Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. Physiology of the Endocannabinoid System. Encyclopedia. Available online: https://encyclopedia.pub/entry/40968 (accessed on 07 February 2026).
Matei D, Trofin D, Iordan DA, Onu I, Condurache I, Ionite C, et al. Physiology of the Endocannabinoid System. Encyclopedia. Available at: https://encyclopedia.pub/entry/40968. Accessed February 07, 2026.
Matei, Daniela, Dan Trofin, Daniel Andrei Iordan, Ilie Onu, Iustina Condurache, Catalin Ionite, Ioana Buculei. "Physiology of the Endocannabinoid System" Encyclopedia, https://encyclopedia.pub/entry/40968 (accessed February 07, 2026).
Matei, D., Trofin, D., Iordan, D.A., Onu, I., Condurache, I., Ionite, C., & Buculei, I. (2023, February 08). Physiology of the Endocannabinoid System. In Encyclopedia. https://encyclopedia.pub/entry/40968
Matei, Daniela, et al. "Physiology of the Endocannabinoid System." Encyclopedia. Web. 08 February, 2023.
Copy Citation
The endocannabinoid system (ECS) is involved in various processes, including brain plasticity, learning and memory, neuronal development, nociception, inflammation, appetite regulation, digestion, metabolism, energy balance, motility, and regulation of stress and emotions.
the endocannabinoid system
physical exercise
CB1
CB2
1. The ECS and the Nervous System
The CB1 receptor is mainly expressed in the brain in areas of primary sensory and motor regions as well as areas of memory, cognition, and emotion, all of which overlap the areas of the autonomic nervous system and neuroendocrine system [1]. Kano et al. suggested that the highest levels of CB1 receptor binding were observed in the olfactory bulb, as well as in the regions of the dentate gyrus, the lateral striatum, the globus pallidus, the entopeduncular nucleus, the substantia nigra (SN) pars reticulata, and the cerebellar molecular layer [2]. Moderate levels were found in the frontal, parietal, and cingulate regions, in the amygdala, in the ventromedial hypothalamus, in the parabrachial nucleus, in the nucleus of the solitary tract, and in the spinal dorsal horn [2]. Low levels of CB1 receptor were found at the level of the thalamus and at the level of the brain stem nuclei [2]. The CB1 receptor is also expressed in peripheral nerve terminals, and in non-neuronal cells, such as endocrine, liver, lung, kidney, endothelial, myocardium, and skeletal muscles; white and brown adipose tissues; glial cells; and astrocytes [3]. As neuromodulators, CB1 receptors modulate synaptic transmission of many neurotransmitters, such as GABA, glutamate, serotonin, acetylcholine (Ach), or cholecystokinin (CCK), among others [4].
The endocannabinoid system is believed to play a major role in neuropathic pain (NP). NP, besides pain, is characterized by sensory impairment that can sometimes lead to extreme phenomena such as allodynia or hyperalgesia. The symptomatology may extend towards affecting cognitive and emotional functions. In spite of all these, spontaneous pain is the main burdensome aspect of NP [5]. The ECS modulates pain control and pathological aspects of NP by the activities of CB1 and CB2 receptors [5]. Peripherally, in nociceptive terminations, CB1R inhibits the nociceptive stimuli and also inhibits neurotransmitter release and pain transmission at the dorsal root ganglia and the dorsal horn of the spinal cord. At the supraspinal level, they inhibit the ascension of nociceptive transmission, especially towards the thalamus. They play a role in activating the descending inhibitory pathway by inhibiting GABA release in the medulla, as well as influencing the emotional pain perceived in the limbic system. In contrast, CB2R was more involved in modulating immune responses associated with chronic pain in the spinal cord [5][6][7][8][9].
Although the mechanisms described above are important for understanding the functionality of the ECS in terms of developing novel therapeutic approaches to treat pain, especially related to receptor agonists, it is of interest to search for possible self-regulatory ways in which the endocannabinoids can help without exogenous intervention. Therefore, the sudden euphoria experienced by endurance sport practitioners is believed to enhance hypoalgesia and even sedation in addition to increasing euphoric levels and reducing anxiety [10][11]. Moreover, euphoria and increased eCBs relate differently to exercise intensity, duration, and individual aspects [12]. Apparently, blocking the opioid receptors with central opioid blockers does not inhibit exercise-dependent hypoalgesia, as opposed to CB1 and CB2 receptors blockade, thus suggesting the eCB signaling role [13][14].
The CB2 receptor can be found mainly in immune cells such as macrophages, B lymphocytes, and blood stem cells as well as in the organs of the immune system, e.g., spleen, tonsils and thymus and to a lesser extent, in the cerebral cortex, cerebellum, and the gastrointestinal tract [15][16]. Recent studies suggested that CB2 receptors are expressed in microglia or glial cells [16]. In the gastrointestinal tract, CB2 receptors regulate intestinal inflammatory reactions [17]. Evidence of a CB3 receptor or “anandamide receptor” in the brain and in the endothelial tissues is still under evaluation [15].
Both the CB1 and CB2 receptors are coupled to Gi/o protein alpha-subunits that inhibit adenylyl cyclase activity and, thereby, depending on the cell type, either inhibit voltage gated calcium channels or activate potassium channels [15][18]. The effect of CB1 activation is neuronal inhibition [15].
The CB2 receptor is an important target for neurodegenerative diseases and cancer; the new radioligand [18F]LU13 is a promising radioligand for the imaging of upregulated CB2R expression using positron emission tomography under pathological conditions in the brain [19].
The ECS is involved in various processes, including brain plasticity, learning and memory, neuronal development, nociception, inflammation, appetite regulation, digestion, metabolism, energy balance, motility, and regulation of stress and emotions (Figure 1).
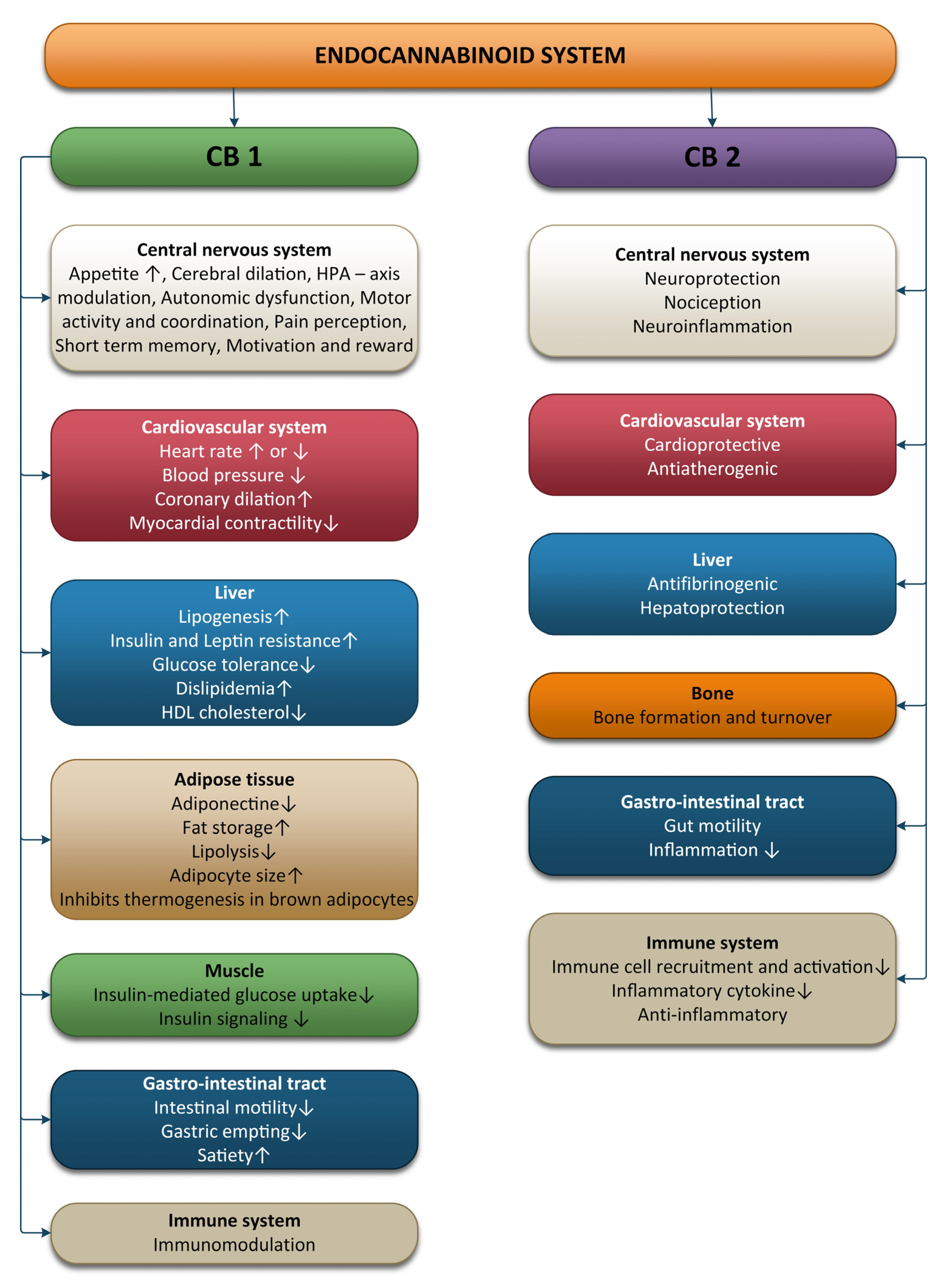
Figure 1. Effects of the endocannabinoid system on different systems and organs.
The ECS modulates the sympathetically driven hypothalamic-pituitary-adrenocortical (HPA) axis as well as the hypothalamic-locus coeruleus-norepinephrine axis. In response to biological stress, the first neurons activated are the NE norepinephrine neurons of the locus coeruleus. Subsequently, the paraventricular nucleus (PVN) of the hypothalamus initiates corticotropin releasing hormone (CRH) secretion, thus inducing adrenocorticotropin hormone (ACTH) release. ACTH stimulates the release of glucocorticoids (cortisol in humans) from the adrenal cortex into general circulation. The amygdala activates the HPA axis, whereas the hippocampus and prefrontal cortex inhibit the HPA axis [20].
Sympathetic nerve terminals contain CB1R, and activation of these receptors has been shown to inhibit norepinephrine release and reduce pain. The SNS mediates the anxiety-like effects observed after CB1R blockade [21]. CB1R activity limits hypothalamic CRH release and restricts ACTH and glucocorticoid release [22]. In contrast, glucocorticoids induce rapid increases in endocannabinoid synthesis in brain areas that shape the perception of psychological stressors [23]. After acute stress, the released glucocorticoids activate G-protein membrane receptors in the basolateral amygdala (BLA), promoting a rapid increase in retrograde 2-AG signaling that leads to the suppression of GABAergic synaptic inputs onto BLA principal neurons, inducing fast increases in anxiety-like behavior [24]. Enhancing FAAH activity results in glucocorticoid release and stress-related anxiety-like behavior [25]. Fatty acid amide hydrolase inhibition or costimulation of CB1R and TRPV1 (transient receptor potential cation channel subfamily V member 1) receptors decreases the release of stress-induced corticoids [26][27].
Stress produces bidirectional changes in the AEA and 2-AG, with stress exposure reducing AEA levels and increasing 2-AG levels in the hypothalamus, prefrontal cortex and hippocampus; these changes are associated with a decreased HPA-axis activity that modulates memory processes and pain perception after stressful stimuli [28].
Prolonged stress may influence the overall health via several different pathways, i.e., alterations in the autonomic nervous system (increased SNS and decreased PNS activity), in the neuroendocrine activity as well as in the immune, behavioral, and cognitive functions. Chronic stress causes hyperactivity of the sympathetic nervous system, which occurs with reduced activation of the HPA axis. Straub et al. termed this physiological phenomenon “uncoupling of SNS and HPA-axis” [29].
The autonomic imbalance during chronic stress described by increased SNS and suppressed PNS activity is associated with cellular stress, including an increase in the levels of cytokine and reactive oxygen species. Exposure to chronic stress reliably causes a downregulation or loss of CB1R in brain regions related with emotional processing. Chronic stress promotes FAAH activity through glucocorticoid stimulation, and anandamide levels are maintained low in the hippocampus, hypothalamus, PFC, and amygdala, which leads to hyperexcitability of the amygdala circuits [30]. The inhibition of FAAH activity prevents these effects of chronic stress and reduces changes that chronic stress promotes in BLA [31][32]. The amygdala sends CB1R-positive projections to the bed nucleus of the stria terminalis (BNST), which is connected with the ventral tegmental area and the locus coeruleus. Glutamatergic and GABAergic projections from the amygdala to the BNST are each sufficient for the development of anxious responses to unpredictable stimuli [30]. CB1R in cortical glutamatergic terminals inhibit glutamate release, and their activation induces anxiolysis, whereas CB1R activation from GABAergic neurons of the forebrain restricts the inhibitory GABA tone and mainly facilitates anxiety [30].
The vagus nerve has afferent and efferent fibers controlling cardiopulmonary, gastrointestinal, metabolism, and many other functions. Vagal afferent neurons transmit visceral information to the nucleus of the solitary tract (NTS) and release glutamate controlled via cannabinoid-sensitive excitatory transient receptor potential (TRP) channels [33]. Three groups of PNS afferent fiber types can be determined using the conduction velocity: slow-conducting unmyelinated C-fibers, moderate-conducting thinly myelinated Aδ-fibers, and fast-conducting heavily myelinated A-fibers. All these afferent subgroups abundantly express a complement of TRP channels, although Transient Receptor Potential Vanilloid Type 1 (TRPV1) is selectively expressed on all C- and some Aδ-fibers, but not in A fibers [33]. TRPV1 is activated by AEA, which reduces synthesis of 2-AG and is implicated in hyperalgesia, chronic pain, inflammation, neurogenesis, and anxiety [34][35]. The sensitivity of TRPV1 to AEA requires CB1-mediated activation of phosphoinositide-3-kinase and phospholipase C [36]. TRPV1 is responsive to both capsaicin and AEA, but TRPV1 requires CB1 activation for sensitivity to AEA but not capsaicin [37].
The activation of vagal afferents via the TRP channels by ECS may have the same therapeutic effects such as electrical vagus nerve stimulation [38][39]. Many studies have shown that acute cannabis use causes hypertension, tachycardia, and a fivefold increase in risk of adverse cardiovascular events including stroke and heart attack [40][41][42][43]. In contrast, chronic cannabis use prevents hypertension, atherosclerosis, and improves cardiovascular disease outcomes [40][41][42][43]. These observations highlight that acute consumption of cannabis leads to harmful effects, because it causes impaired vagal afferent signaling, while the chronic effects of cannabis are associated with increased vagal afferent signaling. These different actions can be explained by the desensitization between TRP channels and CB1 receptors [44]. TRP channels are expressed in primary sensory afferents such as vagal afferents to central circuits, and in non-neuronal tissue such as smooth muscle [45].
Enhancement of vagal afferent signaling may serve to ameliorate autonomic dysfunction associated with a variety of other chronic diseases such as atherosclerosis, diabetes, obesity, epilepsy, anxiety, and depression [40][41][42][43][46][47][48][49]; all of these are each associated with vagal hypofunction, increased heart-rate, and heart-rate variability. Both VNS and ECS increase may improve autonomic dysfunctions by stimulation of vagal afferents that compensate for vagal hypofunction associated with these disorders. The mechanisms by which the vagus may influence these chronic diseases are by reducing oxidative stress and systemic inflammation [50]. Oxidative stress is reduced with the help of antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase, etc.) and non-enzymatic antioxidants (melatonin, lipoic acid, Coenzyme Q10, glutathione, uric acid, bilirubin, etc.). Exercises, depending on the intensity can induce ROS generation, which will result in increased activity of enzymatic antioxidants, thereby leading to increased resistance to oxidative challenges [51][52]. The progenitor cells in the hippocampal region in adults and the embryonic cells produce endocannabinoids, which are expressed upon stimulation of CB1 receptors and the suppression of FAAH enzyme [53]. This demonstrates the involvement of the ECS in neurogenesis. Moreover, the ECS possesses neuroprotective functions, providing protection against acute hypoxia, oxidative stress, and traumatic insults. In mice with a closed head injury, the levels of 2-AG were elevated. Further administration of 2-AG exogenously diminished edema in the brain, decreased the infarct volume, prevented death of hippocampal neuronal cells, and facilitated a better clinical recovery [54].
2. The ECS and the Cardiovascular System
CB1 receptors are well represented at the level of the cardiovascular system. CB1 can be found in the myocardial cells, coronary artery, endothelial cells, and smooth muscle cells. CB2 was also detected in the coronary endothelial cells, smooth muscle cells, and myocardial cells. This means endocannabinoids are produced at this level, are circulating through blood, and play an important role in the management and regulation of the cardiovascular system functions [55]. The results of studies conducted on animals show that endocannabinoids have an impact on blood pressure and heart rate, but the effects are influenced by the condition of the animal (conscious or anesthetized); this implies that endocannabinoids act by modulating the autonomic nervous system [56]. Anandamide has an extended response to hypotension in animals that are anaesthetized, thereby inhibiting the sympathetic activity on the nerve terminals present at the level of the heart and vasculature. CB1 receptors upon activation in arteries inhibit the release of noradrenaline in the mesenterial arterial bed region [56]. In conscious rats, anandamide produces bradycardia with a transient condition of hypertension followed by long-lasting pressor effects, which are accompanied by renal and mesenteric vasoconstriction [57]. Upon activation of CB1, adrenaline blood levels increase via adrenergic beta-2 receptors as well as increased sympathetic activity.
Furthermore, the ECS is involved in the central control of blood pressure associated with the brainstem baroreceptor complexes [58]. Anandamide microinjection can prolong the reflex inhibition of the renal sympathetic nerve activity and suggest that inhibition of the GABAergic tone can lead to an increased sensitivity to baroreflexes. Moreover, an increase in blood pressure due to phenylephrine can enhance the concentrations of anandamide in the region of the nucleus tractus solitarius and support the ECS in controlling and regulating the activity of baroreflexes [59].
A study conducted on mice with blocked CB1 receptors showed significant conditions of elevated heart rate and blood pressure due to irregular breathing during sleep and a disturbed sleep-awake cycle [55]. Studies conducted on rats with hypertension showed that CB1 receptor expression was higher in the cardiac tissue and in the aortic endothelium of rats compared to healthy rats [60].
3. The ECS and the Immune System
The ECS also has an important role in the maintenance of immune homeostasis. Immune cells, especially immune B cells, followed by monocytes, natural killer cells, neutrophils, and CD4 and CD8 lymphocytes, express ECS receptors. CB2 receptors are 10–100 times more present at this level [55][61][62]. Both CB1 and CB2 receptors are important modulators of the immune system, inducing immunosuppression [63].
CB2 receptors have a modulatory role on the immune system, which is related to the induction of apoptosis, suppression of cell proliferation, inhibition of pro-inflammatory cytokines production, increase in anti-inflammatory cytokines, and induction of regulatory T cells [64]. CB2 activation induces a shift from Th1 to Th2 immune response and induced myeloid-derived suppressor and T-regulatory cells [65]. A deficiency of CB2 receptors can activate neutrophils to the inflammation sites. The activity of macrophages associated with tumors is also believed to be inhibited by CB2 receptors. The cytokine implicated in the activity and differentiation of T cells is interleukin 2, which is secreted upon the activation of natural killer cells and T-cells. AEA has a high affinity to CB1, thereby reducing the production of pro-inflammatory IL-6 [66]. Sardinha et al., in 2014, pointed out that the inhibition of MAGL and FAAH, the enzymes that respectively degrade 2-AG and AEA, has CB2-mediated anti-inflammatory effects [67].
Classically activated macrophages (M1) have pro-inflammatory and anti-tumor properties by releasing various types of pro-inflammatory cytokines and chemokines such as TNF, IL-6, and IL-1β. Alternative activated macrophages (M2) perform anti-inflammatory and immunosuppressive effects by releasing anti-inflammatory cytokines (IL-10) and promote tumor progression. An imbalance of M1/M2 is responsible for inflammation [68].
CB2 receptors inhibit immune cell activation and pro-inflammatory mediator release. Moreover, CB2 receptor stimulation with its selective agonists reversed these pathological conditions by reducing both B and T lymphocytes, by promoting anti-inflammatory activities, and by limiting pro-inflammatory cytokine release in macrophages, thereby inhibiting M1 polarization [69][70]. Immunosuppression is the consequence of stimulation of CB2 receptors, which helps to reduce inflammation and prevents associated injury in tissues.
4. The ECS and the Digestive System
One of the best described axes of the body is the bidirectional axis that links the brain and the gastro-intestinal tract (GI); this axis is formed of hormonal and neuronal pathways and is known as the brain-gut axis. The nutrient intake and availability as well as the energy status at the peripheral level are supervised by the GI tract, liver, adipose tissue, pancreas, and skeletal muscle, all of which provide information to the CNS with the aim of maintaining energy homeostasis. The vagus and splanchnic nerves are responsible for the neural signaling of this axis. The communication between the liver and gut, pancreas, and adipose tissue is also ensured by the vagus nerve. Areas of the brain that are stimulated by gut hormones are areas were the blood-brain-barrier is absent or were transporters for the gut hormones that permitted them to signal the CNS. These areas are represented in the brainstem by the area postrema and in the hypothalamus by the arcuate nucleus. Information from the enteric nervous system and from the enteroendocrine cells are transmitted by the vagal afferent neurons to the brainstem in the nucleus of the solitary tract [71].
The ECS is involved in the peripheral and central control of gut functions. Endocannabinoids are not stored like hormones or neurotransmitters at the level of vesicles; moreover, specific signals are needed to induce their synthesis at the lipid membrane level, making them ideal mediators for responding in real-time to the ever-changing feeding state of an organism. The enzymes involved in the synthesis of anandamide, FAAH, and NAPE-PLD can be found in the GI tract, and CB1 and CB2 receptors are also present in the GI tract in the enteric nerves and epithelium. CB1 is highly present in the enteric nervous system on all classes of enteric neurons except inhibitory motor neurons and on some enteroendocrine cells. CB2 is also found on enteric neurons and on immune and epithelial cells in the GI tract [72]. When AEA and 2-AG trigger CB1 and CB2 receptors, the biochemical responses will frequently depend on the type of cell stimulated; the response varies from decreased levels of cAMP thought the inhibition of Ca2+ channels and adenylate cyclase activity to increased activity of mitogen-activated protein kinase pathways, phospholipases, and K+ channels [73].
Normally, under physiological conditions, the ECS activity is ensured by the CB1 receptor, whose activation triggers actions such as increased gastrointestinal motility, mesenteric vasodilatation, and suppression of fluid and acid secretion. Because of the high expression of CB1 in neurons of the CNS, this receptor is also implicated in the regulation of peripheral organs that play an important part in metabolic homeostasis and also control feeding, reward, and energy expenditure. When inflammation is present in the GI tract, the activation of both receptors takes place, resulting in the synthesis of anti-inflammatory cytokines with a role in reducing inflammation and its secondary damages [74][75][76][77][78][79].
Appetite and food intake are locally balanced by the activation of the CB1 receptor that modulates the activity of hypothalamic neurons that cause the release of orexigenic and anorexigenic neuropeptides as well as the function of the mesolimbic and brainstem neurons by directing information to these neurons from the periphery. Therefore, CB1 receptors are implicated both in the homeostatic and hedonic aspects of food intake [73].
The white adipose tissue (WAT) is an essential regulator of energy storage and systemic metabolic homeostasis, and studies conducted in vitro show that the ECS plays a role in the regulation of adipogenesis and lipogenesis, with the CB1 receptor being expressed at the level of mature white adipose cells. The activation of glucose uptake, lipoprotein lipase, and fatty acid synthase; stimulation of PPARg expression and adipogenesis; inhibition of cAMP release, AMPK, and mitochondrial biogenesis; and inhibition of adiponectin production in hypertrophic adipocytes are the mechanisms described in the regulation of adipogenesis and lipogenesis. It was observed that, in animal and human obesity, the CB1 receptor expressed at the level of white adipocytes, WAT AEA, and 2-AG levels are usually deregulated [73][79][80][81][82][83].
The CB1 receptor plays an important role in lipogenesis regulation by enhancing the production of acetyl-Coenzyme A (CoA) and Fas, because it upregulates the sterol regulatory element binding transcription factor 1 (SREBF1). Endocannabinoids and the ECS receptor are also produced in muscle cells and express the enzymes implicated in ECS synthesis and degradation [73].
References
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755.
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380.
- McPartland, J.; Guy, G.; Di Marzo, V. Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System. PloS ONE 2014, 9, e89566.
- Cohen, K.; Weizman, A.; Weinstein, A. Modulatory effects of cannabinoids on brain neurotransmission. Eur. J. Neurosci. 2019, 50, 2322–2345.
- Maldonado, R.; Baños, J.E.; Cabañero, D. The endocannabinoid system and neuropathic pain. Pain 2016, 157, S23–S32.
- Ibrahim, M.M.; Porreca, F.; Lai, J.; Albrecht, P.J.; Rice, F.L.; Khodorova, A.; Davar, G.; Makriyannis, A.; Vanderah, T.W.; Mata, H.P.; et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. USA 2005, 102, 3093–3098.
- Nadal, X.; La Porta, C.; Andreea Bura, S.; Maldonado, R. Involvement of the opioid and cannabinoid systems in pain control: New insights from knockout studies. Eur. J. Pharmacol. 2013, 716, 142–157.
- La Porta, C.; Bura, S.A.; Aracil-Fernández, A.; Manzanares, J.; Maldonado, R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain 2013, 154, 160–174.
- Vaughan, C.W.; Connor, M.; Bagley, E.E.; Christie, M.J. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol. Pharmacol. 2000, 57, 288–295.
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541.
- Siebers, M.; Biedermann, S.V.; Bindila, L.; Lutz, B.; Fussa, J. Exercise-Induced Euphoria and Anxiolysis Do Not Depend on Endogenous Opioids in Humans. Psychoneuroendocrinology 2021, 126, 105173.
- Berger, B.G.; Owen, D.R. Relation of low and moderate intensity exercise with acute mood change in college joggers. Percept. Mot. Skills 1998, 87, 611–621.
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13105–13108.
- Crombie, K.M.; Brellenthin, A.G.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and Opioid System Interactions in Exercise-Induced Hypoalgesia. Pain Med. 2018, 19, 118–123.
- Basavarajappa, B. Neuropharmacology of the Endocannabinoid Signaling System Molecular Mechanisms, Biological Actions and Synaptic Plasticity. Curr. Neuropharmacol. 2007, 5, 81–97.
- Cabral, G.; Raborn, R.; Griffin, L.; Dennis, J.; Marciano-Cabral, F. CB2 receptors in the brain: Role in central immune. Br. J. Pharmacol. 2009, 153, 240–251.
- Wright, K.; Duncan, M.; Sharkey, K. Cannabinoid CB2 receptors in the gastrointestinal tract: A regulatory system in states of inflammation. Br. J. Pharmacol. 2008, 153, 263–270.
- Howlett, A.; Breivogel, C.; Childers, S.; Deadwyler, S.; Hampson, R.; Porrino, L. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 2004, 47, 345–358.
- Gündel, D.; Deuther-Conrad, W.; Ueberham, L.; Kaur, S.; Otikova, E.; Teodoro, R.; Moldovan, R.P. Structure-Based Design, Optimization, and Development of LU13: A Novel Radioligand for Cannabinoid Receptor Type 2 Imaging in the Brain with PET. J. Med. Chem. 2022, 65, 9034–9049.
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409.
- Bellocchio, L.; Soria-Gómez, E.; Quarta, C.; Metna-Laurent, M.; Cardinal, P.; Binder, E.; Marsicano, G. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proc. Natl. Acad. Sci. USA 2013, 110, 4786–4791.
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Pagotto, U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431.
- Hill, M.N.; Karatsoreos, I.N.; Hillard, C.J.; McEwen, B.S. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 2010, 35, 1333–1338.
- Di, S.; Itoga, C.A.; Fisher, M.O.; Solomonow, J.; Roltsch, E.A.; Gilpin, N.W.; Tasker, J.G. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J. Neurosci. 2016, 36, 8461–8670.
- Gray, J.M.; Vecchiarelli, H.A.; Morena, M.; Lee, T.T.; Hermanson, D.J.; Kim, A.B.; Hill, M.N. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 2015, 35, 3879–3892.
- Hartmann, A.; Fassini, A.; Scopinho, A.; Correa, F.M.; Guimarães, F.S.; Lisboa, S.F.; Resstel, L.B. Role of the endocannabinoid system in the dorsal hippocampus in the cardiovascular changes and delayed anxiety-like effect induced by acute restraint stress in rats. J. Psychopharmacol. 2019, 33, 606–614.
- Navarria, A.; Tamburella, A.; Iannotti, F.A.; Micale, V.; Camillieri, G.; Gozzo, L.; Di Marzo, V. The dual blocker of FAAH/TRPV1 N-arachidonoylserotonin reverses the behavioral despair induced by stress in rats and modulates the HPA-axis. Pharmacol. Res. 2014, 87, 151–159.
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological interactions between stress and the endocannabinoid system. Neuropsycho. Pharmacol. 2016, 41, 80–102.
- Straub, R.H.; Herfarth, H.; Falk, W.; Andus, T.; Scholmerich, J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J. Neuroimmunol. 2002, 126, 116–125.
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239.
- Duan, T.; Gu, N.; Wang, Y.; Wang, F.; Zhu, J.; Fang, Y.; Zhang, X. Fatty acid amide hydrolase inhibitors produce rapid anti-anxiety responses through amygdala long-term depression in male rodents. J. Psychiatry Neurosci. 2017, 42, 230–241.
- Yasmin, F.; Colangeli, R.; Morena, M.; Filipski, S.; van der Stelt, M.; Pittman, Q.J.; Chattarji, S. Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc. Natl. Acad. Sci. USA 2020, 117, 650–655.
- Mittleman, M.A.; Lewis, R.A.; Maclure, M.; Sherwood, J.B.; Muller, J.E. Triggering Myocardial Infarction by Marijuana. Circulation 2001, 103, 2805–2809.
- Marsch, R.; Foeller, E.; Rammes, G.; Bunck, M.; Kossl, M.; Holsboer, F.; Wotjak, C.T. Reduced Anxiety, Conditioned Fear, and Hippocampal Long-Term Potentiation in Transient Receptor Potential Vanilloid Type 1 Receptor-Deficient Mice. J. Neurosci. 2007, 27, 832–839.
- Bölcskei, K.; Helyes, Z.; Szabó, Á.; Sándor, K.; Elekes, K.; Németh, J.; Almási, R.; Szolcsányi, J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain 2005, 117, 368–376.
- Ho, B.Y.; Uezono, Y.; Takada, S.; Takase, I.; Izumi, F. Coupling of the expressed cannabinoid CB1 and CB2 receptors to phospholipase C and G protein-coupled inwardly rectifying K+ channels. Recept. Channels 1999, 6, 363–374.
- Fioravanti, B.; De Felice, M.; Stucky, C.L.; Medler, K.A.; Luo, M.-C.; Gardell, L.R.; Vanderah, T.W. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: Antinociceptive actions of CB1 inverse agonists. J. Neurosci. 2008, 28, 11593–11602.
- Zhang, Y.; Popović, Z.B.; Bibevski, S.; Fakhry, I.; Sica, D.A.; Van Wagoner, D.R.; Mazgalev, T.N. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ. Heart Fail. 2009, 2, 692–699.
- Weiss, L.; Zeira, M.; Reich, S.; Slavin, S.; Raz, I.; Mechoulam, R.; Gallily, R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 2008, 54, 244–249.
- Hall, W.; Degenhardt, L. Adverse health effects of non-medical cannabis use. Lancet 2009, 374, 1383–1391.
- Thomas, G.; Kloner, R.A.; Rezkalla, S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 2014, 113, 187–190.
- Singla, S.; Sachdeva, R.; Mehta, J.L. Cannabinoids and atherosclerotic coronary heart disease. Clin. Cardiol. 2012, 35, 329–335.
- Penner, E.A.; Buettner, H.; Mittleman, M.A. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am. J. Med. 2013, 126, 583–589.
- Minke, B.; Cook, B. TRP Channel Proteins and Signal Transduction. Physiol. Rev. 2002, 82, 429–472.
- Kunert-Keil, C.; Bisping, F.; Krüger, J.; Brinkmeier, H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genom. 2006, 7, 159.
- Thayer, J.; Yamamoto, S.; Cardiology, J.B.-I. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Elsevier 2010, 141, 122–131.
- Koenig, J.; Jarczok, M.; Warth, M. Body mass index is related to autonomic nervous system activity as measured by heart rate variability—A replication using short term measurements. J. Nutr. Health Aging 2014, 18, 300–302.
- Lotufo, P.A.; Valiengo, L.; Benseñor, I.M.; Brunoni, A.R. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012, 53, 272–282.
- Sgoifo, A.; Carnevali, L.; Pico Alfonso M de los, A.; Amore, M. Autonomic dysfunction and heart rate variability in depression. Stress 2015, 18, 343–352.
- Matei, D.; Luca, C.; Ilie, O.; Matei, P.; Iordan, D.-A.; Buculei, I. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350.
- Anghel, L.; Baroiu, L.; Popazu, C.R.; Pătraș, D.; Fotea, S.; Nechifor, A.; Ciubara, A.B. Benefits and adverse events of melatonin use in the elderly (Review). Exp. Ther. Med. 2022, 23, 219.
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198.
- Prenderville, J.A.; Kelly, Á.M.; Downer, E.J. The Role of Cannabinoids in Adult Neurogenesis. Br. J. Pharmacol. 2015, 172, 3950–3963.
- Mechoulam, R.; Spatz, M.; Shohami, E. Endocannabinoids and Neuroprotection. Sci. STKE 2002, 2002, re5.
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S. Exploration of Multiverse Activities of Endocannabinoids in Biological Systems. Int. J. Mol. Sci. 2022, 23, 5734.
- Ros, J.; Clària, J.; To-Figueras, J.; Planagumà, A.; Cejudo-Martín, P.; Fernández-Varo, G.; Martín-Ruiz, R.; Arroyo, V.; Rivera, F.; Rodüs, J.; et al. Endogenous Cannabinoids: A New System Involved in the Homeostasis of Arterial Pressure in Experimental Cirrhosis in the Rat. Gastroenterology 2002, 122, 85–93.
- Kunos, G.; Járai, Z.; Bátkai, S.; Goparaju, S.K.; Ishac, E.J.N.; Liu, J.; Wang, L.; Wagner, J.A. Endocannabinoids as Cardiovascular Modulators. Chem. Phys. Lipids 2000, 108, 159–168.
- Mourtakos, S.; Vassiliou, G.; Kontoangelos, K.; Philippou, A.; Tzavellas, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J.; Papageorgiou, C.; Sidossis, L.S.; Papageorgiou, C. Endocannabinoids and Heart Rate Variability Alterations after Exposure to Prolonged Intensive Physical Exercise of the Hellenic Navy Seals. Int. J. Environ. Res. Public Health 2022, 19, 28.
- Jones, R.T. Cardiovascular System Effects of Marijuana. J. Clin. Pharmacol. 2002, 42, 48S–63S.
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Benyó, Z.; Offermanns, S.; Ruisanchez, É.; Szabó, E.; Takáts, Z.; Bátkai, S.; et al. Endocannabinoid-Mediated Modulation of Gq/11 Protein-Coupled Receptor Signaling-Induced Vasoconstriction and Hypertension. Mol. Cell. Endocrinol. 2015, 403, 46–56.
- Rahaman, O.; Ganguly, D. Endocannabinoids in Immune Regulation and Immunopathologies. Immunology 2021, 164, 242–252.
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448.
- Hernandez-Cervantes, R.; Mendez-Diaz, M.; Prospero-Garcia, O.; Morales-Montor, J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation 2017, 24, 183–199.
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605.
- Miller, A.M.; Stella, N. CB2 receptor-mediated migration of immune cells: It can go either way. Br. J. Pharmacol. 2008, 153, 299–308.
- Chang, Y.H.; Lee, S.T.; Lin, W.W. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: Involvement of eicosanoids. J. Cell. Biochem. 2001, 81, 715–723.
- Sardinha, J.; Kelly, M.E.; Zhou, J.; Lehmann, C. Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis. Mediat. Inflamm. 2014, 2014, 978678.
- Snyder, R.J.; Lantis, J.; Kirsner, R.S.; Shah, V.; Molyneaux, M.; Carter, M.J. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair. Regeneration 2016, 24, 613–629.
- Falconer, J.; Murphy, A.N.; Young, S.P.; Clark, A.R.; Tiziani, S.; Guma, M.; Buckley, C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 984–999.
- Du, Y.; Ren, P.; Wang, Q.; Jiang, S.K.; Zhang, M.; Li, J.Y.; Wang, L.L.; Guan, D.W. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J. Inflamm. 2018, 15, 25.
- Cluny, N.L.; Reimer, R.A.; Sharkey, K.A. Cannabinoid signaling regulates inflammation and energy balance: The importance of the brain–gut axis. Brain Behav. Immun. 2012, 26, 691–698.
- Sharkey, K.A.; Wiley, J.W. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology 2016, 151, 252–266.
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490.
- Jansma, J.; Brinkman, F.; van Hemert, S.; El Aidy, S. Targeting the endocannabinoid system with microbial interventions to improve gut integrity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110169.
- Izzo, A.A.; Sharkey, K.A. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol. Ther. 2010, 126, 21–38.
- Fichna, J.; Wood, J.T.; Papanastasiou, M.; Vadivel, S.K.; Oprocha, P.; Sałaga, M.; Storr, M.A. Endocannabinoid and cannabinoid-like fatty acid amide levels correlate with pain-related symptoms in patients with IBS-D and IBS-C: A pilot study. PLoS ONE 2013, 8, e85073.
- Kinsey, S.G.; Nomura, D.K.; O’Neal, S.T.; Long, J.Z.; Mahadevan, A.; Cravatt, B.F.; Grider, J.R.; Lichtman, A.H. Inhibition of monoacylglycerol lipase attenuates nonsteroidal anti-inflammatory drug-induced gastric hemorrhages in mice. J. Pharmacol. Exp. Ther. 2011, 338, 795–802.
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45.
- Di Marzo, V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 2018, 51, 1356–1367.
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. Neuroreport 2003, 14, 2209–2211.
- Silvestri, C.; Ligresti, A.; Di Marzo, V. Peripheral effects of the endocannabinoid system in energy homeostasis: Adipose tissue, liver and skeletal muscle. Rev. Endocr. Metab. Disord. 2011, 12, 153–162.
- Vettor, R.; Pagano, C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 51–63.
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur. J. Appl. Physiol. 2013, 113, 869–875.
More
Information
Subjects:
Sport Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
10 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




