
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Haichang Zhang | -- | 2614 | 2023-02-07 02:59:14 | | | |
| 2 | Conner Chen | Meta information modification | 2614 | 2023-02-07 06:49:29 | | | | |
| 3 | Conner Chen | Meta information modification | 2614 | 2023-02-08 02:01:45 | | |
Video Upload Options
The common fluorescent conjugated materials present weak or quenching luminescent phenomena in the solid or aggregate state (ACQ), which limits their applications in medicine and biology. Certain materials, named aggregation-induced emission (AIE) fluorescent materials, have exhibited strong luminescent properties in the aggregate state, which can overcome the ACQ phenomenon. Due to their intrinsic properties, the AIE materials have been successfully used in biolabeling, where they can not only detect the species of ions and their concentrations in organisms, but can also monitor the organisms’ physiological activity. In addition, these kinds of materials often present non-biological toxicity. Thus, AIE materials have become some of the most popular biofluorescent probe materials and are attracting more and more attention.
1. Introduction
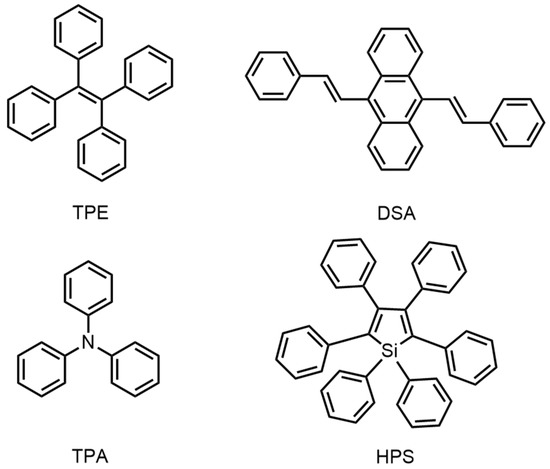
2. AIE Material Design Strategy Based on the Functional Groups
References
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741.
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907.
- Ren, P.; Zhang, Y.; Zhang, H.; Zhang, X.; Li, W.; Yang, W. Synthesis and Characterization of Highly Soluble 9,10-Diphenyl-Substituted Poly(2,6-Anthracenevinylene). Polymer 2009, 50, 4801–4806.
- Qi, J.; Duan, X.; Cai, Y.; Jia, S.; Chen, C.; Zhao, Z.; Li, Y.; Peng, H.-Q.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Simultaneously Boosting the Conjugation, Brightness and Solubility of Organic Fluorophores by Using AIEgens. Chem. Sci. 2020, 11, 8438–8447.
- Wang, D.; Zhou, X.; Ma, C.; Liu, M.; Huang, H.; Zhang, X.; Wei, Y. An Amphiphilic Fluorogen with Aggregation-Induced Emission Characteristic for Highly Sensitive and Selective Detection of Cu2+ in Aqueous Solution and Biological System. Arab. J. Chem. 2021, 14, 10335.
- Zang, T.; Xie, Y.; Su, S.; Liu, F.; Chen, Q.; Jing, J.; Zhang, R.; Niu, G.; Zhang, X. In Vitro Light-Up Visualization of a Subunit-Specific Enzyme by an AIE Probe via Restriction of Single Molecular Motion. Angew. Chem. Int. Ed. 2020, 59, 10003–10007.
- Zhang, R.; Zhang, C.-J.; Feng, G.; Hu, F.; Wang, J.; Liu, B. Specific Light-Up Probe with Aggregation-Induced Emission for Facile Detection of Chymase. Anal. Chem. 2016, 88, 9111–9911.
- Liang, J.; Feng, G.; Kwok, R.T.K.; Ding, D.; Tang, B.; Liu, B. AIEgen Based Light-up Probes for Live Cell Imaging. Sci. China Chem. 2016, 59, 53–56.
- Xu, C.; Zou, H.; Zhao, Z.; Zhang, P.; Kwok, R.T.K.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Tang, B.Z. A New Strategy toward “Simple” Water-Soluble AIE Probes for Hypoxia Detection. Adv. Funct. Mater. 2019, 29, 1903278.
- Guan, W.; Lu, J.; Zhou, W.; Lu, C. Aggregation-Induced Emission Molecules in Layered Matrices for Two-Color Luminescence Films. Chem. Commun. 2014, 50, 11895–11898.
- Zhang, J.; Ma, S.; Fang, H.; Xu, B.; Sun, H.; Chan, I.; Tian, W. Insights into the Origin of Aggregation Enhanced Emission of 9,10-Distyrylanthracene Derivatives. Mater. Chem. Front. 2017, 1, 1422–1429.
- Wang, Z.; Ma, K.; Xu, B.; Li, X.; Tian, W. A Highly Sensitive “Turn-on” Fluorescent Probe for Bovine Serum Albumin Protein Detection and Quantification Based on AIE-Active Distyrylanthracene Derivative. Sci. China Chem. 2013, 56, 1234–1238.
- Zhang, X.; Wang, Y.-X.; Zhao, J.; Duan, P.; Chen, Y.; Chen, L. Structural Insights Into 9-Styrylanthracene-Based Luminophores: Geometry Control Versus Mechanofluorochromism and Sensing Properties. Chem. Asian J. 2017, 12, 830–834.
- Zhao, J.; Chi, Z.; Yang, Z.; Mao, Z.; Zhang, Y.; Ubba, E.; Chi, Z. Recent Progress in the Mechanofluorochromism of Distyrylanthracene Derivatives with Aggregation-Induced Emission. Mater. Chem. Front. 2018, 2, 1595–1608.
- Barot, Y.B.; Anand, V.; Mishra, R. Di-Triphenylamine-Based AIE Active Schiff Base for Highly Sensitive and Selective Fluorescence Sensing of Cu2+ and Fe3+. J. Photochem. Photobiol. A Chem. 2022, 426, 113785.
- Liu, M.-X.; Ma, L.-L.; Liu, X.-Y.; Liu, J.-Y.; Lu, Z.-L.; Liu, R.; He, L. Combination of AneN 3 and Triphenylamine-Benzylideneimidazolone as Nonviral Gene Vectors with Two-Photon and AIE Properties. ACS Appl. Mater. Interfaces 2019, 11, 42975–42987.
- Liu, M.; Cao, J.; Tu, Y.; Huang, C.; Zhang, M.; Zheng, J. An Ultra-Sensitive near-Infrared Fluorescent Probe Based on Triphenylamine with High Selectivity Detecting the Keratin. Anal. Biochem. 2022, 646, 114638.
- Xiang, X.; Zhan, Y.; Yang, W.; Jin, F. Aggregation-Induced Emission Enhancement and Mechanofluorochromism Based on Dicyanovinyl Derivatives Decorated Carbazole or Triphenylamine Units: Effects of Electronic Structures and Spatial Conformations. Dye. Pigment. 2022, 206, 110670.
- Mathivanan, M.; Murugesapandian, B. Substitution Dependent Multi-Color AIE Behavior and ESIPT Active A-D-A Type Triphenylamine Supported Bis Unsymmetrical Azine Derivatives and Their Antibacterial Activity. Dye. Pigment. 2022, 203, 110367.
- Wang, X.; Wang, Y.; Zhan, Y.; Yang, P.; Zhang, X.; Xu, Y. Piezofluorochromism of Triphenylamine-Based Triphenylacrylonitrile Derivative with Intramolecular Charge Transfer and Aggregation-Induced Emission Characteristics. Tetrahedron Lett. 2018, 59, 2057–2061.
- Tang, B.Z.; Zhan, X.; Yu, G.; Sze Lee, P.P.; Liu, Y.; Zhu, D. Efficient Blue Emission from Siloles. J. Mater. Chem. 2001, 11, 2974–2978.
- Chen, J.; Xu, B.; Ouyang, X.; Tang, B.Z.; Cao, Y. Aggregation-Induced Emission of Cis, Cis -1,2,3,4-Tetraphenylbutadiene from Restricted Intramolecular Rotation. J. Phys. Chem. A 2004, 108, 7522–7526.
- Yamaguchi, S.; Tamao, K. Cross-Coupling Reactions in the Chemistry of Silole-Containing p-Conjugated Oligomers and Polymers. J. Organomet. Chem. 2002, 653, 223–228.
- Li, Q.; Li, Z. The Strong Light-Emission Materials in the Aggregated State: What Happens from a Single Molecule to the Collective Group. Adv. Sci. 2017, 4, 1600484.
- Chen, B.; Feng, G.; He, B.; Goh, C.; Xu, S.; Ramos-Ortiz, G.; Aparicio-Ixta, L.; Zhou, J.; Ng, L.; Zhao, Z.; et al. Silole-Based Red Fluorescent Organic Dots for Bright Two-Photon Fluorescence In Vitro Cell and In Vivo Blood Vessel Imaging. Small 2016, 12, 782–792.
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of Fluorescent Sensors Based on Azaheterocyclic Push-Pull Systems towards Nitroaromatic Explosives and Related Compounds: A Review. Dye. Pigment. 2020, 180, 10841.
- Verbitskiy, E.V.; Baranova, A.A.; Lugovik, K.I.; Khokhlov, K.O.; Chuvashov, R.D.; Dinastiya, E.M.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Linear and V-Shaped Push–Pull Systems on a Base of Pyrimidine Scaffold with a Pyrene-Donative Fragment for Detection of Nitroaromatic Compounds. J. Iran. Chem. Soc. 2018, 15, 787–797.
- Chen, Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules 2022, 27, 8628.
- Islam, K.; Narjinari, H.; Kumar, A. Polycyclic Aromatic Hydrocarbons Bearing Polyethynyl Bridges: Synthesis, Photophysical Properties, and Their Applications. Asian J. Org. Chem. 2021, 10, 1544–1566.
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123.
- Zhang, J.; Yan, Z.; Wang, S.; She, M.; Zhang, Z.; Cai, W.; Liu, P.; Li, J. Water Soluble Chemosensor for Ca2+ Based on Aggregation-Induced Emission Characteristics and Its Fluorescence Imaging in Living Cells. Dye. Pigment. 2018, 150, 112–120.
- Zhang, W.; Luo, Y.; Zhao, J.; Lin, W.-H.; Ni, X.-L.; Tao, Z.; Xiao, X.; Xiao, C.-D. TQ-Based AIE Supramolecular Network Polymers as Potential Bioimaging Agents for the Detection of Fe3+ in Live HeLa Cells. Sens. Actuators B Chem. 2022, 354, 131189.
- Xu, J.; Xiong, J.; Qin, Y.; Li, Z.; Pan, C.; Huo, Y.; Zhang, H. A Novel Quinolinyl-Tetraphenylethene-Based Fluorescence “Turn-on” Sensor for Zn2+ with a Large Stokes Shift and Its Applications for Portable Test Strips and Biological Imaging. Mater. Chem. Front. 2020, 4, 3338–3348.
- Zeng, C.; Ouyang, J.; Sun, L.; Zeng, Z.; Tan, Y.; Zeng, F.; Wu, S. An Activatable Probe for Detection and Therapy of Food-Additive-Related Hepatic Injury via NIR-II Fluorescence/Optoacoustic Imaging and Biomarker-Triggered Drug Release. Anal. Chim. Acta 2022, 1208, 339831.
- Yang, S.; Zhou, F.; Yao, X.; Liu, W.; Zhu, W.; Qian, X.; Liu, Y. A Novel Near-Infrared Fluorescent Probe to in Vivo Evaluate the Release Efficiency of H2S Prodrug. Sens. Actuators B Chem. 2021, 339, 129881.
- Wang, R.; Zhou, L.; Wang, W.; Li, X.; Zhang, F. In Vivo Gastrointestinal Drug-Release Monitoring through Second near-Infrared Window Fluorescent Bioimaging with Orally Delivered Microcarriers. Nat. Commun. 2017, 8, 1470.
- He, X.; Wang, X.; Zhang, L.; Fang, G.; Liu, J.; Wang, S. Sensing and Intracellular Imaging of Zn2+ Based on Affinity Peptide Using an Aggregation Induced Emission Fluorescence “Switch-on” Probe. Sens. Actuators B Chem. 2018, 271, 289–299.
- Zhou, H.; Mei, J.; Chen, Y.-A.; Chen, C.-L.; Chen, W.; Zhang, Z.; Su, J.; Chou, P.-T.; Tian, H. Phenazine-Based Ratiometric Hg2+ Probes with Well-Resolved Dual Emissions: A New Sensing Mechanism by Vibration-Induced Emission (VIE). Small 2016, 12, 6542–6546.
- Li, Q.; Li, Z. Molecular Packing: Another Key Point for the Performance of Organic and Polymeric Optoelectronic Materials. Acc. Chem. Res. 2020, 53, 962–973.
- Huang, X.; Zhang, R.; Chen, C.; Kwok, R.T.K.; Tang, B.Z. Wash-Free Detection and Bioimaging by AIEgens. Mater. Chem. Front. 2021, 5, 723–774.
- Tang, F.; Liu, J.-Y.; Wu, C.-Y.; Liang, Y.-X.; Lu, Z.-L.; Ding, A.-X.; Xu, M.-D. Two-Photon Near-Infrared AIE Luminogens as Multifunctional Gene Carriers for Cancer Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 23384–23395.
- Yu, M. On the Application of Incentive Mechanism in Human Resource Management. RERR 2020, 1, 1.
- Asad, M.; Imran Anwar, M.; Abbas, A.; Younas, A.; Hussain, S.; Gao, R.; Li, L.-K.; Shahid, M.; Khan, S. AIE Based Luminescent Porous Materials as Cutting-Edge Tool for Environmental Monitoring: State of the Art Advances and Perspectives. Coord. Chem. Rev. 2022, 463, 214539.
- Kang, X.; Li, Y.; Yin, S.; Li, W.; Qi, J. Reactive Species-Activatable AIEgens for Biomedical Applications. Biosensors 2022, 12, 646.
- Panigrahi, A.; Are, V.N.; Jain, S.; Nayak, D.; Giri, S.; Sarma, T.K. Cationic Organic Nanoaggregates as AIE Luminogens for Wash-Free Imaging of Bacteria and Broad-Spectrum Antimicrobial Application. ACS Appl. Mater. Interfaces 2020, 12, 5389–5402.
- Sun, F.; Liu, J.; Huang, Y.; Zhu, X.; Liu, Y.; Zhang, L.; Yan, J. A Quinoline Derived D-A-D Type Fluorescent Probe for Sensing Tetrameric Transthyretin. Bioorganic Med. Chem. Lett. 2021, 52, 128408.
- Enbanathan, S.; Iyer, S.K. A Novel Phenanthridine and Terpyridine Based D-π-A Fluorescent Probe for the Ratiometric Detection of Cd2+ in Environmental Water Samples and Living Cells. Ecotoxicol. Environ. Saf. 2022, 247, 114272.
- Xu, H.; Wang, A.; Qin, L.; Mo, M.; Zhou, Y.; Lü, C.; Zou, L. D-A-D Based Reversible Fluorescent Probe for Selective Detection and Cell Imaging of Copper Ion. Chem. Phys. 2022, 560, 111571.
- Zhou, Y.; Liu, M.-M.; Li, J.-Y.; Ye, M.-A.; Yao, C. Fluorescence Turn-on Detection of Fluoride Using HPQ-Silyl Ether Reactive Probes and Its in Vivo Application. Dye. Pigment. 2018, 158, 277–284.
- Zhu, L.; Liu, H.-W.; Yang, Y.; Hu, X.-X.; Li, K.; Xu, S.; Li, J.-B.; Ke, G.; Zhang, X.-B. Near-Infrared Fluorescent Furin Probe for Revealing the Role of Furin in Cellular Carcinogenesis and Specific Cancer Imaging. Anal. Chem. 2019, 91, 9682–9968.
- Zhang, E.; Hou, X.; Zhang, Z.; Zhang, Y.; Wang, J.; Yang, H.; You, J.; Ju, P. A Novel Biomass-Based Reusable AIE Material: AIE Properties and Potential Applications in Amine/Ammonia Vapor Sensing and Information Storage. J. Mater. Chem. C 2019, 7, 8404–8411.
- Yan, X.; Zhang, M.; Wei, P.; Zheng, B.; Chi, X.; Ji, X.; Huang, F. PH-Responsive Assembly and Disassembly of a Supramolecular Cryptand-Based Pseudorotaxane Driven by π–π Stacking Interaction. Chem. Commun. 2011, 47, 984.
- Tong, H.; Hong, Y.; Dong, Y.; Häußler, M.; Lam, J.W.Y.; Li, Z.; Guo, Z.; Guo, Z.; Tang, B.Z. Fluorescent “Light-up” Bioprobes Based on Tetraphenylethylene Derivatives with Aggregation-Induced Emission Characteristics. Chem. Commun. 2006, 35, 3705–3707.
- Guan, J.; Wei, R.; Prlj, A.; Peng, J.; Lin, K.; Liu, J.; Han, H.; Corminboeuf, C.; Zhao, D.; Yu, Z.; et al. Direct Observation of Aggregation-Induced Emission Mechanism. Angew. Chem. Int. Ed. 2020, 59, 14903–14909.
- Shi, S.; Zhang, G. Click-Formed Polymer Gels with Aggregation-Induced Emission and Dual Stimuli-Responsive Behaviors. Chin. J. Chem. Phys. 2021, 34, 365–372.
- Xing, Y.; Li, D.; Dong, B.; Wang, X.; Wu, C.; Ding, L.; Zhou, S.; Fan, J.; Song, B. Water-Soluble and Highly Emissive near-Infrared Nano-Probes by Co-Assembly of Ionic Amphiphiles: Towards Application in Cell Imaging. New J. Chem. 2019, 43, 8059–8806.
- Wang, J.; Lu, L.; Wang, C.; Wang, M.; Ju, J.; Zhu, J.; Sun, T. An AIE and PET Fluorescent Probe for Effective Zn(ii) Detection and Imaging in Living Cells. New J. Chem. 2020, 44, 15426–15431.
- Li, B.; Chen, T.; Wang, Z.; Guo, Z.; Peña, J.; Zeng, L.; Xing, J. A Novel Cross-Linked Nanoparticle with Aggregation-Induced Emission Properties for Cancer Cell Imaging. J. Mater. Chem. B 2020, 8, 2431–2437.
- Kim, S.; Zheng, Q.; He, G.S.; Bharali, D.J.; Pudavar, H.E.; Baev, A.; Prasad, P.N. Aggregation-Enhanced Fluorescence and Two-Photon Absorption in Nanoaggregates of a 9,10-BisAnthracene Derivative. Adv. Funct. Mater. 2006, 16, 2317–2323.
- Ma, K.; Wang, H.; Li, H.; Xu, B.; Tian, W. Label-Free Detection for SNP Using AIE Probes and Carbon Nanotubes. Sens. Actuators B Chem. 2017, 253, 92–99.
- Wang, H.; Ma, K.; Xu, B.; Tian, W. Tunable Supramolecular Interactions of Aggregation-Induced Emission Probe and Graphene Oxide with Biomolecules: An Approach toward Ultrasensitive Label-Free and “Turn-On” DNA Sensing. Small 2016, 12, 6613–6622.
- Ma, K.; Wang, H.; Li, X.; Xu, B.; Tian, W. Turn-on Sensing for Ag+ Based on AIE-Active Fluorescent Probe and Cytosine-Rich DNA. Anal. Bioanal. Chem. 2015, 407, 2625–2630.
- Li, H.; Zhang, X.; Zhang, X.; Yang, B.; Yang, Y.; Huang, Z.; Wei, Y. Biocompatible Fluorescent Polymeric Nanoparticles Based on AIE Dye and Phospholipid Monomers. RSC Adv. 2014, 4, 21588.
- Lu, H.; Zhao, X.; Tian, W.; Wang, Q.; Shi, J. Pluronic F127–Folic Acid Encapsulated Nanoparticles with Aggregation-Induced Emission Characteristics for Targeted Cellular Imaging. RSC Adv. 2014, 4, 18460.
- Han, W.; Du, Y.; Song, M.; Sun, K.; Xu, B.; Yan, F.; Tian, W. Fluorescent Nanorods Based on 9,10-Distyrylanthracene (DSA) Derivatives for Efficient and Long-Term Bioimaging. J. Mater. Chem. B 2020, 8, 9544–9554.
- Wang, L.; Pan, X.; Tang, S.; Wang, Y.; Shi, H.; Wang, H.; Liu, W.; Chen, Z. An Intelligent Photosensitizer That Selectively Kills Gram-Positive Pathogenic Cocci While Preventing Harm to Beneficial Bacilli. Dye. Pigment. 2022, 201, 11019.
- Zhang, Y.; Zhuang, W.; Chen, J.; Li, C.; Li, S.; Chen, M. Aggregation-Induced Emission Fluorescent Probes for Lipid Droplets-Specific Bioimaging of Cells and Atherosclerosis Plaques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 12201.
- Zhang, X.; Chen, Y.; Li, C.; Xue, Z.; Wu, H.; Li, J.; Ou, H.; Shen, J.; Ding, D. Root Canal Disinfection Using Highly Effective Aggregation-Induced Emission Photosensitizer. ACS Appl. Bio. Mater. 2021, 4, 3796–3804.
- Gao, H.; Kam, C.; Chou, T.Y.; Wu, M.-Y.; Zhao, X.; Chen, S. A Simple yet Effective AIE-Based Fluorescent Nano-Thermometer for Temperature Mapping in Living Cells Using Fluorescence Lifetime Imaging Microscopy. Nanoscale Horiz. 2020, 5, 488–494.
- Wu, W.; Shen, M.; Liu, X.; Shen, L.; Ke, X.; Li, W. Highly Sensitive Fluorescence-Linked Immunosorbent Assay Based on Aggregation-Induced Emission Luminogens Incorporated Nanobeads. Biosens. Bioelectron. 2020, 150, 111912.




