Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
The targeted delivery of anti-cancer drugs and isotopes is one of the most pursued goals in anti-cancer therapy. One of the prime examples of such an application is the intra-arterial injection of microspheres containing cytostatic drugs or radioisotopes during hepatic embolization procedures. Therapy based on the application of microspheres revolves around vascular occlusion, complemented with local therapy in the form of trans-arterial chemoembolization (TACE) or radioembolization (TARE).
- microspheres
- embolization
- hepatocellular carcinoma
1. Introduction
The targeted delivery of anti-cancer drugs and isotopes is the most pursued goal in anti-cancer therapy. However, a major disadvantage of the systemic application of these drugs is their poor selectivity for cancer cells and their general distribution to non-cancerous tissues, causing toxic side effects to otherwise healthy tissue [1,2]. To this end, novel therapies are constantly being discovered and applied for anti-cancer interventions in pursuit of avoiding toxic side effects. Theoretically, the involvement of non-cancerous tissues can also be reduced via disease-targeted therapeutic delivery. Herein, disease-targeting can be guided by either receptor targeting or via selective administration [3]. Unique for the hepatic system is that cancerous diseases such as HCC and mCRC alter the vascularization of the liver. Normal liver tissue will receive oxygen from the portal system; HCC and mCRC receive oxygen from the artery and are therefore suitable for trans-arterial therapy [4]. Over the past two decades, microspheres have proven themselves effective trans-arterial drug delivery systems [5]. Such microspheres have broad applications in both life science research and medicine [6], e.g., contrast agents [7], tissue fillers [8], and drug delivery vehicles [9]. Stable or biodegradable microspheres are generally utilized to direct drugs to organs by taking advantage of vascular physical restraints [10,11,12,13,14]. Therapeutic microspheres are characteristically small particles or beads with a well-defined size distribution of 50–750 mm, consisting of either glass, synthetic polymers, or proteins [15]. By selectively blocking the blood supply of the targeted tissue via so-called trans-arterial embolization (TAE), the deprived tissue will be starved of nutrients, ultimately resulting in a therapeutic benefit. When lodged in the end vasculature that surrounds the tumor, microspheres can also release encapsulated drugs (trans-arterial chemoembolization (TACE)) or irradiate surrounding tissue via embedded radioisotopes (trans-arterial radioembolization (TARE)).
Being a medicinal product, microspheres are considered medical devices rather than drugs. From a practical perspective, this means that such agents have to comply with different regulations compared to more standard therapeutics. Independent of this classification, the various clinical requirements ultimately drive therapeutical applications, of which the most important ones are stated in Table 1. Caine et al. extensively reviewed more detailed information on the microspheres used for TAE [16].
Table 1. Properties of microspheres: list of requirements for embolization (selected from: https://www.microspheres.us/properties-of-microspheres/ accessed on 1 November 2022).
| Property | Importance |
|---|---|
| Specific gravity (particle density) | Dispersion in other media or occlusion of the micro-vasculature |
| Size | Particle size (diameter = 50–750 mm) that allows occlusion of the microvasculature |
| Durability | Strength during production, solvent resistance, sterilization, chemical stability, or biodegradation, the release of the therapeutical payload |
| Biocompatibility | Safety, toxicity, stability, suitable for intra-arterial delivery |
| Pharmacology | Controlled dosimetry and dosing, full control over release profile by diffusion, zero-order kinetics |
| Surface properties | Hydrophobic vs. hydrophilic surface, surface area, and porosity, ability to coat or functionalize the spheres |
Therapeutical microspheres have been widely implemented for hepatocellular cancer (HCC) and hepatic metastasized disease of different kinds of solid cancers, including colorectal, lung, and breast cancer that metastasize to the liver [5,17]. Non-treatable advanced neoplastic diseases and the development of hepatic metastasis have poor prognoses. Only 10–20% of these patients are suitable candidates for radical resection, as surgical excision cannot be applied in grade 3–5 staged HCC [18]. A substantial portion of inoperable patients who present metastatic liver tumors needs alternative treatment strategies or therapy for bridging surgery [19,20]. Alternative therapies encompass minimally invasive techniques, including percutaneous ablative treatments (radiofrequency ablation, microwave ablation) and trans-catheter intra-arterial therapies [21,22].
2. Trans-Arterial Embolization for Vascular Occlusion
Trans-arterial embolization (TAE) is a technique wherein inert microspheres are used to block the blood supply around the tumor. The use of large microspheres, e.g., Embosphere® (⌀ 300–750 mm, Merit Medical Systems, South Jordan, UT, USA) in tumor-affected liver lobe(s) allows blockage of the blood supply towards the tumor (Figure 1B), ultimately resulting in reduced tumor growth [10,11,12,13,14]. An overview of the different microspheres currently used for hepatic trans-arterial embolization is provided in Table 2. The clinically applied glass and synthetic spheres are non-degradable and remain in the vasculature for life. Recently, in a pre-clinical phase, biodegradable spheres were developed, which allow local occlusion of the vasculature, and after degradation they allow a follow-up injection of embolization treatment. The clinical unfavorable short- and long-term outcomes of patients with large HCCs (≥50 mm) were revealed compared to those with small HCCs (<50 mm). Detailed analyses revealed that the average rates of change in tumor size and shrinkage after TAE were 48.6 ± 35.6 mm and 30.7 ± 17.0%, respectively [23].
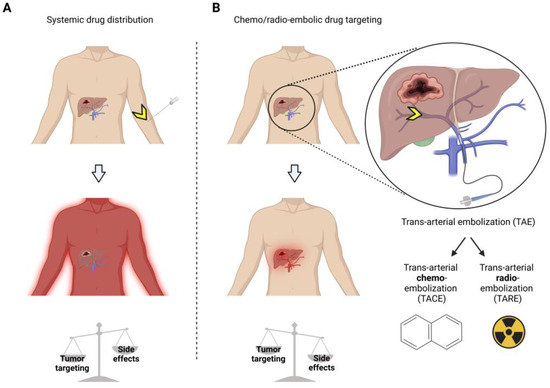
Figure 1. Treatment of hepatocellular carcinoma (HCC) shows the advantages of chemo/radio-embolization drug targeting over systemic drug therapy. Systemic drug therapy (A) yields lower tumor targeting and more side effects than local delivery via trans-arterial chemo/radio embolic drug targeting (B). Yellow arrows = injection site.
Table 2. Overview of non-functionalized microspheres for hepatic trans-arterial embolization (TAE). PC = pre-clinical use, H = Human use, FDA = FDA approved as an active implantable medical device.
| Microspheres Composition | Product Name | Particle Size Range (⌀ μm) | PC/H (FDA Clearance) | Biodegradable | References |
|---|---|---|---|---|---|
| Tris acryl gelatin microspheres (TAGM) | Embosphere® (Merit Medical Systems, South Jordan, UT, USA) | 100–300, 300–500 | H (FDA) | No | [24] |
| Polyvinyl alcohol (PVA) sodium acrylate co-polymer microspheres | Contour SE® (Boston Scientific, Marlborough, MA, USA), Bead Block® (Boston Scientific, Marlborough, MA, USA) Embozene® (Varian Medical Systems, Palo Alto, CA, USA) |
Irregular: 150–250, 250–355, 355–500, 500–710 |
H (FDA) | No | [25] |
| Polyvinyl alcohol-based hydrogel microspheres with sulphonate groups | LC Bead® (Boston Scientific, Marlborough, MA, USA) | 75–150, 100–300, 300–500, 500–700 | H (FDA) | No | [26,27,28,29] |
| Co-polymer of PEG and diacrylamide | Hydropearl® (Terumo Medical Co., Somerset, NJ, USA) | 75–1100 | H (FDA) | No | [1] |
| Starch microspheres | Embocept® (Pharmacept, Berlin, Germany), Spherex® (Magle Life Sciences, Lund, Sweden) |
50 | PC | Yes | [1] |
| Gelatin microspheres | Gel-Bead (Teleflex, Morrisville, NC, USA) | 100–300, 300–500, 500–700, 7000–1000 | H (FDA) | Yes | [1] |
| Collagen-coated poly-(DL-lactic acid-co-glycolic acid (PLGA) microspheres | Occlusin500® (IMBiotechnologies, Edmonton, AL, Canada) | 150–210 | H | Yes | [1] |
Instead of focusing on major vascular occlusion, chemo- or radio-embolic targeting is an alternative treatment option with microspheres that have the potential to manage hepatic tumors effectively [18].
3. Therapeutic Loads Employed during Microsphere-Trans-Arterial Embolization Therapy
3.1. Trans-Arterial Chemoembolization
Trans-arterial chemoembolization (TACE) uses embolization to deliver chemotherapy locally, thus limiting systemic exposure (Figure 1) [30,31]. One prime example are acrylic co-polymer microspheres (Hepasphere™, ⌀ 50–100 µm, Merit Medical Systems, Inc., South Jordan, UT, USA) that can absorb cytostatic drugs such as doxorubicin, irinotecan, epirubicin, mitomycin, cisplatin, and oxaliplatin (Table 3).
Table 3. Drug-eluting microspheres for functionalized hepatic TACE. PC = pre-clinical use, H = human use, FDA = FDA approved as an active implantable medical device.
| Microspheres Composition |
Product Name | Particle Size Range (⌀ µm) | Drug Load | PC/H (FDA) | Biodegradable | References |
|---|---|---|---|---|---|---|
| Polyvinyl alcohol (PVA) sodium acrylate co-polymer microspheres | QuadraSphere® and HepaSphere™ (Merit Medical Systems, Inc., South Jordan, UT, USA) DC Bead® (Boston Scientific, Marlborough, MA, USA), LC Bead®, and Bead Block® (Boston Scientific, Marlborough, MA, USA) |
50–100, 100–300, 200–400 | Doxorubicin, irinotecan, epirubicin, oxaliplatin | PC/H (FDA) | No | [14,26,32,33,34,35,36] |
| Ion-exchange microspheres | CalliSpheres® Beads (Jiangsu Hengrui Medicine Co. Ltd. Jiangsu, China) | 100–300 | Doxorubicin, pirarubicin, oxaliplatin | PC/H | No | [37,38,39,40,41] |
| Tris acryl gelatin microspheres (TAGM) | Embosphere (Merit Medical Systems, South Jor-dan, UT, USA), Embozene®, and Oncozene™ (Varian Medical Systems, Palo Alto, CA, USA) |
40–120, 100–300 | Doxorubicin and Irinotecan | H (FDA) | No | [13,36,42,43] |
| Poly-lactide-co-glycolide (PLGA) | Dexon®, Vicryl®, PerserisTM, Indivior (Indivior Inc. North Chesterfiled, VI, USA), Risperdal Consta® | 20–100 | Mitomycin, doxorubicin, irinotecan, sunitinib, cisplatin | PC/H (FDA) | Yes | [44,45,46,47] |
| Albumin microspheres | Nab-paclitaxel | 10–220 | Mitomycin C, doxorubicin, paclitaxel | PC/H (FDA) | Yes | [48,49] |
After contact with either an ionized environment, such as 0.9% NaCl and blood, or nonionic contrast media, acrylic co-polymer microspheres expand to 200–400 µm and slowly release their cytostatic payload [50]. The advantage of TACE is that it maximizes the concentration of chemotherapeutic agents within the tumor for up to seven days while keeping a minimal concentration in the systemic circulation. This approach reduces systemic side effects and the toxicity of cytostatic drugs compared to systemic chemotherapy. Furthermore, the malignancy’s arterial supply is occluded like in TAE, thus limiting nutrient availability to the tumor [51,52]. In HCC patients [5], this chemoembolization strategy has proven beneficial for patients’ survival, increasing the survival time by up to 12 months [53] and reducing the symptoms related to chemotherapy [28].
3.2. Trans-Arterial Radioembolization (TARE)
Trans-arterial radioembolization (TARE) uses radioisotopes embedded in microspheres to locally irradiate tissue after the vascular occlusion of blood vessels surrounding the tumor [54]. Herein, microspheres carrying b-emitting radioisotopes enable a more pinpointed delivery of radiation to liver tumors than other radiotherapy techniques. Although these options extend patient survival, most remain palliative [10,53]. With 166Ho-microspheres and 3.8 GBq/kg liver tissue in a Phase II study including 38 patients, the target lesions showed complete response or stabilized disease for 27 patients (73%), with a median survival of 15 months [55]. Given these results, a more extensive, randomized Phase III study appears to be required. Although 90Y, 188Re, and 166Ho in microspheres effectively reduce tumor size and patients’ survival, data from large Phase III trials are warranted to prove their benefits compared to other treatment modalities. In addition, the cost-effectiveness between the various radioisotopes and types of microspheres has to be determined. Besides the application of TARE in HCC, patients with intrahepatic cholangiocarcinoma (CC), which is a rare but very aggressive neoplasia with limited therapeutic options, and patients with to liver metastasized colorectal (mCRC) and neuroendocrine disease also benefit from therapy using 90Y-loaded glass or resin microspheres, with a response of more prolonged overall survival of at least 6 months [56,57,58,59]. Various radioembolization microspheres carrying radioisotopes to be delivered in the tumor-bearing hepatic segments of patients are summarized in Table 4.
Table 4. Microspheres used for trans-arterial radioembolization (TARE). PC = pre-clinical use, H = human use, FDA = FDA approved as an active implantable medical device.
| Microspheres Composition |
Product Name | Particle Size Range (⌀ µm) | Radioisotope Load | Pre-Clinical/ Human Use (FDA Clearance) |
Biodegradable | References |
|---|---|---|---|---|---|---|
| Glass | Lipiocis, TheraSphere® (Boston Scientific, Marlborough, MA, USA) | 50–150, 20–30, 25–32 | 32P, 90Y, 177lu, 186Re, 188Re | PC/H (FDA for 90Y, 186Re, and 188Re) | No | [60,61,62,63,64,65] |
| Resin | SIR-Spheres® (Sirtex Medical Inc. Woburn, MA, USA), Amberlite IR-120 (Thermo Fisher Scientific, Landau, Germany) |
20–60 | 90Y, 153Sm | PC/H (FDA for 90Y) | No | [66,67,68,69] |
| Polyhydroxyamic acid polyacrylamide (PHA) | Experimental | 54 | 177lu, 131I | PC | No | [70,71,72] |
| Styrene divinylbenzene | Amberlite IR-120 (Thermo Fisher Scientific, Landau, Germany) | 20–40 | 152Sm | PC | No | [69,73] |
| Poly- DL-lactic acid-co-glycolic acid (PLGA) | YPO4 crystalline particles Radiogel® (Vivos Inc., Richland, WA, USA) | 0.5–2 | 90Y | PC (FDA-approved as a medical device) | Yes | [74] |
| Poly (L-lactic acid) PLLA | Resomer® L104 (Merck, Darmstad, Germany) | 10–45, 20–40 | 188Re/166Ho/175Yb | PC/C | Yes | [75,76,77,78,79,80,81] |
| Poly (glycidyl methacrylate-co-ethylene dimethacrylate & Quinoline-8-ol | G-Gel (Merck, Darmstad, Germany) | 20–40 | 131I, 177lu | PC | No | [66,70,71,72,82] |
| Hydroxyapatite | QuiremSpheres (Quirem Medical, Deventer, The Netherlands) |
20–60 | 166Ho | PC | No | [78,83,84] |
| Albumin | HSA-B20 (Rotop Pharmaka, Dresden, Germany) Vasculosis® (Global Medical Solutions, Auckland, New Zealand) MAA (DRAXIMAGE®, Kirkland, QC, Canada), Pulmocis® (Curium, London, UK) |
25–35, 15–37 | 90Y, 186Re, 188Re | PC/C | Yes | [63,64,65,85,86,87] |
| Chitosan | Millican (Dong Wha Pharmaceutical Co., Soeul, South Korea) | 5–20 | 166Ho | PC/C | Yes | [75,76,77,78,79,88,89,90] |
| Starch-based microparticles (SBMP) | Experimental Kit | 18–42 | 188Re | PC | No | [91,92,93] |
An essential issue in radioembolization studies is preventing shunting to normal tissues, such as the lungs. Shunting displaces a fraction of the administered particles towards the microvasculature of other tissues, mainly the lung, instead of the liver, leading to ineffective dose distribution and irreversible severe adverse effects such as radiation pneumonitis [88,94]. To avoid the shunting of microspheres, radioembolization is performed in a theranostic setting. In this setting, a catheter is (selectively) placed to deliver radiolabeled macro-aggregated albumin (99mTc-MAA; ⌀ 10–40 mm) to the affected tissues. Via a scout scan using single-positron emission computed tomography (SPECT) imaging, the localization and distribution of the 99mTc-MAA are visualized, which is an approach that helps assess the degree of shunting and, at the same time, facilitates dosimetry measurements. When this has been done, therapeutic loads of β-emitting glass or resin microparticles (⌀ 15–25 mm) [95] containing, e.g., 90Y (SIR-Spheres®, Sirtex Medical Inc. Woburn, MA, USA; Therasphere, Boston Scientific, Marlborough, MA, USA) or 166Ho (QuiremSpheres, Quirem Medical Deventer, The Netherlands) are injected via a catheter positioned in the same way [96]. Given the overlap in size and retention properties between 99mTc-MAA and microparticles, a 99mTc-MAA scout scan has been deemed a sufficient standard requirement in the clinical guidelines to predict the accurate delivery of therapeutic microspheres. Despite these guidelines, a mismatch between the scout and therapeutic is inevitable, i.e., given the time span that separates these two procedures. As such, the delivery of microspheres can still lead to adverse side effects and suboptimal dose delivery in about 30% of cases [97,98,99], a complication which highlights the need for innovative solutions that help refine the correlation between the scout scan and therapeutic delivery. In this respect, the physical properties of the used radioisotope, 199Ho as a b/g/paramagnetic element [100] and 90Y as a PET/SPECT imaging agent, post-TARE imaging facilitates mapping of the dose delivery. Where the therapy is insufficient, adjuvant therapy can be considered [101,102]. Furthermore, microspheres containing these isotopes can also serve as a scout scan. Another drawback is the delay of 2 weeks between the execution of the scout scan and the therapeutic intervention due to the need for dosimetry [103,104,105] and the production/delivery time of the β-emitting microspheres [96].
3.3. TACE vs. TARE
Instead of focusing on major vascular occlusion with TAE, in this section, we focus on comparing chemo- or radio-embolic targeting that has the potential to facilitate vascular occlusion and realize chemo- or radio-embolic treatment of hepatic tumors [18].
Four TARE studies determined the overall survival median at 9–11 months [50]. Based on these findings, TARE was not recommended as a first-line therapy for patients with non-resectable colorectal liver metastasis. For HCC, however, the overall survival in a study with unresectable HCC patients was 19.9 months in the 90Y-resin TARE group, which was an improvement compared to the 14 months of survival in a matching TACE group [106]. Recently, the efficacy of TARE combined with TACE was determined in 19 patients with bi-lobar HCC, and no procedure-related major clinical complications were observed, and the mean overall survival yielded a promising 27.3 months compared to untreated patients [107]. Differences between studies comparing TACE and TARE indicate that the outcome in the benefits of treatment may be related to the type of carcinoma, an observation that needs additional research. TARE also proved superior in safety regarding post-embolization syndrome, hospitalization days, and outpatient-based therapy [108,109]. TARE was a safe alternative treatment to TACE [110], especially as using a scout scan helps prevent complications related to shunting with TACE [111]. Applying the scout scan also helps personalize the dosing, a concept that could extend to TACE. Compared to TACE, TARE had a longer time-to-progression, greater ability to downsize tumors, and less post-embolization syndrome [112]. For that reason, it could be an alternative to ablation, surgical resection, or portal vein embolization [113]. On the other hand, TACE is the trans-arterial treatment of choice for patients with marginal hepatic reserve (i.e., hyperbilirubinemia, ascites) or candidates for transplantation [114].
This entry is adapted from the peer-reviewed paper 10.3390/jcm12030918
This entry is offline, you can click here to edit this entry!
