Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mick Welling | -- | 2425 | 2023-02-10 11:10:41 | | | |
| 2 | Rita Xu | Meta information modification | 2425 | 2023-02-13 03:59:59 | | | | |
| 3 | Rita Xu | -3 word(s) | 2422 | 2023-02-13 04:02:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Welling, M.M.; Duszenko, N.; Meerbeek, M.P.V.; Molenaar, T.J.M.; Buckle, T.; Leeuwen, F.W.B.V.; Rietbergen, D.D.D. Microsphere Carrier Systems. Encyclopedia. Available online: https://encyclopedia.pub/entry/41087 (accessed on 08 February 2026).
Welling MM, Duszenko N, Meerbeek MPV, Molenaar TJM, Buckle T, Leeuwen FWBV, et al. Microsphere Carrier Systems. Encyclopedia. Available at: https://encyclopedia.pub/entry/41087. Accessed February 08, 2026.
Welling, Mick. M., Nikolas Duszenko, Maarten P. Van Meerbeek, Tom J. M. Molenaar, Tessa Buckle, Fijs W. B. Van Leeuwen, Daphne D. D. Rietbergen. "Microsphere Carrier Systems" Encyclopedia, https://encyclopedia.pub/entry/41087 (accessed February 08, 2026).
Welling, M.M., Duszenko, N., Meerbeek, M.P.V., Molenaar, T.J.M., Buckle, T., Leeuwen, F.W.B.V., & Rietbergen, D.D.D. (2023, February 10). Microsphere Carrier Systems. In Encyclopedia. https://encyclopedia.pub/entry/41087
Welling, Mick. M., et al. "Microsphere Carrier Systems." Encyclopedia. Web. 10 February, 2023.
Copy Citation
The targeted delivery of anti-cancer drugs and isotopes is one of the most pursued goals in anti-cancer therapy. One of the prime examples of such an application is the intra-arterial injection of microspheres containing cytostatic drugs or radioisotopes during hepatic embolization procedures. Therapy based on the application of microspheres revolves around vascular occlusion, complemented with local therapy in the form of trans-arterial chemoembolization (TACE) or radioembolization (TARE).
microspheres
embolization
hepatocellular carcinoma
1. Introduction
The targeted delivery of anti-cancer drugs and isotopes is the most pursued goal in anti-cancer therapy. However, a major disadvantage of the systemic application of these drugs is their poor selectivity for cancer cells and their general distribution to non-cancerous tissues, causing toxic side effects to otherwise healthy tissue [1][2]. To this end, novel therapies are constantly being discovered and applied for anti-cancer interventions in pursuit of avoiding toxic side effects. Theoretically, the involvement of non-cancerous tissues can also be reduced via disease-targeted therapeutic delivery. Herein, disease-targeting can be guided by either receptor targeting or via selective administration [3]. Unique for the hepatic system is that cancerous diseases such as HCC and mCRC alter the vascularization of the liver. Normal liver tissue will receive oxygen from the portal system; HCC and mCRC receive oxygen from the artery and are therefore suitable for trans-arterial therapy [4]. Over the past two decades, microspheres have proven themselves effective trans-arterial drug delivery systems [5]. Such microspheres have broad applications in both life science research and medicine [6], e.g., contrast agents [7], tissue fillers [8], and drug delivery vehicles [9]. Stable or biodegradable microspheres are generally utilized to direct drugs to organs by taking advantage of vascular physical restraints [10][11][12][13][14]. Therapeutic microspheres are characteristically small particles or beads with a well-defined size distribution of 50–750 mm, consisting of either glass, synthetic polymers, or proteins [15]. By selectively blocking the blood supply of the targeted tissue via so-called trans-arterial embolization (TAE), the deprived tissue will be starved of nutrients, ultimately resulting in a therapeutic benefit. When lodged in the end vasculature that surrounds the tumor, microspheres can also release encapsulated drugs (trans-arterial chemoembolization (TACE)) or irradiate surrounding tissue via embedded radioisotopes (trans-arterial radioembolization (TARE)).
Being a medicinal product, microspheres are considered medical devices rather than drugs. From a practical perspective, this means that such agents have to comply with different regulations compared to more standard therapeutics. Independent of this classification, the various clinical requirements ultimately drive therapeutical applications, of which the most important ones are stated in Table 1. Caine et al. extensively reviewed more detailed information on the microspheres used for TAE [16].
Table 1. Properties of microspheres: list of requirements for embolization (selected from: https://www.microspheres.us/properties-of-microspheres/ accessed on 1 November 2022).
| Property | Importance |
|---|---|
| Specific gravity (particle density) | Dispersion in other media or occlusion of the micro-vasculature |
| Size | Particle size (diameter = 50–750 mm) that allows occlusion of the microvasculature |
| Durability | Strength during production, solvent resistance, sterilization, chemical stability, or biodegradation, the release of the therapeutical payload |
| Biocompatibility | Safety, toxicity, stability, suitable for intra-arterial delivery |
| Pharmacology | Controlled dosimetry and dosing, full control over release profile by diffusion, zero-order kinetics |
| Surface properties | Hydrophobic vs. hydrophilic surface, surface area, and porosity, ability to coat or functionalize the spheres |
Therapeutical microspheres have been widely implemented for hepatocellular cancer (HCC) and hepatic metastasized disease of different kinds of solid cancers, including colorectal, lung, and breast cancer that metastasize to the liver [5][17]. Non-treatable advanced neoplastic diseases and the development of hepatic metastasis have poor prognoses. Only 10–20% of these patients are suitable candidates for radical resection, as surgical excision cannot be applied in grade 3–5 staged HCC [18]. A substantial portion of inoperable patients who present metastatic liver tumors needs alternative treatment strategies or therapy for bridging surgery [19][20]. Alternative therapies encompass minimally invasive techniques, including percutaneous ablative treatments (radiofrequency ablation, microwave ablation) and trans-catheter intra-arterial therapies [21][22].
2. Trans-Arterial Embolization for Vascular Occlusion
Trans-arterial embolization (TAE) is a technique wherein inert microspheres are used to block the blood supply around the tumor. The use of large microspheres, e.g., Embosphere® (⌀ 300–750 mm, Merit Medical Systems, South Jordan, UT, USA) in tumor-affected liver lobe(s) allows blockage of the blood supply towards the tumor (Figure 1B), ultimately resulting in reduced tumor growth [10][11][12][13][14]. An overview of the different microspheres currently used for hepatic trans-arterial embolization is provided in Table 2. The clinically applied glass and synthetic spheres are non-degradable and remain in the vasculature for life. Recently, in a pre-clinical phase, biodegradable spheres were developed, which allow local occlusion of the vasculature, and after degradation they allow a follow-up injection of embolization treatment. The clinical unfavorable short- and long-term outcomes of patients with large HCCs (≥50 mm) were revealed compared to those with small HCCs (<50 mm). Detailed analyses revealed that the average rates of change in tumor size and shrinkage after TAE were 48.6 ± 35.6 mm and 30.7 ± 17.0%, respectively [23].
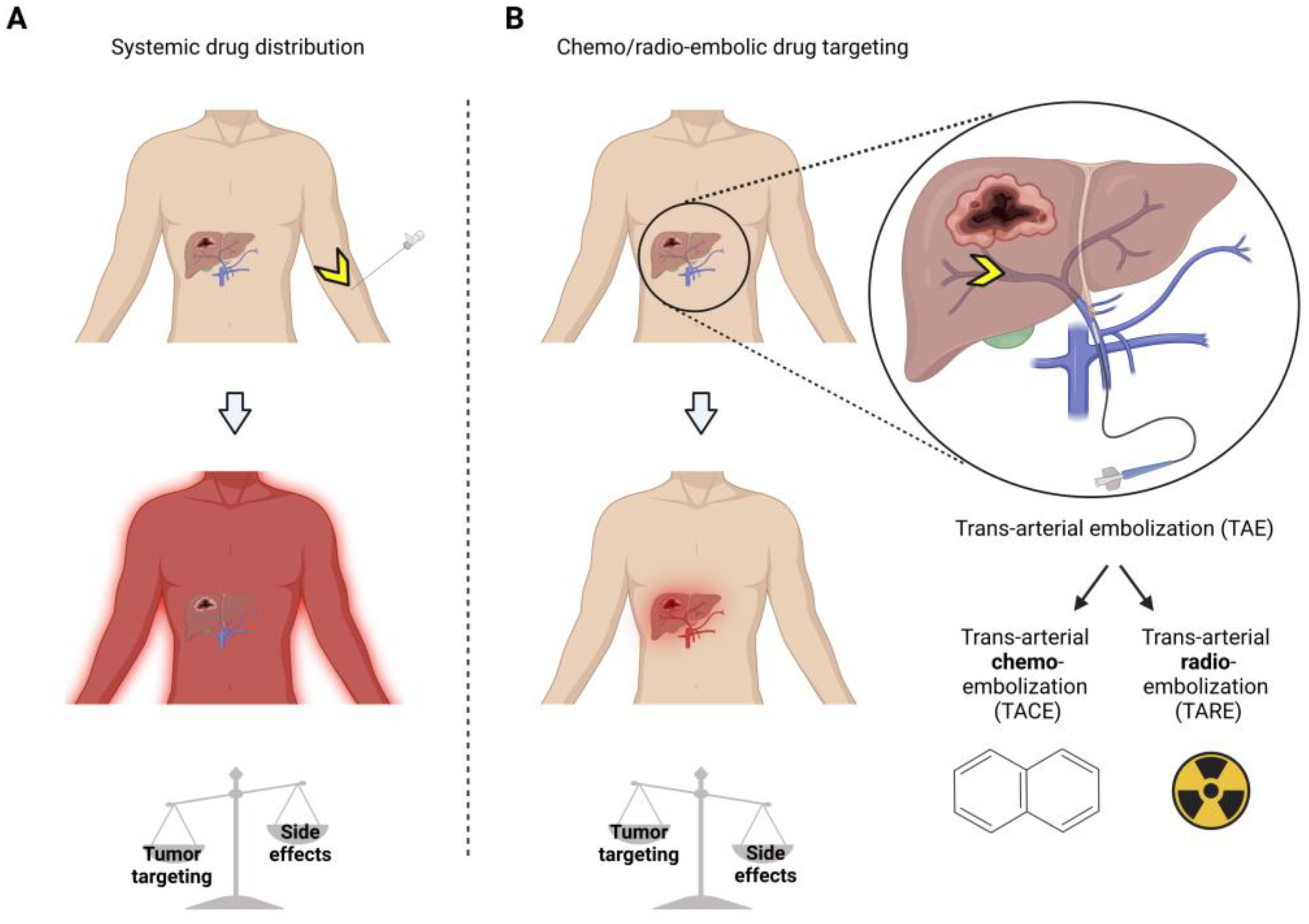
Figure 1. Treatment of hepatocellular carcinoma (HCC) shows the advantages of chemo/radio-embolization drug targeting over systemic drug therapy. Systemic drug therapy (A) yields lower tumor targeting and more side effects than local delivery via trans-arterial chemo/radio embolic drug targeting (B). Yellow arrows = injection site.
Table 2. Overview of non-functionalized microspheres for hepatic trans-arterial embolization (TAE). PC = pre-clinical use, H = Human use, FDA = FDA approved as an active implantable medical device.
| Microspheres Composition | Product Name | Particle Size Range (⌀ μm) | PC/H (FDA Clearance) | Biodegradable | References |
|---|---|---|---|---|---|
| Tris acryl gelatin microspheres (TAGM) | Embosphere® (Merit Medical Systems, South Jordan, UT, USA) | 100–300, 300–500 | H (FDA) | No | [24] |
| Polyvinyl alcohol (PVA) sodium acrylate co-polymer microspheres | Contour SE® (Boston Scientific, Marlborough, MA, USA), Bead Block® (Boston Scientific, Marlborough, MA, USA) Embozene® (Varian Medical Systems, Palo Alto, CA, USA) |
Irregular: 150–250, 250–355, 355–500, 500–710 |
H (FDA) | No | [25] |
| Polyvinyl alcohol-based hydrogel microspheres with sulphonate groups | LC Bead® (Boston Scientific, Marlborough, MA, USA) | 75–150, 100–300, 300–500, 500–700 | H (FDA) | No | [26][27][28][29] |
| Co-polymer of PEG and diacrylamide | Hydropearl® (Terumo Medical Co., Somerset, NJ, USA) | 75–1100 | H (FDA) | No | [1] |
| Starch microspheres | Embocept® (Pharmacept, Berlin, Germany), Spherex® (Magle Life Sciences, Lund, Sweden) |
50 | PC | Yes | [1] |
| Gelatin microspheres | Gel-Bead (Teleflex, Morrisville, NC, USA) | 100–300, 300–500, 500–700, 7000–1000 | H (FDA) | Yes | [1] |
| Collagen-coated poly-(DL-lactic acid-co-glycolic acid (PLGA) microspheres | Occlusin500® (IMBiotechnologies, Edmonton, AL, Canada) | 150–210 | H | Yes | [1] |
Instead of focusing on major vascular occlusion, chemo- or radio-embolic targeting is an alternative treatment option with microspheres that have the potential to manage hepatic tumors effectively [18].
3. Therapeutic Loads Employed during Microsphere-Trans-Arterial Embolization Therapy
3.1. Trans-Arterial Chemoembolization
Trans-arterial chemoembolization (TACE) uses embolization to deliver chemotherapy locally, thus limiting systemic exposure (Figure 1) [30][31]. One prime example are acrylic co-polymer microspheres (Hepasphere™, ⌀ 50–100 µm, Merit Medical Systems, Inc., South Jordan, UT, USA) that can absorb cytostatic drugs such as doxorubicin, irinotecan, epirubicin, mitomycin, cisplatin, and oxaliplatin (Table 3).
Table 3. Drug-eluting microspheres for functionalized hepatic TACE. PC = pre-clinical use, H = human use, FDA = FDA approved as an active implantable medical device.
| Microspheres Composition |
Product Name | Particle Size Range (⌀ µm) | Drug Load | PC/H (FDA) | Biodegradable | References |
|---|---|---|---|---|---|---|
| Polyvinyl alcohol (PVA) sodium acrylate co-polymer microspheres | QuadraSphere® and HepaSphere™ (Merit Medical Systems, Inc., South Jordan, UT, USA) DC Bead® (Boston Scientific, Marlborough, MA, USA), LC Bead®, and Bead Block® (Boston Scientific, Marlborough, MA, USA) |
50–100, 100–300, 200–400 | Doxorubicin, irinotecan, epirubicin, oxaliplatin | PC/H (FDA) | No | [14][26][32][33][34][35][36] |
| Ion-exchange microspheres | CalliSpheres® Beads (Jiangsu Hengrui Medicine Co. Ltd. Jiangsu, China) | 100–300 | Doxorubicin, pirarubicin, oxaliplatin | PC/H | No | [37][38][39][40][41] |
| Tris acryl gelatin microspheres (TAGM) | Embosphere (Merit Medical Systems, South Jor-dan, UT, USA), Embozene®, and Oncozene™ (Varian Medical Systems, Palo Alto, CA, USA) |
40–120, 100–300 | Doxorubicin and Irinotecan | H (FDA) | No | [13][36][42][43] |
| Poly-lactide-co-glycolide (PLGA) | Dexon®, Vicryl®, PerserisTM, Indivior (Indivior Inc. North Chesterfiled, VI, USA), Risperdal Consta® | 20–100 | Mitomycin, doxorubicin, irinotecan, sunitinib, cisplatin | PC/H (FDA) | Yes | [44][45][46][47] |
| Albumin microspheres | Nab-paclitaxel | 10–220 | Mitomycin C, doxorubicin, paclitaxel | PC/H (FDA) | Yes | [48][49] |
After contact with either an ionized environment, such as 0.9% NaCl and blood, or nonionic contrast media, acrylic co-polymer microspheres expand to 200–400 µm and slowly release their cytostatic payload [50]. The advantage of TACE is that it maximizes the concentration of chemotherapeutic agents within the tumor for up to seven days while keeping a minimal concentration in the systemic circulation. This approach reduces systemic side effects and the toxicity of cytostatic drugs compared to systemic chemotherapy. Furthermore, the malignancy’s arterial supply is occluded like in TAE, thus limiting nutrient availability to the tumor [51][52]. In HCC patients [5], this chemoembolization strategy has proven beneficial for patients’ survival, increasing the survival time by up to 12 months [53] and reducing the symptoms related to chemotherapy [28].
3.2. Trans-Arterial Radioembolization (TARE)
Trans-arterial radioembolization (TARE) uses radioisotopes embedded in microspheres to locally irradiate tissue after the vascular occlusion of blood vessels surrounding the tumor [54]. Herein, microspheres carrying b-emitting radioisotopes enable a more pinpointed delivery of radiation to liver tumors than other radiotherapy techniques. Although these options extend patient survival, most remain palliative [10][53]. With 166Ho-microspheres and 3.8 GBq/kg liver tissue in a Phase II study including 38 patients, the target lesions showed complete response or stabilized disease for 27 patients (73%), with a median survival of 15 months [55]. Given these results, a more extensive, randomized Phase III study appears to be required. Although 90Y, 188Re, and 166Ho in microspheres effectively reduce tumor size and patients’ survival, data from large Phase III trials are warranted to prove their benefits compared to other treatment modalities. In addition, the cost-effectiveness between the various radioisotopes and types of microspheres has to be determined. Besides the application of TARE in HCC, patients with intrahepatic cholangiocarcinoma (CC), which is a rare but very aggressive neoplasia with limited therapeutic options, and patients with to liver metastasized colorectal (mCRC) and neuroendocrine disease also benefit from therapy using 90Y-loaded glass or resin microspheres, with a response of more prolonged overall survival of at least 6 months [56][57][58][59]. Various radioembolization microspheres carrying radioisotopes to be delivered in the tumor-bearing hepatic segments of patients are summarized in Table 4.
Table 4. Microspheres used for trans-arterial radioembolization (TARE). PC = pre-clinical use, H = human use, FDA = FDA approved as an active implantable medical device.
| Microspheres Composition |
Product Name | Particle Size Range (⌀ µm) | Radioisotope Load | Pre-Clinical/ Human Use (FDA Clearance) |
Biodegradable | References |
|---|---|---|---|---|---|---|
| Glass | Lipiocis, TheraSphere® (Boston Scientific, Marlborough, MA, USA) | 50–150, 20–30, 25–32 | 32P, 90Y, 177lu, 186Re, 188Re | PC/H (FDA for 90Y, 186Re, and 188Re) | No | [60][61][62][63][64][65] |
| Resin | SIR-Spheres® (Sirtex Medical Inc. Woburn, MA, USA), Amberlite IR-120 (Thermo Fisher Scientific, Landau, Germany) |
20–60 | 90Y, 153Sm | PC/H (FDA for 90Y) | No | [66][67][68][69] |
| Polyhydroxyamic acid polyacrylamide (PHA) | Experimental | 54 | 177lu, 131I | PC | No | [70][71][72] |
| Styrene divinylbenzene | Amberlite IR-120 (Thermo Fisher Scientific, Landau, Germany) | 20–40 | 152Sm | PC | No | [69][73] |
| Poly- DL-lactic acid-co-glycolic acid (PLGA) | YPO4 crystalline particles Radiogel® (Vivos Inc., Richland, WA, USA) | 0.5–2 | 90Y | PC (FDA-approved as a medical device) | Yes | [74] |
| Poly (L-lactic acid) PLLA | Resomer® L104 (Merck, Darmstad, Germany) | 10–45, 20–40 | 188Re/166Ho/175Yb | PC/C | Yes | [75][76][77][78][79][80][81] |
| Poly (glycidyl methacrylate-co-ethylene dimethacrylate & Quinoline-8-ol | G-Gel (Merck, Darmstad, Germany) | 20–40 | 131I, 177lu | PC | No | [66][70][71][72][82] |
| Hydroxyapatite | QuiremSpheres (Quirem Medical, Deventer, The Netherlands) |
20–60 | 166Ho | PC | No | [78][83][84] |
| Albumin | HSA-B20 (Rotop Pharmaka, Dresden, Germany) Vasculosis® (Global Medical Solutions, Auckland, New Zealand) MAA (DRAXIMAGE®, Kirkland, QC, Canada), Pulmocis® (Curium, London, UK) |
25–35, 15–37 | 90Y, 186Re, 188Re | PC/C | Yes | [63][64][65][85][86][87] |
| Chitosan | Millican (Dong Wha Pharmaceutical Co., Soeul, South Korea) | 5–20 | 166Ho | PC/C | Yes | [75][76][77][78][79][88][89][90] |
| Starch-based microparticles (SBMP) | Experimental Kit | 18–42 | 188Re | PC | No | [91][92][93] |
An essential issue in radioembolization studies is preventing shunting to normal tissues, such as the lungs. Shunting displaces a fraction of the administered particles towards the microvasculature of other tissues, mainly the lung, instead of the liver, leading to ineffective dose distribution and irreversible severe adverse effects such as radiation pneumonitis [88][94]. To avoid the shunting of microspheres, radioembolization is performed in a theranostic setting. In this setting, a catheter is (selectively) placed to deliver radiolabeled macro-aggregated albumin (99mTc-MAA; ⌀ 10–40 mm) to the affected tissues. Via a scout scan using single-positron emission computed tomography (SPECT) imaging, the localization and distribution of the 99mTc-MAA are visualized, which is an approach that helps assess the degree of shunting and, at the same time, facilitates dosimetry measurements. When this has been done, therapeutic loads of β-emitting glass or resin microparticles (⌀ 15–25 mm) [95] containing, e.g., 90Y (SIR-Spheres®, Sirtex Medical Inc. Woburn, MA, USA; Therasphere, Boston Scientific, Marlborough, MA, USA) or 166Ho (QuiremSpheres, Quirem Medical Deventer, The Netherlands) are injected via a catheter positioned in the same way [96]. Given the overlap in size and retention properties between 99mTc-MAA and microparticles, a 99mTc-MAA scout scan has been deemed a sufficient standard requirement in the clinical guidelines to predict the accurate delivery of therapeutic microspheres. Despite these guidelines, a mismatch between the scout and therapeutic is inevitable, i.e., given the time span that separates these two procedures. As such, the delivery of microspheres can still lead to adverse side effects and suboptimal dose delivery in about 30% of cases [97][98][99], a complication which highlights the need for innovative solutions that help refine the correlation between the scout scan and therapeutic delivery. In this respect, the physical properties of the used radioisotope, 199Ho as a b/g/paramagnetic element [100] and 90Y as a PET/SPECT imaging agent, post-TARE imaging facilitates mapping of the dose delivery. Where the therapy is insufficient, adjuvant therapy can be considered [101][102]. Furthermore, microspheres containing these isotopes can also serve as a scout scan. Another drawback is the delay of 2 weeks between the execution of the scout scan and the therapeutic intervention due to the need for dosimetry [103][104][105] and the production/delivery time of the β-emitting microspheres [96].
3.3. TACE vs. TARE
Instead of focusing on major vascular occlusion with TAE, researchers focus on comparing chemo- or radio-embolic targeting that has the potential to facilitate vascular occlusion and realize chemo- or radio-embolic treatment of hepatic tumors [18].
Four TARE studies determined the overall survival median at 9–11 months [50]. Based on these findings, TARE was not recommended as a first-line therapy for patients with non-resectable colorectal liver metastasis. For HCC, however, the overall survival in a study with unresectable HCC patients was 19.9 months in the 90Y-resin TARE group, which was an improvement compared to the 14 months of survival in a matching TACE group [106]. Recently, the efficacy of TARE combined with TACE was determined in 19 patients with bi-lobar HCC, and no procedure-related major clinical complications were observed, and the mean overall survival yielded a promising 27.3 months compared to untreated patients [107]. Differences between studies comparing TACE and TARE indicate that the outcome in the benefits of treatment may be related to the type of carcinoma, an observation that needs additional research. TARE also proved superior in safety regarding post-embolization syndrome, hospitalization days, and outpatient-based therapy [108][109]. TARE was a safe alternative treatment to TACE [110], especially as using a scout scan helps prevent complications related to shunting with TACE [111]. Applying the scout scan also helps personalize the dosing, a concept that could extend to TACE. Compared to TACE, TARE had a longer time-to-progression, greater ability to downsize tumors, and less post-embolization syndrome [112]. For that reason, it could be an alternative to ablation, surgical resection, or portal vein embolization [113]. On the other hand, TACE is the trans-arterial treatment of choice for patients with marginal hepatic reserve (i.e., hyperbilirubinemia, ascites) or candidates for transplantation [114].
References
- Pérez-López, A.; Martín-Sabroso, C.; Gómez-Lázaro, L.; Torres-Suárez, A.I.; Aparicio-Blanco, J. Embolization therapy with microspheres for the treatment of liver cancer: State-of-the-art of clinical translation. Acta Biomater. 2022, 149, 1–15.
- Marchal, S.; El Hor, A.; Millard, M.; Gillon, V.; Bezdetnaya, L. Anticancer drug delivery: An update on clinically applied nanotherapeutics. Drugs 2015, 75, 1601–1611.
- Lorscheider, M.; Gaudin, A.; Nakhlé, J.; Veiman, K.L.; Richard, J.; Chassaing, C. Challenges and opportunities in the delivery of cancer therapeutics: Update on recent progress. Ther. Deliv. 2021, 12, 55–76.
- Gritzapis, A.D.; Mahaira, L.G.; Perez, S.A.; Cacoullos, N.T.; Papamichail, M.; Baxevanis, C.N. Vaccination with Human HER-2/neu (435-443) CTL Peptide Induces Effective Antitumor Immunity against HER-2/neu-Expressing Tumor Cells In vivo. Cancer Res. 2006, 66, 5452–5460.
- Rajput, M.; Agrawal, P. Microspheres in cancer therapy. Indian J. Cancer 2010, 47, 458–468.
- Sinha, V.R.; Goyal, V.; Bhinge, J.R.; Mittal, B.R.; Trehan, A. Diagnostic microspheres: An overview. Crit. Rev. Drug Carr. Syst. 2003, 20, 431–460.
- Klibanov, A.L. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv. Drug Deliv. Rev. 1999, 37, 139–157.
- Laeschke, K. Biocompatibility of microparticles into soft tissue fillers. Semin. Cutan Med. Surg. 2004, 23, 214–217.
- Varde, N.K.; Pack, D.W. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 2004, 4, 35–51.
- Niu, H.; Du, T.; Xiao, Q.; Hu, X.; Li, D.; Wang, C.; Gao, W.; Xing, T.; Xu, X. Application of embolization microspheres in interventional therapy of malignant non-hypervascular tumor of liver. Oncotarget 2017, 8, 55593–55599.
- Osuga, K.; Nakajima, Y.; Sone, M.; Arai, Y.; Nambu, Y.; Hori, S. Transarterial embolization of hypervascular tumors using trisacryl gelatin microspheres (Embosphere): A prospective multicenter clinical trial in Japan. Jpn. J. Radiol. 2016, 34, 366–375.
- Vaidya, S.; Tozer, K.R.; Chen, J. An overview of embolic agents. Semin. Interv. Radiol. 2008, 25, 204–215.
- Granberg, D.; Eriksson, L.G.; Welin, S.; Kindmark, H.; Janson, E.T.; Skogseid, B.; Oberg, K.; Eriksson, B.; Nyman, R. Liver embolization with trisacryl gelatin microspheres (embosphere) in patients with neuroendocrine tumors. Acta Radiol. 2007, 48, 180–185.
- Hiraki, T.; Koizumi, J.; Arai, Y.; Sakurai, Y.; Kumada, H.; Nambu, Y.; Hori, S. Transcatheter arterial embolization of hypervascular tumors with HepaSphere: Prospective multicenter open label clinical trial of microspheres in Japan. Jpn. J. Radiol. 2015, 33, 479–486.
- Kettenbach, J.; Stadler, A.; Katzler, I.V.; Schernthaner, R.; Blum, M.; Lammer, J.; Rand, T. Drug-loaded microspheres for the treatment of liver cancer: Review of current results. Cardiovasc. Interv. Radiol. 2008, 31, 468–476.
- Caine, M.; Carugo, D.; Zhang, X.; Hill, M.; Dreher, M.R.; Lewis, A.L. Review of the development of methods for characterization of microspheres for use in embolotherapy: Translating bench to cathlab. Adv. Healthc. Mater. 2017, 6, 1601291.
- Burrill, J.; Hafeli, U.; Liu, D. Advances in radioembolization—Embolics and isotopes. J. Nucl. Med. Radiat. Ther. 2011, 2, 1000107.
- Duran, R.; Chapiro, J.; Schernthaner, R.E.; Geschwind, J.F. Systematic review of catheter-based intra-arterial therapies in hepatocellular carcinoma: State of the art and future directions. Br. J. Radiol. 2015, 88, 20140564.
- Janevska, D.; Chaloska-Ivanova, V.; Janevski, V. Hepatocellular carcinoma: Risk factors, diagnosis and treatment. Open Access Maced. J. Med. Sci. 2015, 3, 732–736.
- Coletta, M.; Nicolini, D.; Benedetti Cacciaguerra, A.; Mazzocato, S.; Rossi, R.; Vivarelli, M. Bridging patients with hepatocellular cancer waiting for liver transplant: All the patients are the same? Transl. Gastroenterol. Hepatol. 2017, 2, 78.
- Li, D.; Kang, J.; Golas, B.J.; Yeung, V.W.; Madoff, D.C. Minimally invasive local therapies for liver cancer. Cancer Biol. Med. 2014, 11, 217–236.
- Freedman, J.; Nilsson, H.; Jonas, E. New horizons in ablation therapy for hepatocellular carcinoma. Hepat. Oncol. 2015, 2, 349–358.
- Saito, R.; Amemiya, H.; Hosomura, N.; Kawaida, H.; Shoda, K.; Furuya, S.; Akaike, H.; Kawaguchi, Y.; Inoue, S.; Kono, H.; et al. Intended preoperative trans-arterial embolization for large hepatocellular carcinoma: A retrospective cohort study. World J. Surg. Oncol. 2022, 20, 90.
- Shimohira, M.; Sato, Y.; Yasumoto, T.; Kodama, Y.; Masada, T.; Inaba, Y.; Yamakado, K. Arterial embolization using microspheres for hypervascular liver metastases refractory to standard treatments: A multicenter prospective clinical trial. Cardiovasc. Interv. Radiol. 2021, 44, 392–400.
- Laurent, A. Microspheres and Nonspherical Particles for Embolization. Tech. Vasc. Interv. Radiol. 2007, 10, 248–256.
- Lee, K.H.; Liapi, E.A.; Cornell, C.; Reb, P.; Buijs, M.; Vossen, J.A.; Ventura, V.P.; Geschwind, J.F. Doxorubicin-loaded QuadraSphere microspheres: Plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc. Interv. Radiol. 2010, 33, 576–582.
- Hecq, J.D.; Lewis, A.L.; Vanbeckbergen, D.; Athanosopoulos, A.; Galanti, L.; Jamart, J.; Czuczman, P.; Chung, T. Doxorubicin-loaded drug-eluting beads (DC Bead®) for use in transarterial chemoembolization: A stability assessment. J. Oncol. Pharm. Pract. 2013, 19, 65–74.
- Lewis, A.L.; Gonzalez, M.V.; Lloyd, A.W.; Hall, B.; Tang, Y.; Willis, S.L.; Leppard, S.W.; Wolfenden, L.C.; Palmer, R.R.; Stratford, P.W.; et al. DC bead: In vitro characterization of a drug-delivery device for transarterial chemoembolization. J. Vasc. Interv. Radiol. 2006, 17, 335–342.
- Wang, Y.; Molin, D.G.M.; Sevrin, C.; Grandfils, C.; van den Akker, N.M.S.; Gagliardi, M.; Knetsch, M.L.; Delhaas, T.; Koole, L.H. In vitro and in vivo evaluation of drug-eluting microspheres designed for transarterial chemoembolization therapy. Int. J. Pharm. 2016, 503, 150–162.
- Liu, Y.S.; Lin, C.Y.; Chuang, M.T.; Lin, C.Y.; Tsai, Y.S.; Wang, C.K.; Ou, M.C. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 2018, 18, 124.
- Ni, J.Y.; Xu, L.F.; Wang, W.D.; Sun, H.L.; Chen, Y.T. Conventional transarterial chemoembolization vs microsphere embolization in hepatocellular carcinoma: A meta-analysis. World J. Gastroenterol. 2014, 20, 17206–17217.
- De Luis, E.; Bilbao, J.I.; de Ciercoles, J.A.; Martinez-Cuesta, A.; de Martino Rodriguez, A.; Lozano, M.D. In vivo evaluation of a new embolic spherical particle (HepaSphere) in a kidney animal model. Cardiovasc. Interv. Radiol. 2008, 31, 367–376.
- Kennoki, N.; Hori, S.; Hori, A.; Takeo, Y.; Oshiro, H. Transcatheter arterial chemoembolization with spherical embolic material for locally advanced breast cancer: First report of HepaSphere treatment for primary breast cancer. BJR Case Rep. 2016, 2, 20150417.
- Sottani, C.; Leoni, E.; Porro, B.; Montagna, B.; Amatu, A.; Sottotetti, F.; Quaretti, P.; Poggi, G.; Minoia, C. Validation of an LC-MS/MS method for the determination of epirubicin in human serum of patients undergoing drug eluting microsphere-transarterial chemoembolization (DEM-TACE). J. Chromatogr. B 2009, 877, 3543–3548.
- Poggi, G.; Quaretti, P.; Minoia, C.; Bernardo, G.; Bonora, M.R.; Gaggeri, R.; Ronchi, A.; Saluzzo, C.M.; Azzaretti, A.; Rodolico, G.; et al. Transhepatic arterial chemoembolization with oxaliplatin-eluting microspheres (OEM-TACE) for unresectable hepatic tumors. Anticancer Res. 2008, 28, 3835–3842.
- Poursaid, A.; Jensen, M.M.; Huo, E.; Ghandehari, H. Polymeric materials for embolic and chemoembolic applications. J. Control. Release 2016, 240, 414–433.
- Duan, X.H.; Li, H.; Ren, J.Z.; Han, X.W.; Chen, P.F.; Li, F.Y.; Huang, G.H.; Ju, S.G. Hepatic Arterial Chemoembolization With Arsenic Trioxide Eluting CalliSpheres Microspheres Versus Lipiodol Emulsion: Pharmacokinetics And Intratumoral Concentration In A Rabbit Liver Tumor Model. Cancer Manag. Res. 2019, 11, 9979–9988.
- Amrein, M.L.; Soong, C.; Liang, N. Upregulated Membrane Expression of a Conserved Voltage—Gated Sodium Channel, Nav1.4a, and Electrical Organ Discharge in Electric Mouse, P. pikachu. PLoS Biol. 2013, 11, 1001501.
- Wu, B.; Zhou, J.; Ling, G.; Zhu, D.; Long, Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: A short-term efficacy and safety study. World J. Surg. Oncol. 2018, 16, 69.
- Ma, Y.; Zhao, C.; Zhao, H.; Li, H.; Chen, C.; Xiang, H.; Zheng, C.; Ma, C.; Luo, C.; Qiu, H.; et al. Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres® microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients. Am. J. Transl. Res. 2019, 11, 7456–7470.
- Zhang, S.; Huang, C.; Li, Z.; Yang, Y.; Bao, T.; Chen, H.; Zou, Y.; Song, L. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 2017, 24, 1011–1017.
- Pelage, J.P.; Fohlen, A.; Mitry, E.; Lagrange, C.; Beauchet, A.; Rougier, P. Chemoembolization of neuroendocrine liver metastases using streptozocin and tris-acryl microspheres: Embozar (EMBOsphere + ZAnosaR) study. Cardiovasc. Interv. Radiol. 2017, 40, 394–400.
- Beaujeux, R.; Laurent, A.; Wassef, M.; Casasco, A.; Gobin, Y.P.; Aymard, A.; Rufenacht, D.; Merland, J.J. Trisacryl gelatin microspheres for therapeutic embolization, II: Preliminary clinical evaluation in tumors and arteriovenous malformations. Am. J. Neuroradiol. 1996, 17, 541–548.
- Qian, J.; Truebenbach, J.; Graepler, F.; Pereira, P.; Huppert, P.; Eul, T.; Wiemann, G.; Claussen, C. Application of poly-lactide-co-glycolide-microspheres in the transarterial chemoembolization in an animal model of hepatocellular carcinoma. World J. Gastroenterol. 2003, 9, 94.
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397.
- Blasi, P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346.
- Fuchs, K.; Duran, R.; Denys, A.; Bize, P.E.; Borchard, G.; Jordan, O. Drug-eluting embolic microspheres for local drug delivery-State of the art. J. Control. Release 2017, 262, 127–138.
- Fujimoto, S.; Miyazaki, M.; Endoh, F.; Takahashi, O.; Okui, K.; Morimoto, Y. Biodegradable mitomycin C microspheres given intra-arterially for inoperable hepatic cancer. With particular reference to a comparison with continuous infusion of mitomycin C and 5-fluorouracil. Cancer 1985, 56, 2404–2410.
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623.
- Tsitskari, M.; Filippiadis, D.; Kostantos, C.; Palialexis, K.; Zavridis, P.; Kelekis, N.; Brountzos, E. The role of interventional oncology in the treatment of colorectal cancer liver metastases. Ann. Gastroenterol. 2019, 32, 147–155.
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255.
- Kloeckner, R.; Weinmann, A.; Prinz, F.; Pinto dos Santos, D.; Ruckes, C.; Dueber, C.; Pitton, M.B. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015, 15, 465.
- Sangro, B.; Carpanese, L.; Cianni, R.; Golfieri, R.; Gasparini, D.; Ezziddin, S.; Paprottka, P.M.; Fiore, F.; van Buskirk, M.; Ignacio Bilbao, J.; et al. Survival after Yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology 2011, 54, 868–878.
- Bouvry, C.; Palard, X.; Edeline, J.; Ardisson, V.; Loyer, P.; Garin, E.; Lepareur, N. Transarterial radioembolization (TARE) agents beyond (90)Y-microspheres. Biomed. Res. Int. 2018, 2018, 1435302.
- Prince, J.F.; van den Bosch, M.A.A.J.; Nijsen, J.F.W.; Smits, M.L.J.; van den Hoven, A.F.; Nikolakopoulos, S.; Wessels, F.J.; Bruijnen, R.C.G.; Braat, M.N.G.J.A.; Zonnenberg, B.A.; et al. Efficacy of Radioembolization with166Ho-Microspheres in Salvage Patients with Liver Metastases: A Phase 2 Study. J. Nucl. Med. 2018, 59, 582–588.
- Filippi, L.; Schillaci, O.; Cianni, R.; Bagni, O. Yttrium-90 resin microspheres and their use in the treatment of intrahepatic cholangiocarcinoma. Future Oncol. 2018, 14, 809–818.
- Robinson, T.J.; Du, L.; Matsuoka, L.; Sze, D.Y.; Kennedy, A.S.; Gandhi, R.T.; Kouri, B.E.; Collins, Z.S.; Kokabi, N.; Grilli, C.J.; et al. Survival and toxicities after Yttrium-90 transarterial radioembolization of Cholangiocarcinoma in the RESiN registry. J. Vasc. Interv. Radiol. 2022, in press.
- Bargellini, I.; Bozzi, E.; Lorenzoni, G.; Boni, G.; Bianchi, F.; Traino, C.A.; Masi, G.; Cioni, R.; Crocetti, L. Role of Transhepatic Arterial Radioembolization in Metastatic Colorectal Cancer. Cardiovasc. Interv. Radiol. 2022, 45, 1579–1589.
- Ingenerf, M.K.; Karim, H.; Fink, N.; Ilhan, H.; Ricke, J.; Treitl, K.M.; Schmid-Tannwald, C. Apparent diffusion coefficients (ADC) in response assessment of transarterial radioembolization (TARE) for liver metastases of neuroendocrine tumors (NET): A feasibility study. Acta Radiol. 2022, 63, 877–888.
- Raoul, J.L.; Guyader, D.; Bretagne, J.F.; Heautot, J.F.; Duvauferrier, R.; Bourguet, P.; Bekhechi, D.; Deugnier, Y.M.; Gosselin, M. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 1997, 26, 1156–1161.
- Pirayesh, E.; Amoui, M.; Akhlaghpoor, S.; Tolooee, S.; Khorrami, M.; Poorbeigi, H.; Sheibani, S.; Assadi, M. Technical considerations of phosphorous-32 Bremsstrahlung SPECT imaging after radioembolization of hepatic tumors: A clinical assessment with a review of imaging parameters. Radiol. Res. Pract. 2014, 2014, 407158.
- Raoul, J.L.; Duvauferrier, R.; Bourguet, P.; Bretagne, J.F.; Coornaert, S.; Darnault, P.; Deugnier, Y.; Herry, J.Y.; Gastard, J. Lipiodolized angiography in hepatocellular carcinomas. Contribution of iodine-131-labelled lipiodol. J. Radiol. 1986, 67, 797–801.
- Hafeli, U.O.; Casillas, S.; Dietz, D.W.; Pauer, G.J.; Rybicki, L.A.; Conzone, S.D.; Day, D.E. Hepatic tumor radioembolization in a rat model using radioactive rhenium (186Re/188Re) glass microspheres. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 189–199.
- Lepareur, N.; Lacoeuille, F.; Bouvry, C.; Hindre, F.; Garcion, E.; Cherel, M.; Noiret, N.; Garin, E.; Knapp, F.F.R., Jr. Rhenium-188 labeled radiopharmaceuticals: Current clinical applications in oncology and promising perspectives. Front. Med. 2019, 6, 00132.
- Liepe, K.; Brogsitter, C.; Leonhard, J.; Wunderlich, G.; Hliscs, R.; Pinkert, J.; Folprecht, G.; Kotzerke, J. Feasibility of high activity rhenium-188-microsphere in hepatic radioembolization. Jpn. J. Clin. Oncol. 2007, 37, 942–950.
- Poorbaygi, H.; Aghamiri, S.M.R.; Sheibani, S.; Kamali-asl, A.; Mohagheghpoor, E. Production of glass microspheres comprising 90Y and 177Lu for treating of hepatic tumors with SPECT imaging capabilities. Appl. Radiat. Isot. 2011, 69, 1407–1414.
- Akram, A.R.; Avlonitis, N.; Scholefield, E.; Vendrell, M.; McDonald, N.; Aslam, T.; Craven, T.H.; Gray, C.; Collie, D.S.; Fisher, A.J.; et al. Enhanced avidity from a multivalent fluorescent antimicrobial peptide enables pathogen detection in a human lung model. Sci. Rep. 2019, 9, 8422.
- Vukadinović, V.; Janković, D.; Radović, M.; Milanović, Z.; Mirković, M.; Stanković, D.; Vranješ-Đurić, S. Optimization of the radiolabelling method for improved in vitro and in vivo stability of 90Y-albumin microspheres. Appl. Radiat. Isot. 2020, 156, 108984.
- Hashikin, N.A.A.; Yeong, C.-H.; Abdullah, B.J.J.; Ng, K.-H.; Chung, L.-Y.; Dahalan, R.; Perkins, A.C. Neutron activated samarium-153 microparticles for transarterial radioembolization of liver tumour with post-procedure imaging capabilities. PLoS ONE 2015, 10, 0138106.
- Hruby, M.; Skodova, M.; Mackova, H.; Skopal, J.; Tomes, M.; Kropacek, M.; Zimova, J.; Kucka, J. Lutetium-177 and iodine-131 loaded chelating polymer microparticles intended for radioembolization of liver malignancies. React. Funct. Polym. 2011, 71, 1155–1159.
- Pandey, U.; Subramanian, S.; Shaikh, S.; Gamre, N.; Kumar, S.; Dash, A. Synthesis and preliminary biological evaluation of 177Lu-labeled polyhydroxamic acid microparticles toward therapy of hepatocellular carcinoma. Cancer Biother. Radiopharm. 2019, 34, 306–315.
- Saxena, S.K.; Kumar, Y.; Shaikh, S.H.; Pandey, U.; Kumar, S.A.; Dash, A. Preparation of radioactive skin patches using polyhydroxamic acid-grafted cellulose films toward applications in treatment of superficial tumors. Cancer Biother. Radiopharm. 2017, 32, 364–370.
- Wong, Y.H.; Tan, H.Y.; Kasbollah, A.; Abdullah, B.J.J.; Yeong, C.H. Preparation and in vitro evaluation of neutron-activated, theranostic samarium-153-labeled microspheres for transarterial radioembolization of hepatocellular carcinoma and liver metastasis. Pharmaceutics 2019, 11, 596.
- Fisher, D.; Fidel, J.; Maitz, C. Direct interstitial treatment of solid tumors using an injectable yttrium-90-polymer composite. Cancer Biother. Radiopharm. 2020, 35, 2947.
- Mumper, R.J.; Ryo, U.Y.; Jay, M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: A potential agent for the internal radiation therapy of hepatic tumors. J. Nucl. Med. 1991, 32, 2139–2143.
- Vente, M.A.; Nijsen, J.F.; de Roos, R.; van Steenbergen, M.J.; Kaaijk, C.N.; Koster-Ammerlaan, M.J.; de Leege, P.F.; Hennink, W.E.; van Het Schip, A.D.; Krijger, G.C. Neutron activation of holmium poly(L-lactic acid) microspheres for hepatic arterial radio-embolization: A validation study. Biomed. Microdevices 2009, 11, 763–772.
- Mumper, R.J.; Jay, M. Biodegradable radiotherapeutic polyester microspheres: Optimization and in-vitro/in-vivo evaluation. J. Control. Release 1992, 18, 193–203.
- Das, T.; Chakraborty, S.; Sarma, H.D.; Venkatesh, M.; Banerjee, S. 166Ho-labeled hydroxyapatite particles: A possible agent for liver cancer therapy. Cancer Biother. Radiopharm. 2009, 24, 7–14.
- Kim, J.K.; Han, K.H.; Lee, J.T.; Paik, Y.H.; Ahn, S.H.; Lee, J.D.; Lee, K.S.; Chon, C.Y.; Moon, Y.M. Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (Milican) for the treatment of small hepatocellular carcinoma. Clin. Cancer Res. 2006, 12, 543–548.
- De la Vega, J.C.; Esquinas, P.L.; Rodríguez-Rodríguez, C.; Bokharaei, M.; Moskalev, I.; Liu, D.; Saatchi, K.; Häfeli, U.O. Radioembolization of hepatocellular carcinoma with built-in dosimetry: First in vivo results with uniformly-sized, biodegradable microspheres labeled with188re. Theranostics 2019, 9, 868–883.
- Jamre, M.; Shamsaei, M.; Erfani, M.; Sadjadi, S.; Maragheh, M.G. Preparation and evaluation of 188Re sulfide colloidal nanoparticles loaded biodegradable poly (L-lactic acid) microspheres for radioembolization therapy. J. Label. Compd. Radiopharm. 2018, 61, 586–594.
- Hrubý, M.; Hradil, J.; Beneš, M.J. Interactions of phenols with silver(I), copper(II) and iron(III) complexes of chelating methacrylate-based polymeric sorbent containing quinolin-8-ol groups. React. Funct. Polym. 2004, 59, 105–118.
- Chinol, M.; Vallabhajosula, S.; Goldsmith, S.J.; Klein, M.J.; Deutsch, K.F.; Chinen, L.K.; Brodack, J.W.; Deutsch, E.A.; Watson, B.A.; Tofe, A.J.; et al. Chemistry and biological behavior of samarium-153 and rhenium-186-labeled hydroxyapatite particles: Potential radiopharmaceuticals for radiation synovectomy. J. Nucl. Med. 1993, 34, 1536–1542.
- Unni, P.R.; Chaudhari, P.R.; Venkatesh, M.; Ramamoorthy, N.; Pillai, M.R. Preparation and bioevaluation of 166Ho labelled hydroxyapatite (HA) particles for radiosynovectomy. Nucl. Med. Biol. 2002, 29, 199–209.
- Nowicki, M.L.; Cwikla, J.B.; Sankowski, A.J.; Shcherbinin, S.; Grimmes, J.; Celler, A.; Buscombe, J.R.; Bator, A.; Pech, M.; Mikolajczak, R.; et al. Initial study of radiological and clinical efficacy radioembolization using 188Re-human serum albumin (HSA) microspheres in patients with progressive, unresectable primary or secondary liver cancers. Med. Sci. Monit. 2014, 20, 1353–1362.
- Bhushan, B.; Khanadeev, V.; Khlebtsov, B.; Khlebtsov, N.; Gopinath, P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv. Colloid Interface Sci. 2017, 246, 13–39.
- Chen, L.-C.; Lee, W.-C.; Ho, C.-L.; Chang, Y.-J.; Chen, S.-J.; Chang, C.-H. Biodistribution, pharmacokinetics and efficacy of (188)re(i)-tricarbonyl-labeled human serum albumin microspheres in an orthotopic hepatoma rat model. In Vivo 2018, 32, 567–573.
- Cremonesi, M.; Chiesa, C.; Strigari, L.; Ferrari, M.; Botta, F.; Guerriero, F.; de Cicco, C.; Bonomo, G.; Orsi, F.; Bodei, L.; et al. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front. Oncol. 2014, 4, 210.
- Memon, K.; Lewandowski, R.J.; Kulik, L.; Riaz, A.; Mulcahy, M.F.; Salem, R. Radioembolization for primary and metastatic liver cancer. Semin. Radiat. Oncol. 2011, 21, 294–302.
- Sohn, J.H.; Choi, H.J.; Lee, J.T.; Lee, J.D.; Kim, J.H.; Moon, Y.M.; Park, K.; Park, K.B.; Kim, E.; Yoo, N.C.; et al. Phase II study of transarterial holmium-166-chitosan complex treatment in patients with a single, large hepatocellular carcinoma. Oncology 2009, 76, 1–9.
- Lacoeuille, F.; Hindré, F.; Denizot, B.; Bouchet, F.; Legras, P.; Couturier, O.; Askiénazy, S.; Benoit, J.P.; le Jeune, J.J. New starch-based radiotracer for lung perfusion scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 146.
- Lacoeuille, F.; Hindré, F.; Venier-Julienne, M.C.; Sergent, M.; Bouchet, F.; Jouaneton, S.; Denizot, B.; Askienazy, S.; Benoit, J.P.; Couturier, O.F.; et al. A starch-based microparticulate system dedicated to diagnostic and therapeutic nuclear medicine applications. Biomaterials 2011, 32, 7999–8009.
- Verger, E.; Drion, P.; Meffre, G.; Bernard, C.; Duwez, L.; Lepareur, N.; Couturier, O.; Hindré, F.; Hustinx, R.; Lacoeuille, F.; et al. 68Ga and 188Re starch-based microparticles as theranostic tool for the hepatocellular carcinoma: Radiolabeling and preliminary in vivo rat studies. PLoS ONE 2016, 11, e0164626.
- Braat, A.J.A.T.; Smits, M.L.J.; Braat, M.N.G.J.A.; van den Hoven, A.F.; Prince, J.F.; de Jong, H.W.A.M.; van den Bosch, M.A.A.J.; Lam, M.G.E.H. 90Y hepatic radioembolization: An update on current practice and recent developments. J. Nucl. Med. 2015, 56, 1079–1087.
- Salem, R.; Lewandowski, R.J.; Gates, V.L.; Nutting, C.W.; Murthy, R.; Rose, S.C.; Soulen, M.C.; Geschwind, J.-F.H.; Kulik, L.; Kim, Y.H.; et al. Research reporting standards for radioembolization of hepatic malignancies. J. Vasc. Interv. Radiol. 2011, 22, 265–278.
- Hickey, R.; Lewandowski, R.J.; Prudhomme, T.; Ehrenwald, E.; Baigorri, B.; Critchfield, J.; Kallini, J.; Gabr, A.; Gorodetski, B.; Geschwind, J.-F.; et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: Safety and survival outcomes from a 531-patient multicenter study. J. Nucl. Med. 2016, 57, 665–671.
- Uliel, L.; Royal, H.D.; Darcy, M.D.; Zuckerman, D.A.; Sharma, A.; Saad, N.E. From the angio suite to the γ-camera: Vascular mapping and 99mTc-MAA hepatic perfusion imaging before liver radioembolization—A comprehensive pictorial review. J. Nucl. Med. 2012, 53, 1736–1747.
- Riaz, A.; Awais, R.; Salem, R. Side effects of Yttrium-90 radioembolization. Front. Oncol. 2014, 4, 00198.
- Xing, M.; Lahti, S.; Kokabi, N.; Schuster, D.M.; Camacho, J.C.; Kim, H.S. 90Y Radioembolization lung shunt fraction in primary and metastatic liver cancer as a biomarker for survival. Clin. Nucl. Med. 2016, 41, 21–27.
- Stella, M.; Braat, A.; van Rooij, R.; de Jong, H.; Lam, M. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardiovasc. Interv. Radiol. 2022, 45, 1634–1645.
- Deidda, D.; Denis-Bacelar, A.M.; Fenwick, A.J.; Ferreira, K.M.; Heetun, W.; Hutton, B.F.; Robinson, A.P.; Scuffham, J.; Thielemans, K. Hybrid kernelised expectation maximisation for Bremsstrahlung SPECT reconstruction in SIRT with (90)Y micro-spheres. EJNMMI Phys. 2022, 9, 25.
- D’Arienzo, M.; Pimpinella, M.; Capogni, M.; de Coste, V.; Filippi, L.; Spezi, E.; Patterson, N.; Mariotti, F.; Ferrari, P.; Chiaramida, P.; et al. Phantom validation of quantitative Y-90 PET/CT-based dosimetry in liver radioembolization. EJNMMI Res. 2017, 7, 94.
- Ilhan, H.; Goritschan, A.; Paprottka, P.; Jakobs, T.F.; Fendler, W.P.; Todica, A.; Bartenstein, P.; Hacker, M.; Haug, A.R. Predictive value of 99mTc-MAA SPECT for 90Y-labeled resin microsphere distribution in radioembolization of primary and secondary hepatic tumors. J. Nucl. Med. 2015, 56, 1654–1660.
- Bult, W.; Vente, M.A.; Zonnenberg, B.A.; van Het Schip, A.D.; Nijsen, J.F. Microsphere radioembolization of liver malignancies: Current developments. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 325–335.
- Elschot, M.; Nijsen, J.F.; Lam, M.G.; Smits, M.L.; Prince, J.F.; Viergever, M.A.; van den Bosch, M.A.; Zonnenberg, B.A.; de Jong, H.W. 99mTc-MAA overestimates the absorbed dose to the lungs in radioembolization: A quantitative evaluation in patients treated with 166Ho-microspheres. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1965–1975.
- She, W.H.; Cheung, T.T.; Yau, T.C.; Chan, A.C.; Chok, K.S.; Chu, F.S.; Liu, R.K.; Poon, R.T.; Chan, S.C.; Fan, S.T.; et al. Survival analysis of transarterial radioembolization with yttrium-90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg. Nutr. 2014, 3, 185–193.
- Kwon, J.H.; Kim, G.M.; Han, K.; Won, J.Y.; Kim, M.D.; Lee, D.Y.; Lee, J.; Choi, W.; Kim, Y.S.; Kim, D.Y.; et al. Safety and efficacy of transarterial radioembolization combined with chemoembolization for bilobar hepatocellular carcinoma: A single-center retrospective study. Cardiovasc. Interv. Radiol. 2018, 41, 459–465.
- Kim, D.Y.; Han, K.H. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: Optimization of selecting treatment modality. Hepatol. Int. 2016, 10, 883–892.
- Moreno-Luna, L.E.; Yang, J.D.; Sanchez, W.; Paz-Fumagalli, R.; Harnois, D.M.; Mettler, T.A.; Gansen, D.N.; de Groen, P.C.; Lazaridis, K.N.; Narayanan Menon, K.V.; et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2013, 36, 714–723.
- Lobo, L.; Yakoub, D.; Picado, O.; Ripat, C.; Pendola, F.; Sharma, R.; ElTawil, R.; Kwon, D.; Venkat, S.; Portelance, L.; et al. Unresectable hepatocellular carcinoma: Radioembolization versus chemoembolization: A systematic review and meta-analysis. Cardiovasc. Interv. Radiol. 2016, 39, 1580–1588.
- Marcacuzco Quinto, A.; Nutu, O.A.; San Román Manso, R.; Justo Alonso, I.; Calvo Pulido, J.; Manrique Municio, A.; García-Sesma, Á.; Loinaz Segurola, C.; Martínez Caballero, J.; Jiménez Romero, L.C. Complications of transarterial chemoembolization (TACE) in the treatment of liver tumors. Cir. Esp. 2018, 96, 560–567.
- Kim, H.C. Radioembolization for the treatment of hepatocellular carcinoma. Clin. Mol. Hepatol. 2017, 23, 109–114.
- Kang, Y.J.; Lee, B.C.; Kim, J.K.; Yim, N.Y.; Kim, H.O.; Cho, S.B.; Jeong, Y.Y. Conventional versus small doxorubicin-eluting bead transcatheter arterial chemoembolization for treating barcelona clinic liver cancer stage 0/A hepatocellular carcinoma. Cardiovasc. Interv. Radiol. 2020, 43, 55–64.
- Fidelman, N.; Kerlan, R.K., Jr. Transarterial chemoembolization and (90)Y radioembolization for hepatocellular carcinoma: Review of current applications beyond intermediate-stage disease. Am. J. Roentgenol. 2015, 205, 742–752.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
789
Revisions:
3 times
(View History)
Update Date:
13 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




