zhang X, Li X, Fang H, Guo F, Li F, Chen A, Huang S. Flavonoids as inducers of white adipose tissue browning and ther-mogenesis: signalling pathways and molecular triggers. Nutr Metab (Lond). 2019, 16:47. doi:10.1186/s12986-019-0370-7.
- Zhang Y, Sowers JR, Ren J. Targeting autophagy in obesity: From pathophysiology to management. Nat Rev Endocrinol. 2018, 14(6):356-376. doi:10.1038/s41574-018-0009-1.
- Yan Q, Li X. Relationship between mitophagy and browning of white adipose. International J Endocrinol. Metabolism 2019, 39(02):87-90.
- Alan P, Vandevoorde KR, Joshi B, Cardoen B, Gao G, Mohammadzadeh Y, Hamarneh G, Nabi IR. Gp78-mediated basal mitophagy promotes mitochondrial health and limits mitochondrial ROS production. bioRxiv 2021. doi: https://doi.org/10.1101/2021.09.17.460825
- Leiva-Rodríguez T, Romeo-Guitart D, Herrando-Grabulosa M, Muñoz-Guardiola P, Polo M, Bañuls C, Petegnief V, Bosch A, Lizcano JM, Apostolova N, Forés J, Casas C. GRP78 overexpression triggers PINK1-IP3R-mediated neuroprotective mi-tophagy. Biomedicines 2021, 9(8):1039. doi:10.3390/biomedicines9081039.
- Girona J, Rodríguez-Borjabad C, Ibarretxe D, Vallvé JC, Ferré R, Heras M, Rodríguez-Calvo R, Guaita-Esteruelas S, Mar-tínez-Micaelo N, Plana N, Masana L. The circulating GRP78/BiP is a marker of metabolic diseases and atherosclerosis: Bringing endoplasmic reticulum stress into the clinical scenario. J Clin Med. 2019, 8(11):1793. doi:10.3390/jcm8111793.
- Kim JH, Lee E, Friedline RH, Suk S, Jung DY, Dagdeviren S, Hu X, Inashima K, Noh HL, Kwon JY, Nambu A, Huh JR, Han MS, Davis RJ, Lee AS, Lee KW, Kim JK. Endoplasmic reticulum chaperone GRP78 regulates macrophage function and insulin resistance in diet-induced obesity. FASEB J. 2018, 32(4):2292-2304. doi:10.1096/fj.201701017R.
- Khadir A, Kavalakatt S, Abubaker J, Cherian P, Madhu D, Al-Khairi I, Abu-Farha M, Warsame S, Elkum N, Dehbi M, Tiss A. Physical exercise alleviates ER stress in obese humans through reduction in the expression and release of GRP78 chaperone. Metabolism 2016, 65(9):1409-1420. doi:10.1016/j.metabol.2016.06.004.
- Yung HW, Charnock-Jones DS, Burton GJ. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic retic-ulum stress modulates substrate specificity in a severity dependent manner. PLoS One 2011, 6(3):e17894. doi:10.1371/journal.pone.0017894.
- Angelini G, Salinari S, Bertuzzi A, Iaconelli A, Mingrone G. Metabolic surgery improves insulin resistance through the re-duction of gut-secreted heat shock proteins. Commun Biol. 2018, 1:69. doi:10.1038/s42003-018-0069-8.
- Zhang L, Lai E, Teodoro T, Volchuk A. GRP78, but not protein-disulfide isomerase, partially reverses hyperglycemia-induced inhibition of insulin synthesis and secretion in pancreatic β-Cells. J Biol Chem. 2009, 284(8):5289-5298. doi:10.1074/jbc.M805477200.
- Chen WT, Zhu G, Pfaffenbach K, Kanel G, Stiles B, Lee AS. GRP78 as a regulator of liver steatosis and cancer progression mediated by loss of the tumor suppressor PTEN. Oncogene 2014, 33(42):4997-5005. doi:10.1038/onc.2013.437.
- Gentile CL, Frye M, Pagliassotti MJ. Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid Redox Signal 2011, 15(2):505-521. doi:10.1089/ars.2010.3790.
- Chalasani N, Szabo G. Alcoholic and non alcoholic fatty liver disease pathogenesis of NAFLD and NASH. 2016, 10.1007/978-3-319-20538-0(Chapter 4):71-101. doi:10.1007/978-3-319-20538-0_4
- Teodoro-Morrison T, Schuiki I, Zhang L, Belsham DD, Volchuk A. GRP78 overproduction in pancreatic beta cells protects against high-fat-diet-induced diabetes in mice. Diabetologia 2013, 56(5):1057-1067. doi:10.1007/s00125-013-2855-7.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
13 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




