
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefania Pieroni | -- | 1895 | 2023-02-06 15:28:06 | | | |
| 2 | Rita Xu | Meta information modification | 1895 | 2023-02-07 03:35:00 | | |
Video Upload Options
The healthy state is guaranteed by the fine-tuning of genes controlling cell proliferation, differentiation, and development, whose alteration induces cellular behavioral changes finally leading to cancer. A major challenge to the cell is to guarantee that proteins are made, folded, assembled and delivered to function properly, like and even more when referring to oncogenes and onco-suppressors products. Ubiquitination and ubiquitin-like modifications modulate the stability and control the activity of most of the proteins that manage cell cycle, immune responses, apoptosis, and senescence.
1. Introduction
2. Transcriptional and Translational Control of Protein Synthesis
2.1. Genetic and Epigenetic Control
2.2. Translational Control
3. Post-Translational Modifications
3.1. Types of PTMs
3.2. PTMs Crosstalk
3.3. PTMs in the Control of Oncogenes and TSGs Activity
| Gene | Description | Oncogenic Alteration | Related Cancers | |
|---|---|---|---|---|
| RAS | -KRAS | RAS Proto-Oncogenes GTPase activity Signal transduction |
Mutation Amplification Deep deletion |
Pancreatic Colorectal Lung NSCLC |
| -NRAS | Mutation Amplification Deep deletion |
Melanoma AML Endometrial NSCLC |
||
| -HRAS | Amplification Deep deletion Mutation |
Lung Head and Neck Soft tissue Esophagogastric |
||
| MYC | MYC Proto-Oncogene BHLH Transcription Factor |
Amplification | Endometrial Ovarian Brest Head and Neck |
|
| RB | Transcriptional Corepressor 1 Tumor suppressor Cell proliferation |
Amplification Deep deletion Mutation |
Bladder Colorectal Esophagogastric Soft tissue |
|
| TP53 | Tumor suppressor growth arrest apoptosis induction cell cycle regulation |
Mutation | Ovarian Endometrial NSCLC Esophagogastric |
|
- -
-
RAS proteins as molecules integrating receptor signaling along pathways that control cellular growth;
- -
-
c-MYC as a transcription factor acting as a key player in the development of many human cancers;
- -
-
RB, as the first tumor suppressor gene identified;
- -
-
p53, as the master gene in the preservation of genetic integrity, so far called “the guardian of the genome”.
4. p53
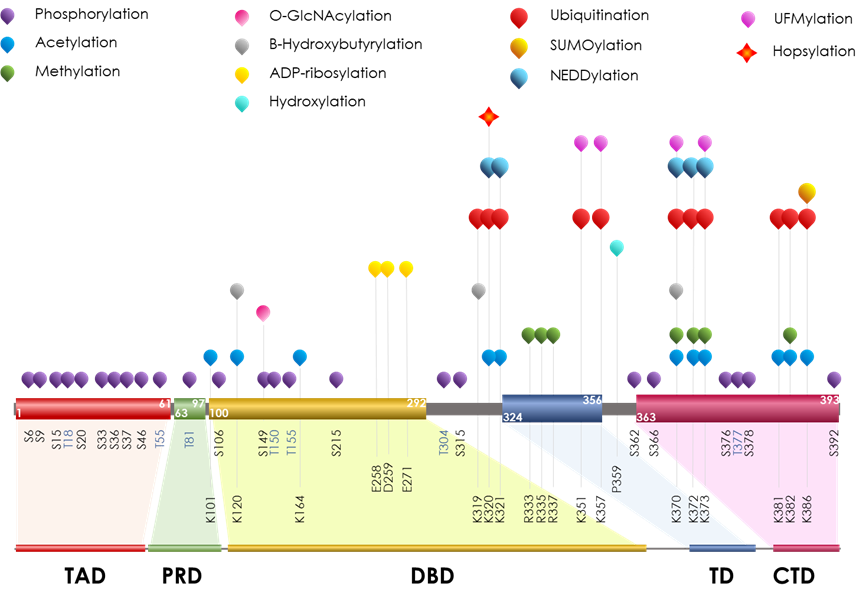
Figure 1. Schematic representation of p53 protein domains with the main PTMs. p53 is a 393 aa long protein whose functional domains are: N-terminal Trans Activation Domain (TAD, aa 1-61), Proline-Rich Domain (PRD, aa 64-92), central DNA-Binding Domain (DBD, aa 101-292), Tetramerization Domain (TD, aa 325–356), and an intrinsically disordered C-Terminal Domain (CTD, aa 363-393). Given its regulatory function, the CTD is strongly affected by PTMs. The specific PTMs are functional to the role of the domain involved. All the six p53 domains and relative linker sequences undergo PTMs: phosphorylation, acetylation, methylation, O-GlcNAcylation, ADP-ribosylation, hydroxylation, β-hydroxybutyrylation, ubiquitination, SUMOylation, NEDDylation, UFMylation and Hopsylation are reported and labeled as indicated in the legend. The localization and the modified aas are reported. The higher PTM frequency in the last third of sequence implies that the picture is not drawn to scale. The real size of each domain is shown at the bottom.
The characterization of each functional domain, with the modification sites within, was widely and deeply studied in past years and allowed researchers to consider PTM crosstalk as a key mechanism to balance p53 functional output [35].
Recently, other than SUMO and NEDD, a novel ubiquitin-like protein has been identified. HOPS/TMUB1 (Hepatocyte Odd Protein Shuttling/Trans Membrane Ubiquitin-Like Protein 1, hereafter referred to as HOPS) [36] is able to impact the control of p53 stability by inhibiting its recruitment to the proteasome and lengthening its half-life [37]. Firstly, HOPS has been shown to interact and control the stabilization and the nucleolar localization of the tumor suppressor p19Arf, also acting in its functional binding to the onco-suppressor nucleophosmin (NPM) [38]. Given the nature of HOPS interaction to p53 and other targets, it has been referred as HOPSylation, since it acts through the HOPS UBL domain. HOPSylation appears to occur at Lys320 and is reported to be engaged in ubiquitination, NEDDylation and acetylation, and is involved in the management of p53 cellular distribution. Functionally, HOPS interaction to p53 leads to the control of its apoptotic potential upon DNA damaging stresses, impairing p53 localization as a critical event for specific functional outcomes [37][39].
Emerging research shows that p53 can be a substrate for UFMylation. Analogously to other UBLs, the ubiquitin-fold modifier (UFM) is a recently identified ubiquitin-like protein [40][41]. UFMylation occurs at CTD both at lysine sites, and is reported to be interested in other Ub or UBL conjugations (K370 and K373), and two novel ones (K351 and K357). Since p53 UFMylation and other ubiquitin-like modifications compete for the same lysine sites in p53, they act in modulating the stability, by interfering with p53 ubiquitination-mediated proteasomal degradation and possibly in controlling other functional outcomes [42].
5. Conclusions
The fine tuning of protein expression and function is a key step in cellular homeostasis and healthy state preservation. Alterations affecting such events including protein biosynthesis and turnover are at the origin of a wide spectrum of pathologies and diseases, including cancer.
The PTMs represent a powerful mechanism to rapidly modulate specific protein amounts in cells, thus controlling their localization, function, and intracellular dislocation. Since they play a critical role in the control of cellular homeostasis and there are negative outcomes for the alteration of proper protein modifications, their role in cancer progression is definitively assessed.
For this purpose, in the past years, more significant evidence has been produced to suggest their use in new therapeutical approaches in the field of oncologic diseases. So far, a number of new molecules (i.e., ubiquitination pathway inhibitors/modulators) have been developed for clinical use and are now available in current medical practice.
References
- Spandidos, D.A.; Anderson, M.L.M. Oncogenes and onco-suppressor genes: Their involvement in cancer. J. Pathol. 1989, 157, 1–10.
- Chen, L.; Liu, S.; Tao, Y. Regulating tumor suppressor genes: Post-translational modifications. Signal Transduct. Target. Ther. 2020, 5, 90.
- Macaluso, M.; Paggi, M.G.; Giordano, A. Genetic and epigenetic alterations as hallmarks of the intricate road to cancer. Oncogene 2003, 22, 6472–6478.
- Baxter, E.; Windloch, K.; Gannon, F.; Lee, J.S. Epigenetic regulation in cancer progression. Cell Biosci. 2014, 4, 45.
- Brandman, O.; Hegde, R.S. Ribosome-associated protein quality control. Nat. Struct. Mol. Biol. 2016, 23, 7–15.
- Ingolia, N.T.; Hussmann, J.A.; Weissman, J.S. Ribosome Profiling: Global Views of Translation. Cold Spring Harb. Perspect. Biol. 2018, 11, a032698.
- Han, Z.-J.; Feng, Y.-H.; Gu, B.-H.; Li, Y.-M.; Chen, H. The post-translational modification, SUMOylation, and cancer (Review). Int. J. Oncol. 2018, 52, 1081–1094.
- Stram, A.R.; Payne, R.M. Post-translational modifications in mitochondria: Protein signaling in the powerhouse. Cell. Mol. Life Sci. 2016, 73, 4063–4073.
- Lo, W.-S.; Trievel, R.C.; Rojas, J.R.; Duggan, L.; Hsu, J.-Y.; Allis, C.; Marmorstein, R.; Berger, S.L. Phosphorylation of Serine 10 in Histone H3 Is Functionally Linked In Vitro and In Vivo to Gcn5-Mediated Acetylation at Lysine 14. Mol. Cell 2000, 5, 917–926.
- Fujiki, R.; Hashiba, W.; Sekine, H.; Yokoyama, A.; Chikanishi, T.; Ito, S.; Imai, Y.; Kim, J.; He, H.H.; Igarashi, K.; et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature 2011, 480, 557–560.
- Minguez, P.; Parca, L.; Diella, F.; Mende, D.R.; Kumar, R.; Helmer-Citterich, M.; Gavin, A.-C.; van Noort, V.; Bork, P. Deciphering a global network of functionally associated post-translational modifications. Mol. Syst. Biol. 2012, 8, 599.
- Huang, K.-Y.; Lee, T.-Y.; Kao, H.-J.; Ma, C.-T.; Lee, C.-C.; Lin, T.-H.; Chang, W.-C.; Huang, H.-D. dbPTM in 2019: Exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res. 2018, 47, D298–D308.
- Vu, L.D.; Gevaert, K.; De Smet, I. Protein Language: Post-Translational Modifications Talking to Each Other. Trends Plant Sci. 2018, 23, 1068–1080.
- Venne, A.S.; Kollipara, L.; Zahedi, R.P. The next level of complexity: Crosstalk of posttranslational modifications. Proteomics 2014, 14, 513–524.
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J., Jr. Protein Posttranslational Modifications: The Chemistry of Proteome Diversifications. Angew. Chem. Int. Ed. 2005, 44, 7342–7372.
- Horita, H.; Law, A.; Middleton, K. Utilizing Optimized Tools to Investigate PTM Crosstalk: Identifying Potential PTM Crosstalk of Acetylated Mitochondrial Proteins. Proteomes 2018, 6, 24.
- Minguez, P.; Letunic, I.; Parca, L.; Garcia-Alonso, L.; Dopazo, J.; Huerta-Cepas, J.; Bork, P. PTMcode v2: A resource for functional associations of post-translational modifications within and between proteins. Nucleic Acids Res. 2014, 43, D494–D502.
- Leutert, M.; Entwisle, S.W.; Villén, J. Decoding Post-Translational Modification Crosstalk with Proteomics. Mol. Cell. Proteom. 2021, 20, 100129.
- Johnson, L.N. The regulation of protein phosphorylation. Biochem. Soc. Trans. 2009, 37, 627–641.
- Boopathy, G.T.; Lynn, J.L.S.; Wee, S.; Gunaratne, J.; Hong, W. Phosphorylation of Mig6 negatively regulates the ubiquitination and degradation of EGFR mutants in lung adenocarcinoma cell lines. Cell. Signal. 2017, 43, 21–31.
- Van der Laarse, S.A.; Leney, A.C.; Heck, A.J.R. Crosstalk between phosphorylation and O-GlcNAcylation: Friend or foe. FEBS J. 2018, 285, 3152–3167.
- Leroy, C.; Shen, Q.; Strande, V.; Meyer, R.; E McLaughlin, M.; Lezan, E.; Bentires-Alj, M.; Voshol, H.; Bonenfant, D.; Gaither, L.A. CUB-domain-containing protein 1 overexpression in solid cancers promotes cancer cell growth by activating Src family kinases. Oncogene 2015, 34, 5593–5598.
- Olivier-Van Stichelen, S.; Dehennaut, V.; Buzy, A.; Zachayus, J.-L.; Guinez, C.; Mir, A.-M.; El Yazidi-Belkoura, I.; Copin, M.-C.; Boureme, D.; Loyaux, D.; et al. O-GlcNAcylation stabilizes β-catenin through direct competition with phosphorylation at threonine 41. FASEB J. 2014, 28, 3325–3338.
- Hunter, T. The Age of Crosstalk: Phosphorylation, Ubiquitination, and Beyond. Mol. Cell 2007, 28, 730–738.
- Swaney, D.L.; Beltrao, P.; Starita, L.; Guo, A.; Rush, J.; Fields, S.; Krogan, N.J.; Villen, J. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 2013, 10, 676–682.
- Wang, S.; Huang, X.; Sun, D.; Xin, X.; Pan, Q.; Peng, S.; Liang, Z.; Luo, C.; Yang, Y.; Jiang, H.; et al. Extensive Crosstalk between O-GlcNAcylation and Phosphorylation Regulates Akt Signaling. PLoS ONE 2012, 7, e37427.
- Morrow, J.K.; Lin, H.-K.; Sun, S.-C.; Zhang, S. Targeting ubiquitination for cancer therapies. Futur. Med. Chem. 2015, 7, 2333–2350.
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015, 16, 393–405.
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078.
- Li, L.; Mao, Y.; Zhao, L.; Li, L.; Wu, J.; Zhao, M.; Du, W.; Yu, L.; Jiang, P. p53 regulation of ammonia metabolism through urea cycle controls polyamine biosynthesis. Nature 2019, 567, 253–256, Publisher Correction in Nature 2019, 569, E10.
- Liu, Y.; Gu, W. The complexity of p53-mediated metabolic regulation in tumor suppression. Semin. Cancer Biol. 2021, 85, 4–32.
- Liu, Y.; Tavana, O.; Gu, W. p53 modifications: Exquisite decorations of the powerful guardian. J. Mol. Cell Biol. 2019, 11, 564–577.
- Lane, D.P. p53, guardian of the genome. Nature 1992, 358, 15–16.
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210.
- Joerger, A.C.; Fersht, A.R. Structural Biology of the Tumor Suppressor p53. Annu. Rev. Biochem. 2008, 77, 557–582.
- Della-Fazia, M.A.; Castelli, M.; Piobbico, D.; Pieroni, S.; Servillo, G. The Ins and Outs of HOPS/TMUB1 in biology and pathology. FEBS J. 2020, 288, 2773–2783.
- Castelli, M.; Piobbico, D.; Chiacchiaretta, M.; Brunacci, C.; Pieroni, S.; Bartoli, D.; Gargaro, M.; Fallarino, F.; Puccetti, P.; Soddu, S.; et al. HOPS/TMUB1 retains p53 in the cytoplasm and sustains p53-dependent mitochondrial apoptosis. EMBO Rep. 2019, 21, e48073.
- Castelli, M.; Pieroni, S.; Brunacci, C.; Piobbico, D.; Bartoli, D.; Bellet, M.M.; Colombo, E.; Pelicci, P.G.; A Della Fazia, M.; Servillo, G. Hepatocyte odd protein shuttling (HOPS) is a bridging protein in the nucleophosmin-p19Arf network. Oncogene 2012, 32, 3350–3358.
- Della-Fazia, M.A.; Castelli, M.; Piobbico, D.; Pieroni, S.; Servillo, G. HOPS and p53: Thick as thieves in life and death. Cell Cycle 2020, 19, 2996–3003.
- Komatsu, M.; Chiba, T.; Tatsumi, K.; Iemura, S.-I.; Tanida, I.; Okazaki, N.; Ueno, T.; Kominami, E.; Natsume, T.; Tanaka, K. A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J. 2004, 23, 1977–1986.
- Banerjee, S.; Kumar, M.; Wiener, R. Decrypting UFMylation: How Proteins Are Modified with UFM1. Biomolecules 2020, 10, 1442.
- Liu, J.; Guan, D.; Dong, M.; Yang, J.; Wei, H.; Liang, Q.; Song, L.; Xu, L.; Bai, J.; Liu, C.; et al. UFMylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nat. Cell Biol. 2020, 22, 1056–1063.




