Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rahim Hirani | -- | 2122 | 2023-02-08 16:58:46 | | | |
| 2 | Sirius Huang | Meta information modification | 2122 | 2023-02-09 02:14:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hirani, R.; Smiley, A. Risk Factors of Sleep Apnea. Encyclopedia. Available online: https://encyclopedia.pub/entry/40995 (accessed on 07 February 2026).
Hirani R, Smiley A. Risk Factors of Sleep Apnea. Encyclopedia. Available at: https://encyclopedia.pub/entry/40995. Accessed February 07, 2026.
Hirani, Rahim, Abbas Smiley. "Risk Factors of Sleep Apnea" Encyclopedia, https://encyclopedia.pub/entry/40995 (accessed February 07, 2026).
Hirani, R., & Smiley, A. (2023, February 08). Risk Factors of Sleep Apnea. In Encyclopedia. https://encyclopedia.pub/entry/40995
Hirani, Rahim and Abbas Smiley. "Risk Factors of Sleep Apnea." Encyclopedia. Web. 08 February, 2023.
Copy Citation
Obstructive sleep apnea (OSA), a condition in which there is a recurrent collapse of the upper airway while sleeping, is a widespread disease affecting 5% to 10% people worldwide. Despite several advances in the treatment modalities for OSA, morbidity and mortality remain a concern. Common symptoms include loud snoring, gasping for air during sleep, morning headache, insomnia, hypersomnia, attention deficits, and irritability. Obese individuals, male gender, older age (65+), family history, smoking, and alcohol consumption are well recognized risk factors of OSA.
sleep apnea
morbidity
upper airway
risk factors
1. Sleep Apnea: A Brief History
Although the official naming and discovery of sleep apnea reportedly occurred in the 1960s, it is by no means a new disorder [1]. While it is true that it is only receiving relatively more attention due to further advances in diagnosing and managing the disease, the symptoms first appeared approximately 2000 years ago and were lumped together using the term “Pickwickian syndrome” in the 19th century [2][3]. For years, the initial focus was on the process of understanding the intermittent closure of the upper airway; however, the late 1960s brought a fresh perspective to observe the various symptoms and risk factors at the same time, though limited by methodological difficulties at the time [1][4]. Research studies were mainly conducted via observing dogs and treating the condition with tracheotomies [5]. Though an earlier concept of continuous positive airway pressure (CPAP) using a customized mask in the 1970 and 1980s further advanced modern management [6]. To date, polysomnography, including electrocardiogram, sleep staging, electromyogram, and electroencephalogram, is the gold standard for diagnosing OSA, while home sleep apnea testing (HSAT) is an alternative method with some limitations [7][8].
There are four reported endotypes of OSA: loop gain, upper-airway collapsibility, arousal threshold, and upper-airway dilator muscle response, which is also knows as compensation [9]. Loop gain is basically the ventilatory response-to-disturbance ratio estimated by ventilation characteristics during obstructed breathing episodes [10]. It is usually noted as a drop in CPAP, suggesting a decrease in ventilation as compared to the holding pressure. This drop in ventilation leads to the accumulation of CO2, and thus, an increase in ventilatory drive. This increase can be estimated by measuring the ventilatory overshoot from the holding pressure of CPAP, providing a ratio for response and disturbance in ventilation [9][11][12]. Upper-airway collapsibility measures the propensity for collapse as reported in patients among OSA. Although there are several techniques to measure this mechanistic variable, negative pressure pulses seem to provide a reliable estimate as it is rapid and thus less likely to be influenced by external behaviors [13]. Arousal threshold is essentially the compensatory drive of ventilation that produces arousal [10]. This variable is a measure of the propensity to wake up from sleep given the changes in negative intra-thoracic pressure [10][12][14]. Lastly, patients with ineffective upper-airway dilator muscle endotypes have a decreased tone of dilator muscles, particularly genioglossus, the largest extrinsic muscle of the tongue [15]. The absence or presence of these endotypes can manifest differently in individuals that can subsequently have an impact on the severity of the disease. These individual endotypes can also be targeted individually, or in combination.
Over the years, there have been several advances, including the identification of symptoms (Figure 1) and various risk factors, to better diagnose and manage individual conditions. Loud snoring, gasping for air during sleep, xerostomia, insomnia, hypersomnia, nocturnal choking, and attention deficits are some of the many symptoms that can be observed in patients with OSA [16][17][18][19]. Some of the most important risk factors of sleep apnea are discussed herein (Figure 2).
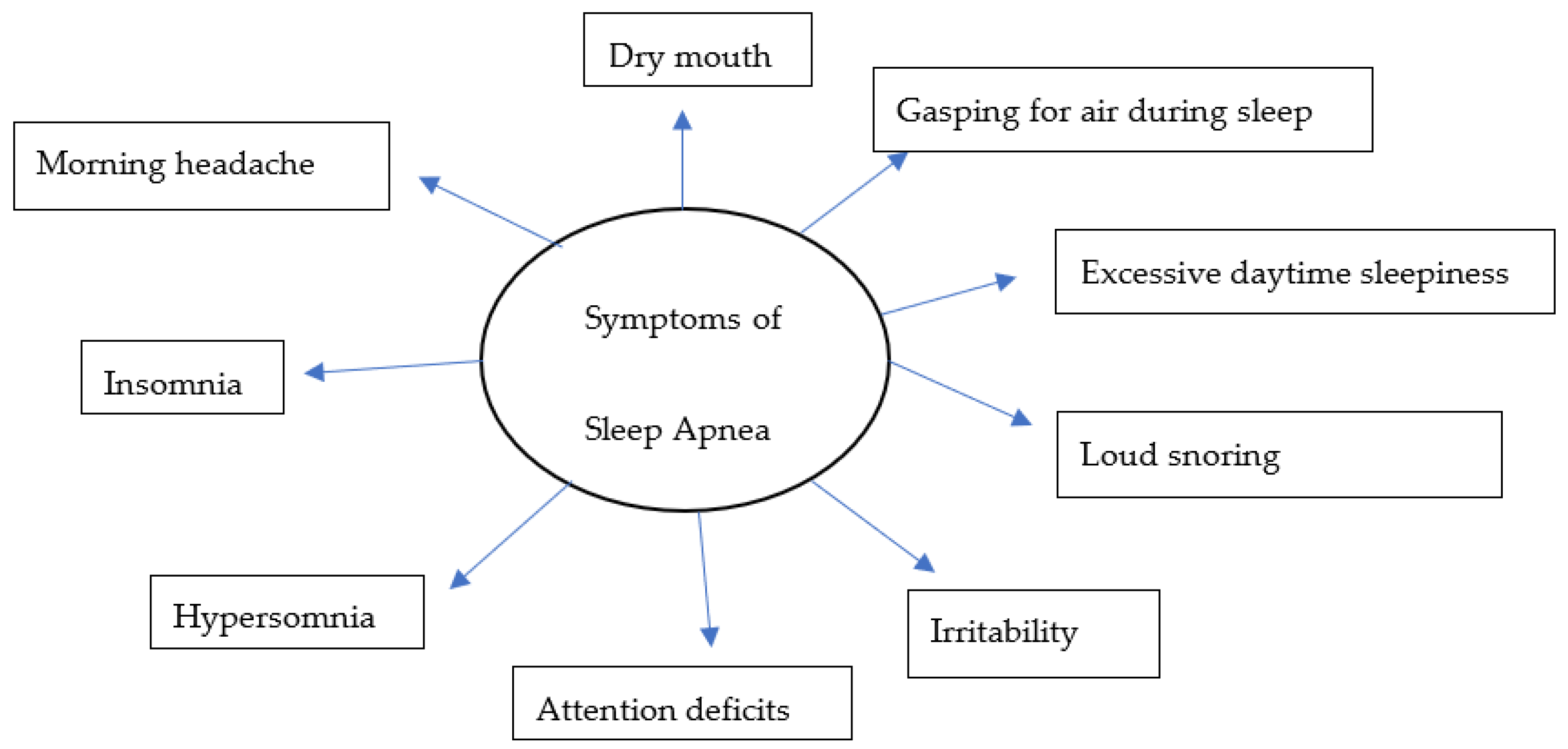
Figure 1. A model for common symptoms of sleep apnea.
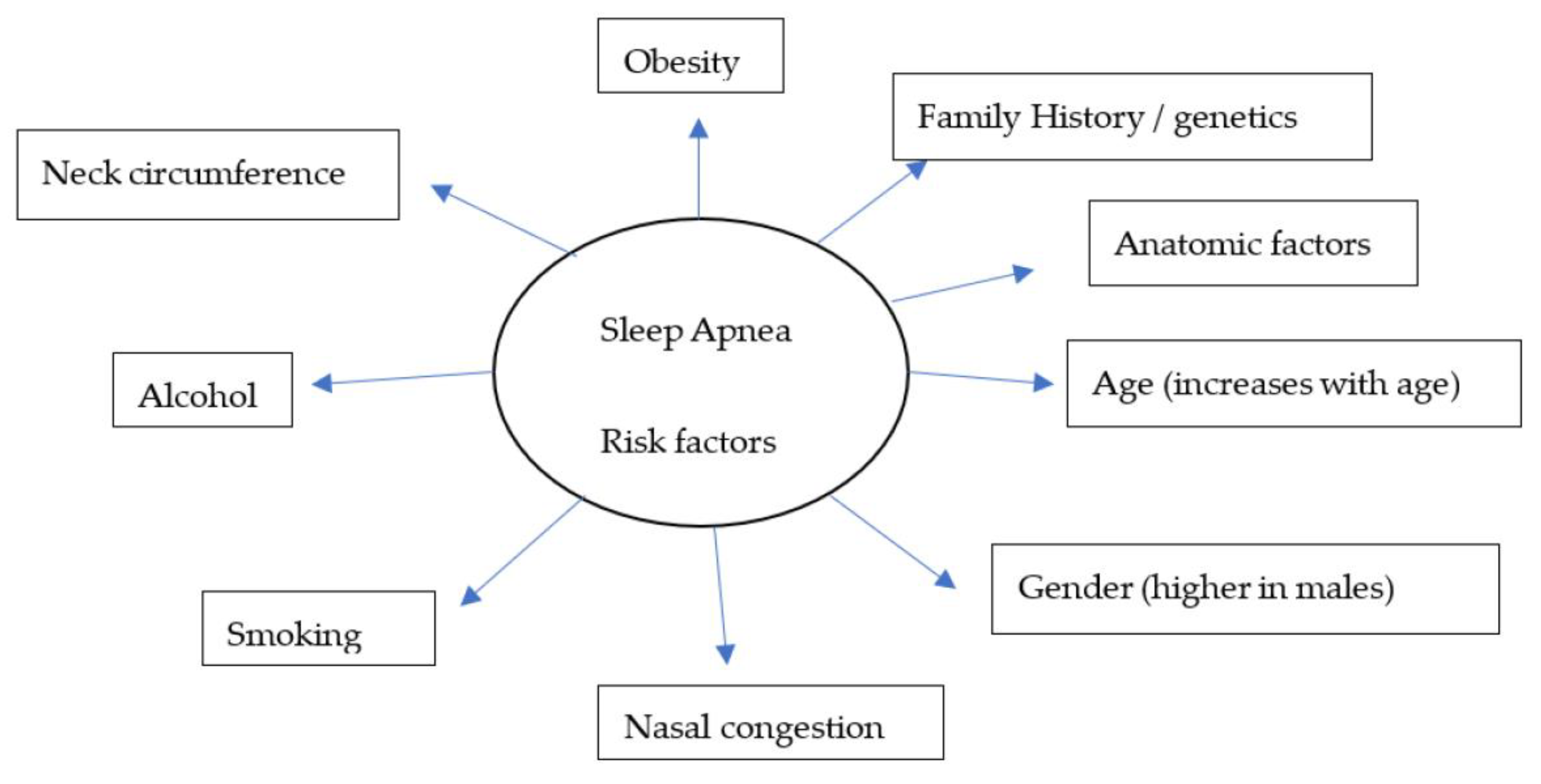
Figure 2. A model for widely recognized risk factors of sleep apnea.
2. Risk Factors of Sleep Apnea
2.1. Obesity
Obesity has been identified as one of the main components contributing to OSA [20][21]. Many correlations have been established between weight, BMI, waist-to-hip ratio, neck circumference, and severity of OSA. The sleep heart healthy study was one of the landmark studies that established the same ideas; the study showed an increase in the apnea-hypopnea index (AHI) by approximately five-fold in men and two-fold in women over the course of their study [22]. Peppard et al. in their population-based prospective cohort study of 690 randomly selected Wisconsin residents also demonstrated a six-fold increase in the odds of developing OSA with a mere 10% increase in weight, while weight loss resulted in decreasing severity among patients [23], suggesting a reciprocal relationship between these two variables.
2.2. Family History/Genetics
Several studies have reported some underlying causes of OSA to have a genetic component, suggesting its hereditary nature. However, these results should be carefully evaluated as OSA as a disorder is a complex interplay between genetics and environmental factors. The Cleveland Study was another landmark study in investigating OSA and its link to genetics. This genetic–epidemiologic study concluded that OSA is more prevalent in relatives of index probands of OSA as compared to their control counterparts [24]. Additionally, Ferini-Strambi et al. in their comparative study showed a higher prevalence of snoring among monozygotic twins, which is one of the primary symptoms of OSA [25]. Other biomarkers via genome-wide linkage studies have also been investigated to establish an association between OSA and various genes. One study showed such an association between a polymorphism in the angiopoietin-2 gene (ANGPT2) and mean nocturnal oxygen saturation, which is a commonly used marker to determine severity in OSA [26]. Furthermore, similar studies have established polymorphisms in tumor necrosis factor-a (TNF-a), prostaglandin E2 receptor EP3 subtype (PTGER3), and Lysophosphatidic acid receptor 1 (LPAR1) to be a risk factor associated with OSA [27][28]. Therefore, further studies and the biological significance of these polymorphisms in conjunction with OSA are warranted.
2.3. Age and Gender
Generally, OSA has been shown to be more prevalent in men with a two-fold-greater likelihood in people older than 65 years as compared to middle-aged adults aged 30–50 years [29]. Additionally, the prevalence was shown to be around 5% in middle-aged females and 12% in their male counterparts [30]. These estimates are around 12–32% in patients aged 65 years or older [29][31]. However, the severity of the disease varies among elderly individuals and could even be milder than the severity observed in adults [32]. In the elderly, the disease is said to be manifested differently, resembling behavioral and cognitive impairments mimicking dementia [33][34].
According to the current literature, there is a higher prevalence of OSA among men compared to women [35][36][37][38]. In a recent study of 1208 people between 20 and 81 years of age with 46% of the cohort being female, an estimated prevalence of OSA was 33% among women when AHI was more than equal to 5%, while it was 59% among men [36]. Even with an AHI of more than or equal to 15%, the prevalence was higher in men when compared to women; 30% vs. 13%, respectively [36]. This effect was not only restricted to prevalence; OSA has been reported to be more severe in men with more specific symptoms suggestive of OSA when compared to women. While men frequently report snoring, gasping, attention deficits, insomnia, snorting, and apnea, women are reportedly presented with more non-specific symptoms, such as headache, fatigue, depression, and anxiety [35]. The presence of only non-specific symptoms then could make it challenging for a physician to perform a correct diagnosis. This also helps explain the results that women are diagnosed at advanced ages and with a higher BMI as compared to men. The difference in gender, generally speaking, could also be due to a differing body-fat distribution in males vs. females, with males having more adipose tissue in the neck region, resulting in a higher susceptibility to airway collapse [39][40]. Although the pharyngeal cross-sectional area is reported to be similar among men and women, men are noted to exhibit greater upper-airway collapsibility. This could be accounted for by the presence of a longer airway length and larger volume of soft tissues on the lateral pharyngeal walls in men [35][41]. Hormonal differences are yet another factor that plays a role in the differing prevalence of OSA among men and women. Previous studies have shown how ventilatory response is affected and AHI is increased in hypogonadal men with an acute administration of testosterone [35][42]. In one study of testosterone replacement therapy among hypogonadal men, Matsumoto et al. reported not only a significant decrease in the ventilatory drive in patients receiving testosterone, but also noted the new induction of OSA, and an exacerbation of symptoms in patients previously diagnosed with OSA [42]. Years later, after this study, it was deciphered that an acute administration of testosterone enhances the ventilatory instability and the loop-gain of the ventilatory system as a consequence of an increase in the ventilatory response to hypoxia [11], increasing the predisposition to OSA in men.
OSA among pregnant and post-menopausal women is one area where there is a lack of research. While it has been reported that sleep-disordered breathing is more severe in postmenopausal when compared to premenopausal women [43], it is unclear whether a decreased production of female hormones plays a role in this exacerbation. Moreover, symptoms of OSA can also be more difficult to identify or interpreted as menopausal manifestations, leading to misdiagnosis [37]. Therefore, these gender differences could mostly be explained by anatomic and physiologic variabilities, a difficulty identifying and categorizing non-specific symptoms, and underdiagnosis due to physician biases [40].
2.4. Smoking and Alcohol
Several studies have cited smoking and alcohol as risk factors for OSA. This could be explained by a general decrease in sleep latency, difficulty in initiating sleep, and irregular sleeping patterns after smoking or drinking [44]. The chemicals consumed during smoking can also result in local inflammation and fluid retention in the upper airway, which could exacerbate these symptoms. Where many studies have observed a positive correlation between smoking and OSA, such as in a study by Kashyap et al., who reported the occurrence of OSA to be approximately twice as likely in current smokers as compared to previous smokers and non-smokers combined [45], many other studies have shown the opposite association, or did not observe smoking to be an independent variable for OSA. However, the number of cigarettes consumed per day was still reported to be higher among more severe forms of OSA [46]. Some studies have also reported smoking addiction due to untreated OSA [47]. This variability in results suggests an inconclusive consensus about the role of smoking in OSA progression and severity; thus, further studies are required to elucidate the relevant mechanisms involved.
On the other hand, the role of alcohol seems to be relatively established among OSA patients. The studies show a general consensus that alcohol is positively correlated with an increased risk and severity of OSA by 25% [48]. The likely mechanisms include the relaxation of muscles in the neck and throat leading to airway collapse, decreased ventilatory responses to an increase in higher partial pressure of CO2 and lower pressure of oxygen, and reduction in muscle activity in the tongue [40]. While we know that alcohol can increase the risk of OSA, it would be beneficial to understand if these effects are impacted by individual race, metabolic and immune status, number of drinks consumed per week, and whether individuals are suffering from any other comorbidities. Answers to these questions will allow for a better public health policy.
2.5. Inflammation
Inflammation plays an integral role in the induction, progression, and exacerbation of OSA. Over the years, several inflammatory mediators have been corelated with the pathogenesis of OSA; however, some are more extensively researched and notably reported, including CRP, IL-6, IL-8, IL-33 and its receptor ST2, Pentraxin-3 (PTX-3), procalcitonin (ProCT), and TNF-a [49][50]. These mediators can play an important role as a biomarker to decipher the severity of OSA among patients. Particularly, PTX-3 as a predictor of OSA severity has garnered attention due to its consistent specificity and sensitivity across studies. For example, Sozer et al. reported a specificity and sensitivity of 91.7% for PTX-3 as a predictor of OSA among other inflammatory mediators [49]. They also reported a positive correlation between PTX-3 and BMI, suggesting a potential link between these two variables in the subsequent progression of the disease. Other studies have noted the importance of morning levels of PTX-3 as a sensitive biomarker, as patients with OSA could have a higher hypoxic state during sleep [51]. PTX-3 is essentially from the same family as CRP, an acute phase protein with a role in innate immunity, the regulation of inflammatory reactions, and apoptosis. Its role in the pathogenesis of OSA was further elucidated after treating patients with CPAP. In a study by Kobukai et al., there was a marked reduction in the morning levels of PTX-3 and CRP; however, only PTX-3 levels were shown to be significantly correlated with the severity of OSA using AHI [51].
CRP is another important biomarker that has been extensively researched in the pathogenesis of OSA. However, its association and specificity have been questioned in the last two decades, given the variable results across studies [50][52][53][54]. A strong relationship between OSA and obesity was established in earlier studies, and perhaps this relationship could distort the data if the patients were not optimally matched for BMI [55].
References
- Bahammam, A. Obstructive Sleep Apnea: From Simple Upper Airway Obstruction to Systemic Inflammation. Ann. Saudi Med. 2011, 31, 1–2.
- Littleton, S.W.; Mokhlesi, B. The Pickwickian Syndrome—Obesity Hypoventilation Syndrome. Clin. Chest Med. 2009, 30, 467–478.
- Sleep Eos. A Brief History of Sleep Apnea and Sleep Apnea Signs & Symptoms. Eos Sleep, 2015. Available online: https://www.eossleep.com/2015/05/26/a-brief-history-of-the-causes-of-sleep-apnea/ (accessed on 22 October 2022).
- Redline, S.; Young, T. Epidemiology and Natural History of Obstructive Sleep Apnea. Ear Nose Throat J. 1993, 72, 20–26.
- Yaremchuk, K.; Garcia-Rodriguez, L. The History of Sleep Surgery. In Sleep-Related Breathing Disorders; Karger Publishers: Basel, Switzerland, 2017; Volume 80, pp. 17–21.
- Silber, M.H. Tracheostomy Can Fatally Exacerbate Sleep-Disordered Breathing in Multiple System Atrophy. Available online: https://n.neurology.org/content/tracheostomy-can-fatally-exacerbate-sleep-disordered-breathing-multiple-system-atrophy-0 (accessed on 22 October 2022).
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504.
- Laratta, C.R.; Ayas, N.T.; Povitz, M.; Pendharkar, S.R. Diagnosis and treatment of obstructive sleep apnea in adults. Can. Med. Assoc. J. 2017, 189, E1481–E1488.
- Finnsson, E.; Ólafsdóttir, G.H.; Loftsdóttir, D.L.; Jónsson, S.Æ.; Helgadóttir, H.; Ágústsson, J.S.; Sands, S.A.; Wellman, A. A scalable method of determining physiological endotypes of sleep apnea from a polysomnographic sleep study. Sleep 2021, 44, zsaa168.
- Wellman, A.; Eckert, D.; Jordan, A.; Edwards, B.; Passaglia, C.; Jackson, A.C.; Gautam, S.; Owens, R.L.; Malhotra, A.; White, D.P. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J. Appl. Physiol. 2011, 110, 1627–1637.
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining Phenotypic Causes of Obstructive Sleep Apnea. Identification of Novel Therapeutic Targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004.
- Carberry, J.; Amatoury, J.; Eckert, D.J. Personalized Management Approach for OSA. Chest 2018, 153, 744–755.
- Malhotra, A.; Pillar, G.; Fogel, R.; Beauregard, J.; Edwards, J.; White, D.P. Upper-Airway Collapsibility*. Chest 2001, 120, 156–161.
- Malhotra, A.; Jordan, A. The importance of arousal in obstructive sleep apnea—Updates from the American Thoracic Society. J. Thorac. Dis. 2016, 8, S542–S544.
- Subramani, Y.; Singh, M.; Wong, J.; Kushida, C.A.; Malhotra, A.; Chung, F. Understanding Phenotypes of Obstructive Sleep Apnea: Applications in Anesthesia, Surgery, and Perioperative Medicine. Anesth. Analg. 2017, 124, 179–191.
- Vanek, J.; Prasko, J.; Genzor, S.; Ociskova, M.; Kantor, K.; Holubova, M.; Nesnidal, V.; Kolek, A.; Sova, M. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020, 72, 50–58.
- De Felício, C.M.; da Silva Dias, F.V.; Trawitzki, L.V.V. Obstructive sleep apnea: Focus on myofunctional therapy. Nat. Sci. Sleep 2018, 10, 271–286.
- Mukundan, T.H. Pediatric Obstructive Sleep Apnea and Pediatric Hypersomnia. In Sleep Disorders in Women: A Guide to Practical Management; Attarian, H., Viola-Saltzman, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 115–128, (Current Clinical Neurology).
- Sweetman, A.; Lack, L.; McEvoy, R.D.; Smith, S.; Eckert, D.J.; Osman, A.; Carberry, J.C.; Wallace, D.; Nguyen, P.D.; Catcheside, P. Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA). Sleep Med. Rev. 2021, 60, 101519.
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions Between Obesity and Obstructive Sleep Apnea: Implications for Treatment. Chest 2010, 137, 711–719.
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. 2017, 1, 00019.
- Newman, A.B.; Foster, G.; Givelber, R.; Nieto, F.J.; Redline, S.; Young, T. Progression and regression of sleep-disordered breathing with changes in weight: The Sleep Heart Health Study. Arch. Intern. Med. 2005, 165, 2408–2413.
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal Study of Moderate Weight Change and Sleep-Disordered Breathing. JAMA 2000, 284, 3015–3021.
- Redline, S.; Tishler, P.V.; Tosteson, T.D.; Williamson, J.; Kump, K.; Browner, I.; Ferrette, P.; Krejci, P. The familial aggregation of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 151, 682–687.
- Ferini-Strambi, L.; Calori, G.; Oldani, A.; Della Marca, G.; Zucconi, M.; Castronovo, V.; Gallus, G.; Smirne, S. Snoring in twins. Respir. Med. 1995, 89, 337–340.
- Mukherjee, S.; Saxena, R.; Palmer, L.J. The genetics of obstructive sleep apnea. Respirology 2018, 23, 18–27.
- Zhong, A.; Xiong, X.; Xu, H.; Shi, M. An Updated Meta-Analysis of the Association between Tumor Necrosis Factor-α-308G/A Polymorphism and Obstructive Sleep Apnea-Hypopnea Syndrome. PLoS ONE 2014, 9, e106270.
- Patel, S.R.; Goodloe, R.; De, G.; Kowgier, M.; Weng, J.; Buxbaum, S.G.; Cade, B.; Fulop, T.; Gharib, S.A.; Gottlieb, D.J.; et al. Association of genetic loci with sleep apnea in European Americans and African-Americans: The Candidate Gene Association Resource (CARe). PLoS ONE 2012, 7, e48836.
- Glasser, M.; Bailey, N.; McMillan, A.; Goff, E.; Morrell, M. Sleep apnoea in older people. Breathe 2011, 7, 248–256.
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235.
- Hader, C.; Schroeder, A.; Hinz, M.; Micklefield, G.H.; Rasche, K. Sleep disordered breathing in the elderly: Comparison of women and men. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2005, 56, 85–91.
- Shochat, T.; Pillar, G. Sleep apnoea in the older adult: Pathophysiology, epidemiology, consequences and management. Drugs Aging 2003, 20, 551–560.
- Mant, A.; Saunders, N.A.; Eyland, A.E.; Pond, C.D.; Chancellor, A.H.; Webster, I.W. Sleep-Related Respiratory Disturbance and Dementia in Elderly Females. J. Gerontol. 1988, 43, M140–M144.
- Enright, P.L.; Newman, A.B.; Wahl, P.W.; Manolio, T.A.; Haponik, F.E.; Boyle, P.J.R. Prevalence and Correlates of Snoring and Observed Apneas in 5201 Older Adults. Sleep 1996, 19, 531–538.
- Kim, S.W.; Taranto-Montemurro, L. When do gender differences begin in obstructive sleep apnea patients? J. Thorac. Dis. 2019, 11 (Suppl. 9), S1147–S1149.
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J. Sleep Res. 2018, 28, e12770.
- Bonsignore, M.R.; Borel, A.-L.; Machan, E.; Grunstein, R. Sleep apnoea and metabolic dysfunction. Eur. Respir. Rev. 2013, 22, 353–364.
- Zhou, X.; Zhou, B.; Li, Z.; Lu, Q.; Li, S.; Pu, Z.; Luo, F. Gender differences of clinical and polysomnographic findings with obstructive sleep apnea syndrome. Sci. Rep. 2021, 11, 1–6.
- Millman, R.P.; Carlisle, C.C.; McGarvey, S.T.; Eveloff, S.E.; Levinson, P.D. Body Fat Distribution and Sleep Apnea Severity in Women. Chest 1995, 107, 362–366.
- Mehra, R.; Gharibeh, T. Obstructive sleep apnea syndrome: Natural history, diagnosis, and emerging treatment options. Nat. Sci. Sleep 2010, 2, 233–255.
- Malhotra, A.; Huang, Y.; Fogel, R.B.; Pillar, G.; Edwards, J.K.; Kikinis, R.; Loring, S.H.; White, D.P. The male predisposition to pharyngeal collapse: Importance of airway length. Am. J. Respir. Crit. Care Med. 2002, 166, 1388–1395.
- Matsumoto, A.M.; Sandblom, R.E.; Schoene, R.B.; Lee, K.A.; Giblin, E.C.; Pierson, D.J.; Bremner, W.J. Testosterone replacement in hypogonadal men: Effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin. Endocrinol. 1985, 22, 713–721.
- Anttalainen, U.; Saaresranta, T.; Aittokallio, J.; Kalleinen, N.; Vahlberg, T.; Virtanen, I.; Polo, O. Impact of menopause on the manifestation and severity of sleep-disordered breathing. Acta Obstet. Gynecol. Scand. 2006, 85, 1381–1388.
- Liao, Y.; Xie, L.; Chen, X.; Kelly, B.C.; Qi, C.; Pan, C.; Yang, M.; Hao, W.; Liu, T.; Tang, J. Sleep quality in cigarette smokers and nonsmokers: Findings from the general population in central China. BMC Public Health 2019, 19, 808.
- Kashyap, R.; Hock, L.M.; Bowman, T.J. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath 2001, 5, 167–172.
- Ioannidou, D.; Kalamaras, G.; Kotoulas, S.-C.; Pataka, A. Smoking and Obstructive Sleep Apnea: Is There An Association between These Cardiometabolic Risk Factors?—Gender Analysis. Medicina 2021, 57, 1137.
- Krishnan, V.; Dixon-Williams, S.; Thornton, J.D. Where There Is Smoke…There Is Sleep Apnea: Exploring the relationship between smoking and sleep apnea. Chest 2014, 146, 1673–1680.
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol and the risk of sleep apnoea: A systematic review and meta-analysis. Sleep Med. 2018, 42, 38–46.
- Sozer, V.; Kutnu, M.; Atahan, E.; Ozturk, B.C.; Hysi, E.; Cabuk, C.; Musellim, B.; Simsek, G.; Uzun, H. Changes in inflammatory mediators as a result of intermittent hypoxia in obstructive sleep apnea syndrome. Clin. Respir. J. 2017, 12, 1615–1622.
- McNicholas, W.T. Obstructive Sleep Apnea and Inflammation. Prog. Cardiovasc. Dis. 2009, 51, 392–399.
- Kobukai, Y.; Koyama, T.; Watanabe, H.; Ito, H. Morning pentraxin3 levels reflect obstructive sleep apnea-related acute inflammation. J. Appl. Physiol. 2014, 117, 1141–1148.
- Yokoe, T.; Minoguchi, K.; Matsuo, H.; Oda, N.; Minoguchi, H.; Yoshino, G.; Hirano, T.; Adachi, M. Elevated Levels of C-Reactive Protein and Interleukin-6 in Patients with Obstructive Sleep Apnea Syndrome Are Decreased by Nasal Continuous Positive Airway Pressure. Circulation 2003, 107, 1129–1134.
- Taheri, S.; Austin, D.; Lin, L.; Nieto, F.J.; Young, T.; Mignot, E. Correlates of Serum C-Reactive Protein (CRP)—No Association with Sleep Duration or Sleep Disordered Breathing. Sleep 2007, 30, 991–996.
- Hayashi, M.; Fujimoto, K.; Urushibata, K.; Takamizawa, A.; Kinoshita, O.; Kubo, K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology 2006, 11, 24–31.
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
828
Revisions:
2 times
(View History)
Update Date:
09 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




