Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sofia Morozova | -- | 2161 | 2023-02-09 08:58:14 | | | |
| 2 | Sirius Huang | -2 word(s) | 2159 | 2023-02-10 02:03:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Morozova, S.M. Hydrogels via Diels–Alder Crosslinking. Encyclopedia. Available online: https://encyclopedia.pub/entry/41019 (accessed on 07 February 2026).
Morozova SM. Hydrogels via Diels–Alder Crosslinking. Encyclopedia. Available at: https://encyclopedia.pub/entry/41019. Accessed February 07, 2026.
Morozova, Sofia M.. "Hydrogels via Diels–Alder Crosslinking" Encyclopedia, https://encyclopedia.pub/entry/41019 (accessed February 07, 2026).
Morozova, S.M. (2023, February 09). Hydrogels via Diels–Alder Crosslinking. In Encyclopedia. https://encyclopedia.pub/entry/41019
Morozova, Sofia M.. "Hydrogels via Diels–Alder Crosslinking." Encyclopedia. Web. 09 February, 2023.
Copy Citation
The Diels–Alder (DA) reaction is a promising tool for obtaining covalently crosslinked hydrogels due to its reaction bioorthogonality, the absence of by-products, and the application of mild conditions without a catalyst. The resulting hydrogels are in demand for use in various fields of materials science and biomedicine.
hydrogel
Diels–Alder reaction
self-healing
cell culture
1. Introduction
The Diels–Alder reaction (DA) was discovered by German scientists Otto Diels and Kurt Alder in 1928 [1], for which they received the Nobel Prize in 1950. This reaction consists of the [2+4]-cycloaddition of compounds with a conjugated system of double bonds (diene component) with compounds having a double or triple bond (dienophilic component), leading to the formation of a six-membered cycle. Since its discovery, the DA reaction has gained increasing demand in various fields of chemistry, including organic synthesis for the production of cyclo-containing building blocks [2], and in macromolecular design for the production of gels, dendrimers, and brush-like polymers [3]; the thermal reversibility of the DA reaction led to the creation of a class of self-healing materials [4], and the speed and completeness of the interaction allowed the reaction to be used for the introduction of functional components (fluorescent dyes, proteins, physiologically active molecules) in various biomaterials [5]. Over the past 5 years, there has been an increased interest in the use of the DA reaction in biomaterials for the fabrication of biocompatible and biodegradable hydrogels [6][7]. However, existing reviews either considered the DA reaction only for self-healing applications [4], for bioapplications in general (not limited to gels) [6], or were aimed at synthesizing the initial components [7]. In connection with the active development of the research direction, it is interesting to summarize the results based specifically on DA hydrogels for bio-applications. Figure 1 illustrates the advantages of DA chemistry, both normal and inverse electron-demand, for biomaterials and their possible applications.

Figure 1. Illustration of various types of Diels–Alder-based hydrogels and their possible bioapplications.
2. Hydrogels via Diels–Alder Crosslinking
2.1. Classification
The Diels–Alder reaction exhibits the following features: (i) regioselectivity, resulting mainly in ortho-para isomers relative to the position of the functional groups in the diene–dienophile pair; (ii) partial stereospecificity and stereoselectivity, depending on the exact example; in principal, it is possible to obtain two stereospecific isomers, i.e., the presence of two endo- and exo-adducts, depending on the position of the most significant substituent (electron acceptor and/or conjugating group) relative to the diene π-system; (iii) thermal reversibility, i.e., reversible decomposition of the resulting 6-membered cycle back to diene and dienophile; and (iv) attributability of the reaction to normal or inverse electron-demand type, depending on the nature of the substituents (electron donor or electron acceptor) of diene and dienophile [1][2][3]. Since the focus here is on the gel materials, it considers only the last feature of the reaction, while the first two have not yet shown a significant role in the application of hydrogels for biomedicine [7], and the third type is characterized by high temperatures of the reverse reaction (>100 °C) [8], which is not suitable for biological systems which are unstable at this temperature. Thus, the hydrogels are classified primarily by the electronic type of reaction DA—normal or inverse.
Figure 2 illustrates the schematics of normal and inverse electron-demand DA, with examples of the reactions of real compounds.
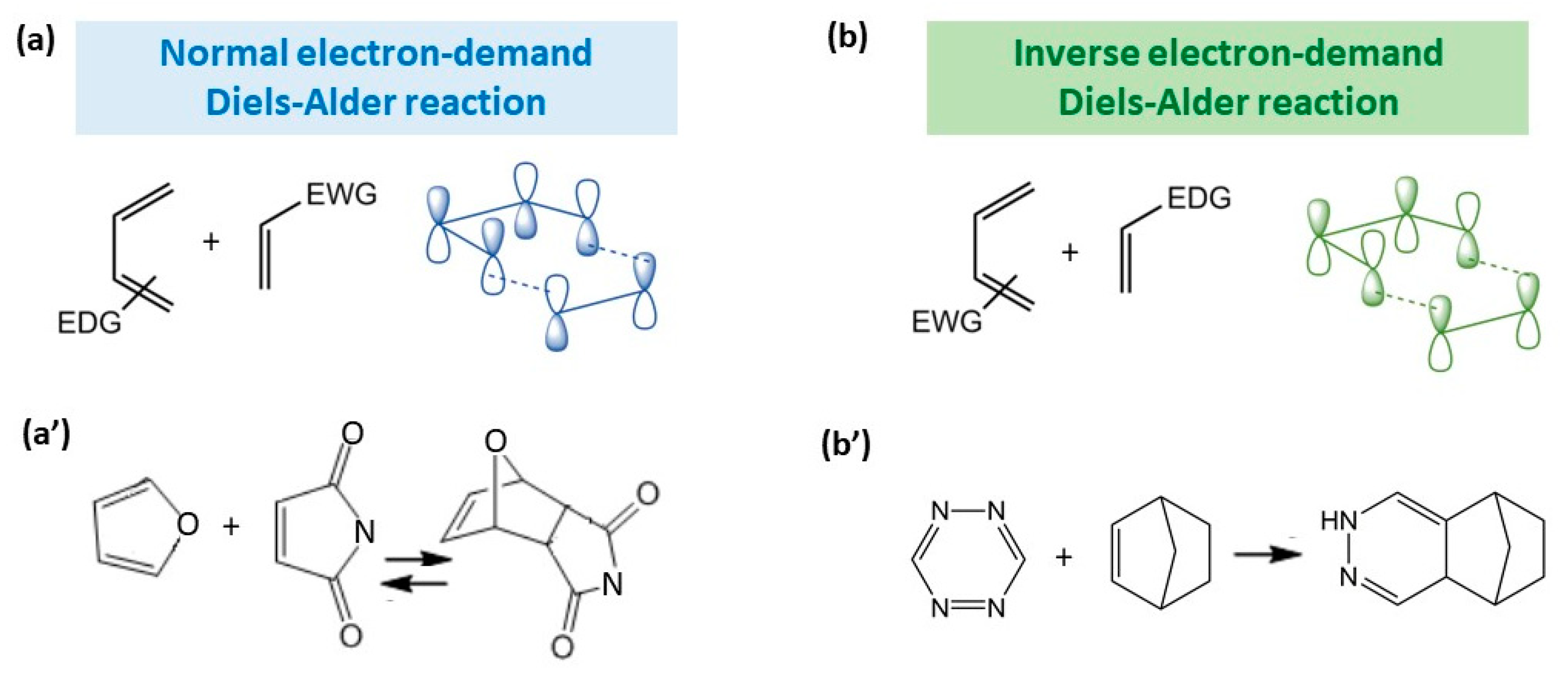
Figure 2. Illustration of the orbital interactions in normal (a) and inverse (b) electron-demand Diels–Alder reactions and the most common examples of these reactions for normal (a′) and inverse (b′) electron-demand Diels–Alder reactions.
The normal electron-demand Diels–Alder reaction involves electron-rich donating dienes (EDG group) and electronpoor withdrawing dienophiles (EWG group) owing to the matching of the diene’s highest occupied molecular orbital (HOMO) with the dienophile’s lowest unoccupied molecular orbital (LUMO) [9] (Figure 2a). The inverse electron-demand DA reaction was first discovered by Bachmann and Deno in 1949 [10], and it involves the opposite groups for diene and dienophile in comparison with normal electron-demand DA reaction, namely, the interaction of an dienophile with EDG group with an diene with EWG group. In frontier molecular orbital theory, this corresponds to the interaction of the LUMO of the dienophile with the HOMO of the diene [11] (Figure 2b). Examples of the most commonly used diene–dienophile functional pairs in modern polymer chemistry is the furan–maleimide coupling for normal electron-demand DA (Figure 2a′) and norbornene-tetrazine for inverse electron-demand DA (Figure 2b′).
In addition to the DA type of reaction, hydrogels are classified according to the type of components used for gelation (Figure 3).
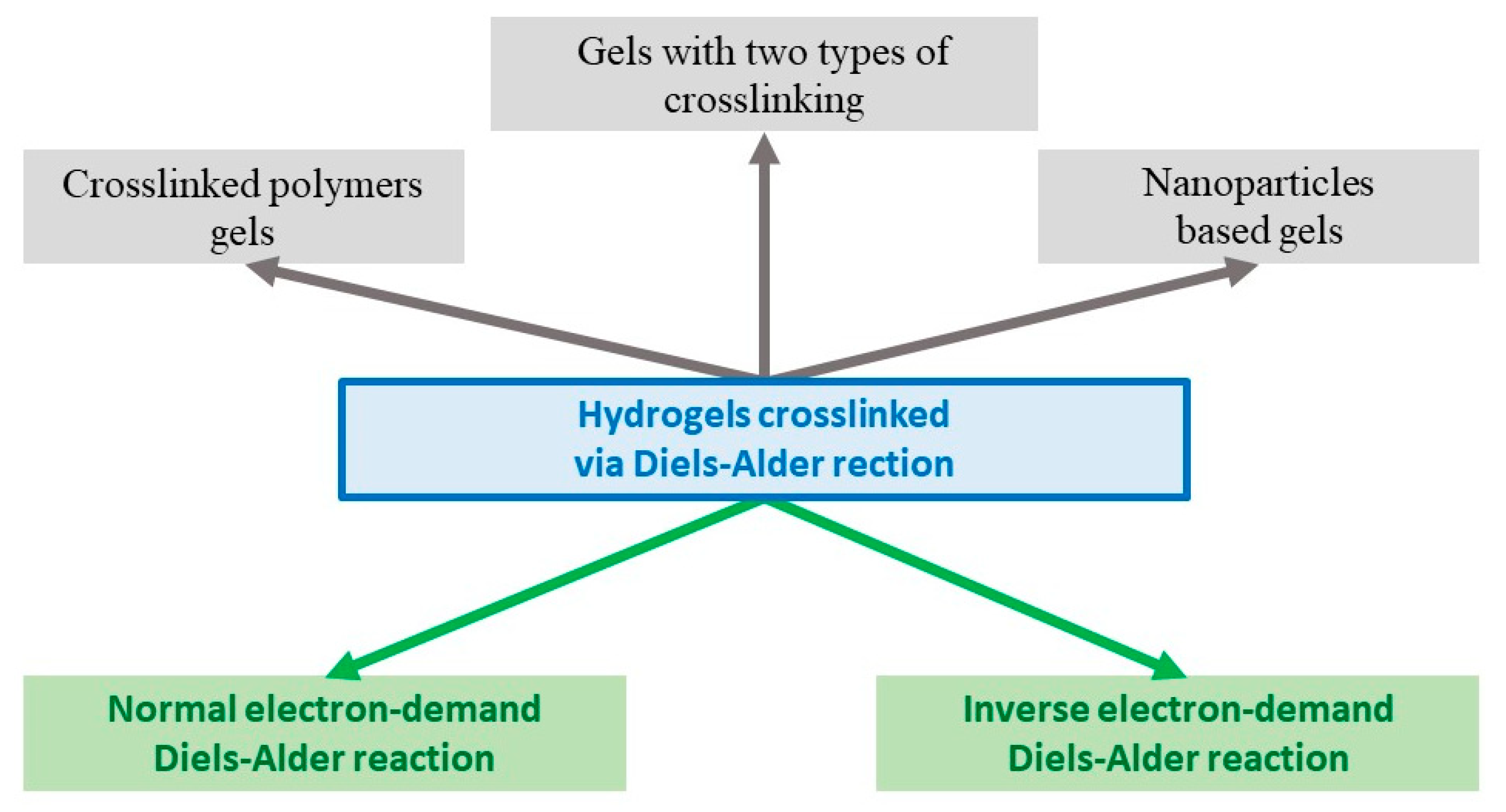
Figure 3. Classification of hydrogels obtained via Diels–Alder crosslinks.
A subclass of crosslinked polymers gels has been identified, which are either two crosslinked polymers [12], or a polymer crosslinked with a low molecular weight crosslinker [13]. Gels with two types of crosslinking are gels with interpenetrating polymer networks with crosslinks of a different nature; for example, when a strong and pH-resistant DA crosslinking is used in the gel with degradable crosslinking due to disulfide bonds [14]. Another distinguished subclass is the group called nanoparticle-based gels, in which the latter acts as a crosslinking agent using the DA reaction [15]. The following section provides examples of specific systems, according to the classification described above.
2.2. Hydrogel Design
2.2.1. Normal Electron-Demand DA
The DA reaction of furan and maleimide is the most common, and it is studied in view of its simplicity and the accessibility of its initial components [3]. Thus, most of the examples of hydrogels reported in the literature describe the use of maleimide as the dienophile and furan as the diene. An additional advantage is that furan derivatives can be obtained from agricultural and forestry wastes; thus, furan-based chemistry has garnered recent attention due to its sustainability component [6]. Other pairs of dienophiles and dienes, for example, fulvene–maleimide [16], have been rarely used; however, the potentially application of other derivatives could lead to the kinetic control and the reversibility of the reaction.
Crosslinked polymers gels: hydrogels formed by the crosslinking of two polymer components, or a polymer and a low molecular weight crosslinker, are the most common types of DA-based gels [17][18][19].
Table 1 shows reported examples of diene and dienophile components used in hydrogel formation.
Table 1. Components for DA-based hydrogels. Diene (a) and dienophile (b) components, which are commonly used for hydrogel formation.
| (a) Diene Component | (b) Dienophile Component | ||
|---|---|---|---|
| Description | Reference | Description | Reference |
| Furyl modified Chitin | [20] | 4-arm maleimide-terminated PEG and PEG dimaleimide | [15][16][18][20][21][22][23][24][25][26][27] |
| Furyl modified hyaluronan | [18][23][24][27] | Maleimide-terminated Jeffamine | [12][28] |
| Furyl modified hydroxypropylcellulose | [29] | Maleimide-modified cyclodextrin | [19][29] |
| Furyl modified poly(glutamic acid) | [22] | Bismaleimide | [30] |
| Furyl modified poly(caprolactone) | [13] | Maleimide-modified cellulose nanocrystals | [31] |
| Furyl modified gelatin | [28][31][32][33] | Maleimide-modified Ag NPs | [33] |
| Furyl modified cellulose nanocrystals | [15] | Maleimide-modified TiO2 NPs | [32] |
| Fulven modified PEG | [16] | ||
Bioapplications require biocompatibility and, in some cases, even biodegradability of the initial polymers, which limits the choice of the starting materials to the FDA approved substances [7]. Thus, the majority of examples described in the literature are based on well-known biocompatible synthetic polymers such as poly(ethylene glycol) (PEG) [18], poly(glutamic acid) (PGA) [22], poly(caprolactone) [13], Jeffamine [12], or nature-derived polymers, such as gelatin [28], chitin [20], chitosan [19], and hyaluronan [23]. Some maleimide crosslinkers, such as maleimide-terminated 4-arm PEG, PEG dimaleimides, and bismaleimide are commercially available. However, the synthesis of furan, fulvene, and maleimide-modified polymers and nanoparticles (NPs) are in many cases, 1–2 step processes based on rather simple chemistry involving epoxy ring opening in furfuryl glycidyl ether by NH2 groups largely present in biopolymers [20][28], amide bond formation [18][24], Schiff’s base reaction, with the subsequent reduction of imine bonds [12], ester bond formation [29], or acetal formation [15].
Gels with two types of crosslinking. On the one hand, the resistance of the DA reaction to pH or catalytic decomposition can be considered as an advantage for creating strong and resistant gels; on the other hand, this could be a disadvantage if it is required to create a stimuli-responsive crosslinking. The strategy of creating gels that are different in chemical nature using two types of crosslinking can be used to create functional and stimuli-responsive gels [21][25][26][27][29] or to accelerate the formation of a gel to prevent excessive swelling [20], i.e., the introduction of pH-sensitive imine fragments [26][27] introduce to the gel a programmable sequential degradation under acidic environment and UV irradiation, which is beneficial for controlled drug release. A double cross-linked network hydrogel was prepared by combining a DA reaction and the coordination of catechol fragments with iron ions [25]. This hydrogel showed anti-EDTA performance and self-healing properties due to its supramolecular Fe3+-catechol bonds. An interesting example was the combination of both normal and inverse electron-demand DA reactions in the same hydrogel [21]. The first type of crosslink was responsible for the mechanical framework of the gel, and the second one, which was based on a faster reaction, was used for the introduction of a fluorescent dye.
Nanoparticle-based gels. Recently, there has been an increased interest in gels based on nanoparticles, including those covalently crosslinked with the gel [34]. Currently, there are only a few examples of hydrogels crosslinked with NPs via the DA reaction [15][31][32][33], and this is due to the greater complexity of the modification and characterization of NPs compared to linear polymers or low molecular weight compounds. Inorganic NPs could be modified by reacting with a dopamine-maleimide linker [32][33]. By applying this strategy, hydrogels based on the DA reaction of benzotriazole maleimide (BTM) functionalized Ag NPs and furan-containing gelatin were reported [33]. The incorporation of Ag NPs as cross-linkers led to an increase in the storage modulus of the gel, and a decrease in the swelling ratio in comparison to the NPs-free control. The obtained hydrogel also demonstrated improved cell viability (L-929 murine fibroblast cells) and enhanced drug release, which opens a new route to a number of potential biomedical applications, such as controlled therapeutic delivery or tissue engineering. By using a similar strategy, DA hydrogels formed from dopamine-maleimide modified TiO2 NPs and furan-modified gelatin were reported [32]. The use of nanocrystalline cellulose (CNC) nanoparticles in hydrogel formation is highly promising, due to their natural origin—sourced from natural wood, their biocompatibility, and their anisotropic rod shape [35]. The modification of CNC could be performed by ester formation with 3-maleimidopropionic acid [31], acetal formation with furfural [15], or by carbamation with isocyanate-modified furan or maleimide derivatives [36]. However, only the first two strategies were used to obtain DA cross-linked hydrogels [15][31]. At the same time, the authors used a large excess of polymer to CNC (>10), while an increase in the amount of CNC leads to the fibrillar structure of the gel [37], which plays an important role in cell growth [38]. Moreover, the biocompatibility of the gels remained undetermined.
2.2.2. Inverse Electron-Demand DA
One of the disadvantages of a normal reaction DA crosslinking is the slow gelation time (several hours), due to which the gels undergo significant swelling [39]. One of the solution is to switch to a faster inverse electron-demand DA reaction [14]. However, the disadvantage of this reaction is the specificity of the compounds used and their lower availability compared to the normal electron-demand DA reaction. Few examples are known, and all of them are based on tetrazine–norbornene interaction [14][21][30]. This “click” reaction is carrying out without a catalyst, and produces only negligible quantities of nitrogen gas, without any no other toxic side products, making it very promising for bioapplications. Moreover, the nitrogen release could positively affect the formation of porous structures inside the hydrogel networks [30].
Crosslinked polymers gels. In a recent work [39], hydrogels formed by the crosslinking of two types of hyaluronan components, where one was modified by 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride, and the other with 5-norbornene-2-methylamine through the formation of amide bonds. Tunable gelation, with gelation times from 4.4 min to 46.2 min, were achieved by tuning the composition and molar mass of the initial HA. The obtained gels were transparent, mechanically strong (Young modulus up to 1000 Pa), biodegradable, and cytocompatible, making them promising for 3D cell culture and imaging.
Gels with two types of crosslinking. This gel contains two types of crosslinks—an irreversible DA-based gel and a multi-stimuli diselenide crosslink gel were reported [30]. The hyaluronan polymer was modified, as described previously, with 5-norbornene-2-methylamine [39], and was crosslinked with a novel diselenide-ditetrazine cross-linker. By varying the polymer/crosslinker ratios from 1 to 4, gelation times increased from 155 to 509 s. Similarly, the mechanical strength of the hydrogel decreased by decreasing the molar ratio of the cross-linker from 3000 to 750 dyne/cm2. Due to the presence of the diselenide bond, the hydrogels possessed a stimuli-responsive drug release related to the degradation of the S–Se bond under the presence of 4-dithiothreitol or H2O2, as well as under near-infrared (NIR) irradiation, making them promising for use in photothermal therapy for tumor treatment. A similar approach was realized in methylcellulose- (MC) based gels with disulfide and DA crosslinks [14]. One component was based on MC modified with a carboxylic group, which then was reacted with 5-norbornene-2-methylamine, and the other component was based on MC-methylphenyltetrazine containing disulfide bonds. The obtained gels have a gelation time of <15 min at a physiological temperature and pH, and possess a Young’s modulus similar to that of brain tissue (1–3 kPa). The disulfide bonds in the hydrogel were degradable in the presence of thiols (which are naturally occurring in the biological environment) and demonstrated the ability to release proteins and chondroitinase ABC.
References
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122.
- Mali, G.; Chauhan, A.N.S.; Chavan, K.A.; Erande, R.D. Development and Applications of Double Diels-Alder Reaction in Organic Synthesis. Asian J. Org. Chem. 2021, 10, 2848–2868.
- Briou, B.; Ameduri, B.; Boutevin, B. Trends in the Diels–Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097.
- Ratwani, A.; Abdelkader, C.R. Self-healing by Diels-Alder cycloaddition in advanced functional polymers: A review. Prog. Mater. Sci. 2022, 131, 101001.
- Ganz, H.; Harijan, D.; Wagenknecht, D. Labelling of DNA and RNA in the cellular environment by means of bioorthogonal cycloaddition chemistry. RSC Chem. Biol. 2020, 1, 86–97.
- Nihal, T.; Sanyal, A. Furan-containing polymeric Materials: Harnessing the Diels-Alder chemistry for biomedical applications. Eur. J. Org. Chem. 2021, 153, 110514.
- Cadamuro, F.; Russo, L.; Nicotra, F. Biomedical Hydrogels Fabricated Using Diels–Alder Crosslinking. Biomaterials 2021, 3, 374–382.
- Vauthier, F.; Jierry, M.; Oliveira, L.; Hassouna, J.C.; Roucoules, L.; Gall, V.B.-L. Interfacial thermoreversible chemistry on functional coatings: A focus on the Diels–Alder reaction. Adv. Funct. Mater. 2019, 29, 1806765.
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–16698.
- Bachmann, W.E.; Deno, N.C. The Diels–Alder Reaction of 1-Vinylnaphthalene with α,β- and α,β,γ,δ-Unsaturated Acids and Derivatives. J. Am. Chem. Soc. 1949, 71, 3062–3072.
- Png, Z.M.; Zeng, H.; Ye, Q.; Xu, J. Inverse-Electron-Demand Diels–Alder Reactions: Principles and Applications. Chem. Asian J. 2019, 12, 2142–2159.
- Guaresti, O.; Astrain, C.; Aguirresarobe, R.; Eceiza, A.; Gabilondo, N. Synthesis of stimuli–Responsive chitosan–Based hydrogels by Diels–Alder cross–Linking ‘click’ reaction as potential carriers for drug administration. Carbohydr. Polym. 2018, 183, 278–286.
- Farhat, W.; Venditti, R.; Becquart, F.; Ayoub, A.; Majeste, J.; Taha, M.; Mignard, N.; De Lyon, U. Synthesis and Characterization of Thermoresponsive Xylan Networks by Diels–Alder Reaction. ACS Appl. Polym. Mater. 2019, 1, 856–866.
- Delplace, V.; Pickering, A.; Hettiaratchi, M.; Zhao, S.; Kivija, T.; Shoichet, M. Inverse Electron-Demand Diels–Alder Methylcellulose Hydrogels Enable the Co-Delivery of Chondroitinase ABC and Neural Progenitor Cells. Biomacromolecules 2020, 21, 2421–2431.
- Shao, C.; Wang, M.; Chang, H.; Xu, F.; Yang, J. A Self-Healing Cellulose Nanocrystal-Poly(ethylene glycol) Nanocomposite Hydrogel via Diels–Alder Click Reaction. ACS Sustain. Chem. Eng. 2017, 5, 6167–6174.
- Yan, A.; Gundsambuu, J.; Krasowska, B.; Paltts, M.; Marina, K.; Gerber, P.F.; Barry, C.; Blencowe, S.C. Injectable Diels-Alder cycloaddition hydrogels with tuneable gelation, stiffness and degradation for the sustained release of T-lymphocytes. J. Mater. Chem. B 2022, 10, 3329–3343.
- Li, D.; Wang, S.; Meng, Y.; Guo, Z.; Cheng, M.; Li, J. Fabrication of self-healing pectin/chitosan hybrid hydrogel via Diels-Alder reactions for drug delivery with high swelling property, pH-responsiveness, and cytocompatibility. Carbohydr. Polym. 2021, 268, 118244.
- Yang, Y.; Zhu, Z.; Gao, R.; Yuan, J.; Zhang, J.; Li, H. Acta Biomaterialia Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic acid/PEG hydrogel for osteoarthritis improvement. Acta Biomater. 2021, 128, 163–174.
- Zhang, M.; Wang, J.; Jin, Z. Supramolecular hydrogel formation between chitosan and hydroxypropyl β-cyclodextrin via Diels-Alder reaction and its drug delivery. Int. J. Biol. Macromol. 2018, 114, 381–391.
- Bi, B.; Ma, M.; Lv, S.; Zhuo, R.; Jiang, X. In-situ forming thermosensitive hydroxypropyl chitin-based hydrogel crosslinked by Diels-Alder reaction for three dimensional cell culture. Carbohydr. Polym. 2019, 212, 368–377.
- Madl, C.; Heilshorn, S. Rapid Diels–Alder Cross-linking of Cell Encapsulating Hydrogels. Chem. Mater. 2019, 31, 8035–8043.
- Wei, M.; Hsu, Y.; Asoh, T.; Sung, M.; Uyama, H. Injectable poly(c-glutamic acid)-based biodegradable hydrogels with tunable gelation rate and mechanical strength. J. Mater. Chem. B 2021, 9, 3584–3594.
- Zhou, C.; Sheng, C.; Chen, J.; Liang, Y.; Liu, Q.; Li, P.; Huang, X.; Liu, B. Gradual hydrogel degradation for programable repairing full-thickness skin defect wound. Chem. Eng. J. 2022, 450, 138200.
- Ilochonwu, B.; Mihajlovic, M.; Maas-bakker, R.; Rousou, C.; Tang, M.; Chen, M.; Hennink, W.; Vermonden, T. Hyaluronic Acid-PEG-Based Diels–Alder In Situ Forming Hydrogels for Sustained Intraocular Delivery of Bevacizumab. Biomacromolecules 2022, 23, 2914–2929.
- Li, S.; Wang, L.; Yu, X.; Wang, C.; Wang, Z. Synthesis and characterization of a novel double cross-linked hydrogel based on Diels-Alder click reaction and coordination bonding. Mater. Sci. Eng. C 2018, 82, 299–309.
- Wei, Q.; Bai, J.; Wang, H.; Ma, G.; Li, X.; Zhang, W.; Hu, Z. Photo-induced programmable degradation of carboxymethyl chitosan-based hydrogels. Carbohydr. Polym. 2021, 256, 117609.
- Hu, X.; Gao, Z.; Tan, H.; Wang, H.; Mao, X. An Injectable Hyaluronic Acid-Based Composite Hydrogel by DA Click Chemistry With pH Sensitive Nanoparticle for Biomedical Application. Front. Chem. 2019, 7, 477.
- Online, V.; Gandini, A.; Algar, I.; Eceiza, A.; Corcuera, M.; Gabilondo, N. Diels–Alder “click” chemistry for the cross-linking of furfuryl-gelatin-polyetheramine hydrogels. RSC Adv. 2014, 4, 35578–35587.
- Wei, H.; Li, S.; Liu, Z.; Chen, H.; Liu, Y.; Li, W. Preparation and characterization of starch-cellulose interpenetrating network hydrogels based on sequential Diels-Alder click reaction and photopolymerization. Int. J. Biol. Macromol. 2022, 194, 962–973.
- Jo, Y.; Gulfam, M.; Jo, S.; Gal, Y.; Oh, C.; Park, S.; Taek, K. Multi-stimuli responsive hydrogels derived from hyaluronic acid for cancer therapy application. Carbohydr. Polym. 2022, 286, 119303.
- González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016, 149, 94–101.
- Miljevic, M.; Ahmed, I.; Martin, L.; Eceiza, A.; Fruk, L.; Corcuera, M. Designing hydrogel nanocomposites using TiO2 as clickable cross-linkers. J. Mater. Sci. 2016, 51, 5073–5081.
- Garc, C.; Chen, C.; Palomares, T.; Eceiza, A.; Fruk, L. Biocompatible Hydrogel Nanocomposite with Covalently Embedded Silver Nanoparticles. Biomacromolecules 2015, 16, 1301–1310.
- Matter, M.; Luna, F.; Niederberg, A. From colloidal dispersions to aerogels: How to master nanoparticle gelation. Nano Today 2020, 30, 100827.
- He, Q.; Lu, X. Design and fabrication strategies of cellulose nanocrystal-based hydrogel and its highlighted application using 3D printing: A review. Carbohydr. Polym. 2022, 301, 120351.
- de Oliveira, M.; Rigolet, J.; Marichal, S.; Roucoules, C.; Laborie, V. Grafting Diels-Alder moieties on cellulose nanocrystals through carbamation. Carbohydr. Polym. 2020, 250, 116966.
- Prince, E.; Chen, Z.; Khuu, N.; Kumacheva, E. Nano fi brillar Hydrogel Recapitulates Changes Occurring in the Fibrotic Extracellular Matrix. Biomacromolecules 2021, 22, 2352–2362.
- Storm, P.; Pastore, C.; MacKintosh, J.; Lubensky, F.; Janmey, T. Nonlinear elasticity in biological gels. Nature 2005, 435, 191–194.
- Delplace, V.; Nickerson, P.; Ortin-martinez, A.; Baker, A.; Wallace, V.; Shoichet, M. Nonswelling, Ultralow Content Inverse Electron-Demand Diels–Alder Hyaluronan Hydrogels with Tunable Gelation Time: Synthesis and In Vitro Evaluation. Adv. Funct. Mater. 2020, 30, 1903978.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
10 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




