1. Introduction
The red fox (
Vulpes vulpes, Linnaeus, 1758) is the medium-size canid with the widest world distribution
[1]. This species is present throughout the northern hemisphere and regions of North Africa and has been introduced into Australia, where it is considered a plague
[1][2]. It is listed as least concern by the International Union Conservation of Nature (IUCN), and in some countries is hunted by its fur and meat
[1]. It is highly adaptable to local environmental conditions so that this animal can be found in urban, suburban, and rural areas. Red foxes live in small family groups and are more active at night
[2]. They are opportunist predators who can adjust their diet to seasonal and local availability. Their heterogeneous diet can include fruits, invertebrates, small mammals, birds, and even rubbish
[2][3]. Their main predators are large carnivores (e.g., wolves, bears), large birds of prey (e.g., golden eagles), and humans
[2][4]. The major cause of the admission of these animals to wildlife rehabilitation centers is traffic accidents and poisoning
[5][6]. Foxes also harbor a number of pathogens, including some zoonotic
[2].
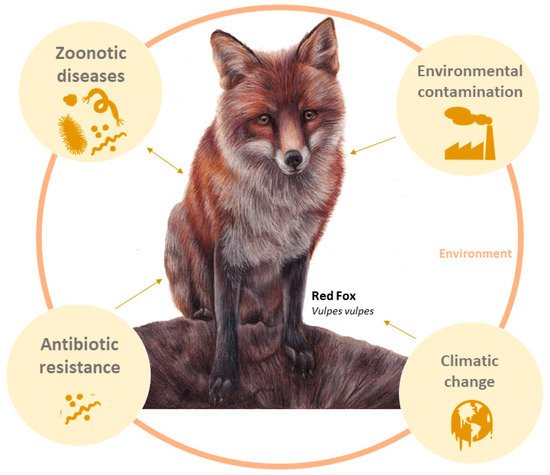
Because the red fox is one of the most widely distributed wild mammals, feeds on a broad range of food resources, and lives in close contact with humans, it has been proposed as a sentinel species in several studies (
Figure 1). A sentinel species is used to detect and monitor the presence and effects of contaminants in the animals introduced or living in their habitat
[3][7][8]. It also allows the identification of threats, namely infectious agents (e.g., viruses, bacteria), or other anthropogenic hazards, that represent a risk to the fauna and flora of the ecosystem and potentially to humans
[9].
Figure 1. Red fox (Vulpes vulpes) as bioindicator sentinel of environmental ecosystem health: zoonotic diseases, environmental contamination, antibiotic resistance, zoonotic diseases, climate changes, and anthropogenic changes.
A sentinel species, in a One Health context, can give people the tools to predict environmental changes and disease outbreaks. As a result, early actions could be taken to prevent catastrophic consequences, as you saw in the case of the COVID-19 pandemic. Thus, the aim is to compile the studies that use the red fox (V. vulpes) as a sentinel species.
2. Research Methods
Researchers conducted a literature search through the main web search engines, which included Google Web, Google Scholar, Web of Knowledge, Re-search Gate, and PubMed, as well as in the more relevant ecological ecology, chemistry, veterinary, and similar themed journals. To collect articles related to red fox (V. vulpes) as environmental sentinel bioindicators, the search terms included combinations of fox, red fox, Vulpes vulpes, bioindicator, environmental sentinel, one health, pollution, toxics, antimicrobial resistance, environmental contaminants, heavy metals, disease, poison, morbidity, mortality, persistent organic pollutants, zoonosis, infectious diseases, parasites, organochlorides. As inclusion criteria, only works that describe information regarding Vulpes vulpes as environmental sentinels were included.
3. Results Obtained from the Consulted Papers
Overall, researchers analyzed a total of 112 research works published between the years 1963 to 2021. To facilitate the description of the studies, they were grouped under an integrated “One Health” vision, taking into consideration the main threats to environmental, human, and animal health.
3.1. Red Fox as a Sentinel of Environmental Contamination
Organochlorine pesticides, polychlorinated biphenyls (PCBs), polybrominated di-phenyl ethers (PBDEs), and heavy metals are ubiquitous environmental contaminants that originated from human activities, such as agriculture, burning of fossil fuels, industrial activities, and transportation
[3][10][11]. They are toxic and have the potential to persist in ecosystems. Due to high lipophilia, they can accumulate in the adipose tissues of vertebrates and bio magnify in food chains
[10].
Foxes are significant elements in the food chain
[12]. Carnivores, such as foxes, tend to have higher levels of polluting residues than herbivorous animals as a result of bioaccumulation effects between trophic levels. It is, therefore, necessary to trace the presence and the number of chemical substances, such as heavy metals and pesticides, in their tissues. Contaminants studies make it possible to understand the organic changes in the animal, but also the potential dangers for human health
[13][14]. Contaminants’ exposition, during long times and at low doses, can alter physiological processes (e.g., metabolism, hormonal changes), decrease animal body condition (e.g., small and weak animals), immunotoxic effects, decrease reproductive success (e.g., infertility, abortion, malformations), and can result in genotoxic and mutagenic effects (cancer)
[8][15][16].
Table 1 presents the published works associated with environmental contaminants studies in
V. vulpes. The majority of the studies (out of 35) were conducted in Europe (
n = 34), with the largest number in Poland (
n = 12) and Italy (
n = 7). The research focused on wild foxes, with the exception of two cases where red foxes were raised on farms for fur
[17].
Table 1. Review of articles that evaluated environmental contaminates in red fox (Vulpes vulpes) regarding the number of animals substance type, year, sample type analyzed, country, the origin of the animals (wild or fur farm).
| Substance |
Number of Animals |
Origin Animal |
Sample |
Country |
Year |
Reference |
| Cr, Cu, Ni, Pb, Zn |
20 |
Wild and Fur farm |
Hair and skin |
Poland |
2011 |
[17] |
| Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn |
48 |
Wild |
Small intestines |
Czech Republic |
2010–2011 |
[18] |
| Cd, Pb, Cu, Zn |
87 |
Wild |
Kidney and liver |
Switzerland |
1997–1998 |
[19] |
| Pb, Cd, Hg |
30 |
Fur Farm |
Kidney |
Poland |
2008 |
[20] |
| Cu, Ni, Zn, Co, Cd, Pb |
10 |
Wild |
Kidney |
Hungary |
2008 |
[21] |
| Hg |
37 |
Fur farm |
Hair and skin |
Poland |
2014 |
[17] |
| Hg, Pb, Cd, Cr, As |
18 |
Wild |
Liver, kidneys, and muscles |
Slovak Republic |
1998–1999 |
[12] |
| Cd, Pb, Zn |
250 |
Wild |
Kidney |
Spain |
2003–2011 |
[22] |
| Cd, Pb, Zn. |
36 |
Wild |
Kidney, liver and muscle |
Poland |
2002–2003 |
[23] |
| Hg |
6 |
Wild |
Liver and kidney |
Russia |
2007–2011 |
[14] |
| Al, Ca, Cr, Cu, Fe, Mg, Mn, Ni, Pb |
56 |
Wild |
Liver |
Romania |
May–September 2014 |
[24] |
| Hg |
200 |
Wild |
Liver, muscle, kidney, hair, bone |
Alaska |
2010–2011 |
[25] |
| Zn, Cu, Pb, Cd, Hg |
30 |
Wild |
Cartilage, compact bone, and spongy bone |
Poland |
2008–2009 |
[26] |
| Pb, Cu |
42 |
Wild |
Muscle and skin |
Italy |
2010 |
[13] |
| Cd, Pb, Cr, Cu, Zn, Mn, Ni |
27 |
Wild |
Intestine |
Czech Republic |
2009 to 2010 |
[27] |
| Hg |
27 |
Wild |
Liver, muscle, and kidney |
Poland |
2004–2006 |
[28] |
| Mn, Fe, Sr |
38 |
Wild |
Bone |
Poland |
2008–2009 |
[29] |
| Hg, Cd, Pb |
46 |
Wild |
Liver |
Italy |
1992 |
[30] |
| As, Cd, Cu, Pb, Hg |
28 |
Wild |
Liver, kidney and muscle |
Croatia |
2008–2009 |
[31] |
| Pb, Cd, Cr, Hg |
Unknown |
Wild |
Heart, liver, diaphragm, kidney, muscle, and adipose tissue |
Italy |
1994–1995 |
[10] |
| PCB, DDE |
Unknown |
Wild |
Heart, liver, diaphragm, kidney, muscle, and adipose tissue |
Italy |
1994–1995 |
[10] |
| PCB, Dieldrin, DDT, Endosulfan, HCB, Heptachlor |
192 |
Wild |
Perirenal adipose tissue, Kidney |
Switzerland |
1999–2000 |
[32] |
| PCB |
80 |
Wild |
Muscle |
Germany |
1983–1991. |
[33] |
| PCBs, DDT |
23 |
Wild |
Adipose tissue |
Italy |
1991–1992 |
[3] |
| HCB, DDT, PBC |
57 |
Wild |
Muscle and adipose tissue |
Italy |
1992–1993 |
[3] |
| PBDEs |
33 |
Wild |
Adipose tissue, liver, and muscle |
Belgium |
2003–2004 |
[34] |
| HCB, DDT, PCB |
36 |
Wild |
Adipose tissues and muscle |
Italy |
1992 |
[30] |
| PCB |
20 |
Wild |
Liver, lungs |
Poland |
2008–2009 |
[35] |
| Aldrin, cis-chlordane, trans-chlordane, DDE, DDD, DDT, dieldrin, endosulfan, endrin, HCB, heptachlor, heptachlor-exo-epoxide, iso-drin, methoxychlor, mirex, PBC |
18 |
Wild |
Plasma, liver, and adipose tissue |
Spain |
2004–2006 |
[36] |
| Fluoride |
32 |
Wild |
Bone |
Poland |
2014 |
[37] |
| Fluoride |
34 |
Wild |
Teeth |
Poland |
Unknown |
[38] |
| Fluoride |
182 |
Wild |
Mandible |
Great Britain |
Unknown |
[38] |
| Fluoride |
35 |
Wild |
Teeth |
Poland |
2004/2005 and 2005/2006 |
[7] |
| 90Sr, 238,239+240Pu, 241Am and 137Cs |
183 |
Wild |
Jaw bones |
Poland |
2008 |
[39] |
With respect to the type of contaminant, 20 studies were performed on heavy metals, 14 on pesticides/PCBs/PBDEs, and one on radioactive compounds.
Research works on pesticides using the fox as a sentinel are more common for the Arctic fox than the red fox
[40][41][42][43][44]. Acute toxicity probably appears to be more common in these animals than the non-lethal chronic effects of pesticides
[2]. Indeed, accidental or deliberate poisoning by organochlorine pesticides, biocides, and rodenticides is one of the main causes of red fox admission to wildlife rehabilitation centers
[45]. Contrary to expectations, since these animals live near farms and agricultural fields, the pesticides levels in red foxes’ tissues seem to be low, and probably are not associated with adverse health effects. In a study performed in Germany, the investigation of samples from 1983, 1987, and 1991 showed a reduction in the levels of the highly chlorinated biphenyls 138, 153, and 180
[33]. A similar study conducted in Zurich showed a general reduction in exposure to PCBs, with lower levels of PCBs in samples obtained from 1999 to 2000
[32]. In Poland, the ΣPCBs (sum of PCBs: 28, 52, 101, 118, 138, 153, 180) levels in the liver and lungs were 389.99 ng/g and 110.57 ng/g of lipid weight
[35]. In Italy, the investigators found in muscle concentrations of 20.2 µg g µ1 lipid in muscle and 7.2 µg g µ1 lb in adipose tissue
[46]. While the levels were found to be low, ongoing studies are important for monitoring pesticides environmental pollution.
Several studies have been carried out to determine heavy metal concentrations (Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn) in red foxes. Mercury (Hg) is one of the most studied and presents variations according to the geographic location of the animals. Wild foxes living near water sources have naturally higher levels of mercury in their tissues, possibly as a result of feeding higher up the food chain
[25]. Studies in Slovakia
[14], Russia
[14], Poland
[28], and Spain
[30] have shown toxic levels of mercury in red fox fever, which appear to increase with age. Wild foxes have elevated levels of mercury in their tissues, even in populations living in isolated areas as Alaska
[25].
Fluoride (F−) pollution has been increasing over the last several decades. In excess, fluoride can cause toxic effects on living organisms, such as dental and bone fluorosis and bone tumors
[7]. In Poland, two different studies detected concentrations from 176 to 3668 mg/kg dw in bone
[7][38] and 230 and 296 mg/kg dw in mandibular first molars. The interpretation of these values reflects moderate fluoride contamination in the area and makes red foxes a promising sentinel to access industrial pollution
[7][37][38].
The study of radioactivity was carried out in the Ukraine, where the Chernobyl nuclear accident occurred. Fox bones did not show a high level of contamination in comparison to the results obtained on the bones of small animals (rodents or insectivorous mammals) previously determined. This suggests that there is no accumulation of bone isotopes at the top of the food chain
[39].
3.2. Red Foxes as a Sentinel of Antimicrobial Resistance (AMR)
Antimicrobial resistance (AMR) is a major public health problem of modern times and has increased worldwide, not only in humans but also in animals, due to a continued spread of antimicrobial-resistant bacteria in the environment through different pathways
[47][48]. The production of extended-spectrum b-lactamases (ESBLs) by Enterobacteriaceae, in particular by
Escherichia coli, vancomycin-resistant Enterococci (VRE), Methicillin-resistant
Staphylococcus pseudointermedius (MRSP), and Methicillin-resistant
Staphylococcus aureus (MRSA) have been some of the main public health concerns in the last years
[49][50].
Some red fox populations are urban and may therefore acquire antimicrobial-resistant bacteria directly from man and other animals or indirectly through reservoirs, such as food waste, garbage, sewage, and wastewater
[47]. Consequently, the red fox can be a hopeful sentinel for monitoring the occurrence of AMR, providing a better understanding of resistance dynamics and factors
[49][50].
The studies of AMR conducted in foxes are still scarce. Escherichia coli displaying carbapenem or colistin resistance was isolated in 387 out of 528 samples of wild red foxes evaluated in Denmark. In addition, the total occurrence of AMR was significantly higher in areas where the population density was higher
[51].
In a Portuguese study, cefotaxime-resistant
E. coli was isolated from 2 of the 52 fecal samples (4%), being both ESBL producers. The b-lactamase genes found in the two isolates were
blaSHV-12+blaTEM-1b. The
tet (A) and
sul2 genes were also detected in these isolates, together with the non-classical class 1 integrin (
intI1-dfrA12-orfF-aadA2-cmlA1- aadA1-qacH-IS440-sul3) with the
PcH1 promoter
[49]. In other study, 14 VRE were detected in 7 of 52 fecal samples (13.5%)
[50][52].
In an investigation carried out in the United Kingdom, including 38 foxes (
Vulpes vulpes) samples from rural and semirural areas, 35 presented isolates of coagulase-negative
Staphilococcus sciuri group (35%),
S. equorum (27%), and
S. capitis (22%). All were phenotypically resistant to methicillin, and
mecA was detected in 33 (89%) of the isolates and 10 (27%) showed broad b-lactam antibiotic resistance
[53]. Resistance/intermediate resistance to at least one class of antibiotics and the highest resistance values were observed in the tetracycline class, with 33 strains being MDR.