Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marialuisa Zedde | -- | 3577 | 2023-02-06 04:25:57 | | | |
| 2 | Catherine Yang | -6 word(s) | 3571 | 2023-02-06 04:32:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zedde, M.; Grisendi, I.; Pezzella, F.R.; Napoli, M.; Moratti, C.; Valzania, F.; Pascarella, R. Brainstem Midline Stroke. Encyclopedia. Available online: https://encyclopedia.pub/entry/40847 (accessed on 07 February 2026).
Zedde M, Grisendi I, Pezzella FR, Napoli M, Moratti C, Valzania F, et al. Brainstem Midline Stroke. Encyclopedia. Available at: https://encyclopedia.pub/entry/40847. Accessed February 07, 2026.
Zedde, Marialuisa, Ilaria Grisendi, Francesca Romana Pezzella, Manuela Napoli, Claudio Moratti, Franco Valzania, Rosario Pascarella. "Brainstem Midline Stroke" Encyclopedia, https://encyclopedia.pub/entry/40847 (accessed February 07, 2026).
Zedde, M., Grisendi, I., Pezzella, F.R., Napoli, M., Moratti, C., Valzania, F., & Pascarella, R. (2023, February 06). Brainstem Midline Stroke. In Encyclopedia. https://encyclopedia.pub/entry/40847
Zedde, Marialuisa, et al. "Brainstem Midline Stroke." Encyclopedia. Web. 06 February, 2023.
Copy Citation
Most medullary and pontine strokes are sharply marginated and paramedian, with the long axis being oriented in the sagittal plane. This pattern is related to the distribution of the paramedian-penetrating branches arising from the BA and distal VAs, which perforate the paramedian brainstem and never cross the midline. Lateral infarcts, in the distribution of the short circumferential arteries, are seen less frequently than paramedian lesions are. At the midbrain level, midline infarctions can be visualized, as the many involved branches are not limited to a paramedian distribution. Characteristically, brainstem vascular syndromes are also called alternating syndromes due to the presence of crossed neurological signs (ipsilateral cranial nerve signs and contralateral signs of the ascending and descending tracts) which are hallmarks of the brainstem location.
cerebrovascular disease
medial medullary infarction
heart appearance sign
stroke
1. Medullary Infarction
The medulla oblongata can be classified into three major portions, which are summarized in Table 1.
Table 1. Anatomical portions of the medulla oblongata.
| Portions | Structures |
|---|---|
| Anterior portion | Fibers of the corticospinal tract, which most of them cross over to the contralateral side. |
| Tegmentum | Olivary complex, nuclei of cranial nerves (VIII–XII and part of V), parts of the reticular formation and ascending and descending fiber tracts (e.g., sympathetic fibers). |
| Posterior portion | The lower part is anatomically similar to the spinal cord, and it contains ascending fiber tracts that mostly end in the nuclei gracilis and cuneatus. |
The upper part of the posterior surface of the medulla oblongata also forms the lower floor of the fourth ventricle which contains the area, postrema.
The caudal regions of the medial medulla oblongata are supplied by paramedian branches of the ASA (which arises from both the VAs), whereas more rostrally located regions of the medial medulla oblongata are supplied by the paramedian branches of the VAs [1]. The lateral medulla oblongata is mostly supplied by the circumferentially penetrating branches from the VA, while the PICA supplies the remaining lateral and posterior portion of the medulla oblongata.
Two main vascular medullary syndromes can be distinguished: the lateral medullary syndrome (Wallenberg’s syndrome), which is the most common one (about 2% of ischemic strokes), and the medial medullary syndrome (Dejerine’s syndrome), which has an incidence up to four times lower than that of the lateral medullary syndrome (less than 1% of all brainstem strokes) [2]. At least three other less frequent and overlapping syndromes may occur by the spreading of an ischemic lesion and occlusions of several VA branches.
Medullary infarctions account for 7% of all ischemic brainstem strokes [3]. The most frequent cause of medial medullary infarctions (MMI) is the atherosclerosis of the VA and its branches (mainly ASA), but VA dissection too has been reported in a selected population as a cause.
Unilateral MMIs produce Dejerine’s Syndrome, whose clinical features are represented by the classical triad of contralateral hemiparesis/hemiplegia, contralateral loss of position and vibration sense and ipsilateral tongue weakness; in addition, oculomotor abnormalities and dysarthria may be present. The involved structures and corresponding deficits in MMI are summarized in Table 2.
Table 2. Structures involved in MMI and corresponding neurological deficit (modified from [1]).
| Structures | Main Neurological Deficit |
|---|---|
| Corticobulbar tract | Dysarthria. |
| Corticospinal tract | Contralateral hemiparesis/hemiplegia. |
| Medial Lemniscus | Contralateral loss of position sense and vibration. |
| Medial longitudinal fasciculus (MLF) | Ipsilateral internuclear ophtalmoplegia. |
| Hypoglossal (CN XII) nucleus | Ipsilateral weakness of the hemitongue. |
Abbreviations: Medial Medullar Infarction (MMI).
The most common presentation of unilateral MMI in a case series was hemiparesis; the second most common clinical manifestation was hemisensory symptoms, which in a few patients involved the face [4]. Tongue weakness was an uncommon finding, and it was less frequent than facial weakness.
Since the pyramidal tract, medial lemniscus and hypoglossal nucleus are arranged anterior-posteriorly in the medial medulla, the clinical presentation is dependent upon the anteroposterior extent of the lesion; a lesion involving the anterior medulla sparing the posterior aspect will result in the sparing of the hypoglossal nucleus, thereby explaining the absence of tongue weakness. Nystagmus was another finding which was seen in a few patients.
Bilateral MMI (BMMI) is a rare event, and basically, the features summarized for the unilateral MMI are present on both sides, with the characteristics of quadriplegia and gaze paralysis, which may be confused with some features of locked-in syndrome. If quadriplegia/quadriparesis is a characteristic clinical hallmark, an upper motor neuron type of facial weakness may be unilateral, and the sensory manifestations or nystagmus are not constant. The facial palsy in patients with a medullary infarction has been postulated to result from the interruption of aberrant cortico-facial fibers which descend to the level of the upper-middle medulla and are located ventromedially, decussate, and then ascend in the dorsolateral medulla to supply the facial nucleus [5] (Figure 1).
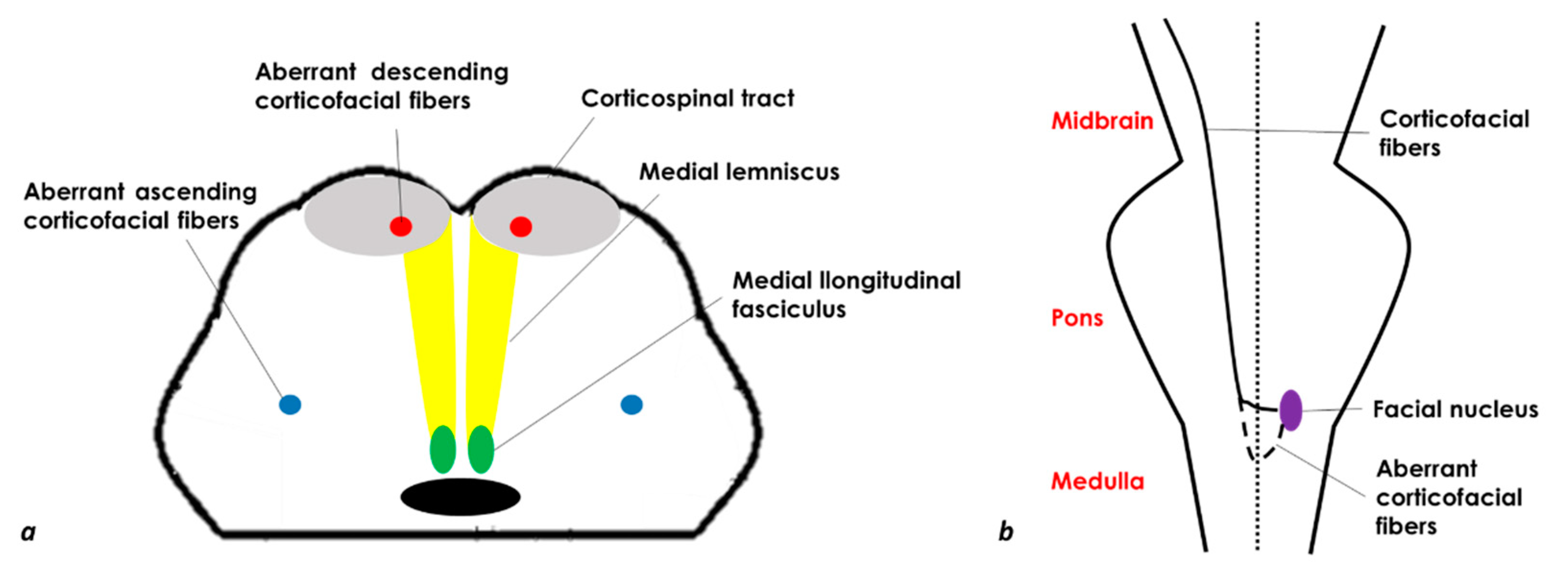
Figure 1. Schematic drawing of a medulla axial section (a) and a coronal section of the brainstem with the aberrant corticofacial fibers (b) (modified and redesigned from [6]).
In a systematic review of 38 cases of MRI-proven BMMIs [7] described from 1992 to 2011, the mean age was 62.2 years, and 74.2% of them were male. The most common clinical presentations were motor weakness in 78.4% of them, dysarthria in 48.6% of them, and hypoglossal palsy in 40.5% of them as rostral medullary lesions. Thirty-eight point-five percent of the patients had VA atherosclerosis as a putative etiologic mechanism, which was followed by branch occlusive disease. A characteristic, although uncommon, is the neuroimaging sign in brain MRI which has been described as having a “heart appearance sign” [8][9][10]. A good example of the MRI of the “heart appearance sign” both on Diffusion Weighted Imaging (DWI) and in Fluid Attenuated Inversion recovery (FLAIR) sequences is showed in Figure 2.
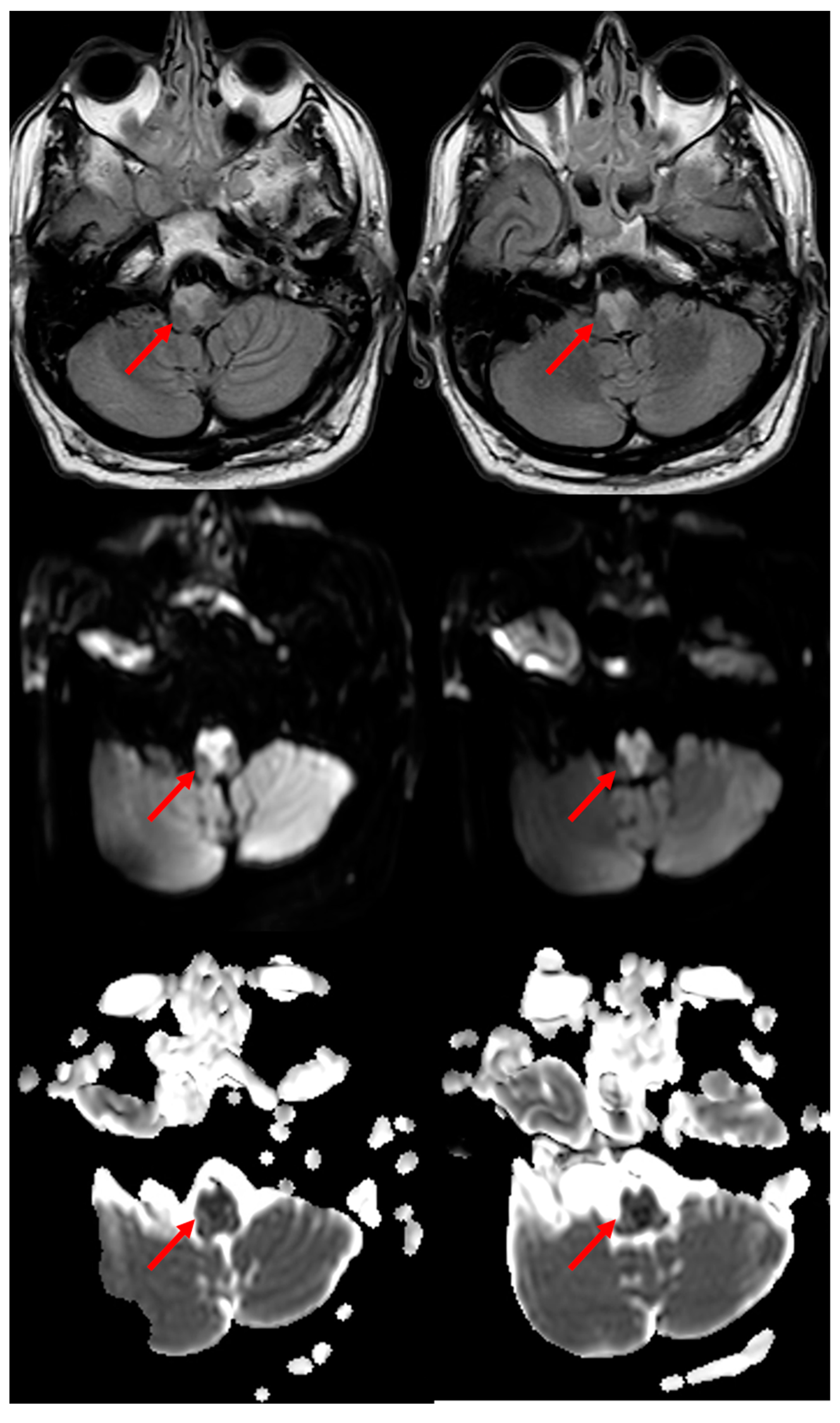
Figure 2. “Heart appearance sign” in acute BMMI, which is well evident in MRI on FLAIR axial images (first row), DWI images (second row) and Apparent Diffusion Coefficient (ADC) images (third row) (red arrows).
The patient whose MRI was shown in Figure 2 was admitted to the ED because he had a transient right hemisensory syndrome and he developed during the hospital stay, the abrupt onset of hypotonic quadriplegia, severe dysarthria and respiratory failure, and he required ventilator support. In the following hours, bilateral hypoglossal palsy, complex gaze abnormalities with horizontal gaze limitation and profound anesthesia for touch and pain in the four limbs and trunk developed. The cause of the BMMI was an atheromatous right V4 VA occlusion (Figure 3).
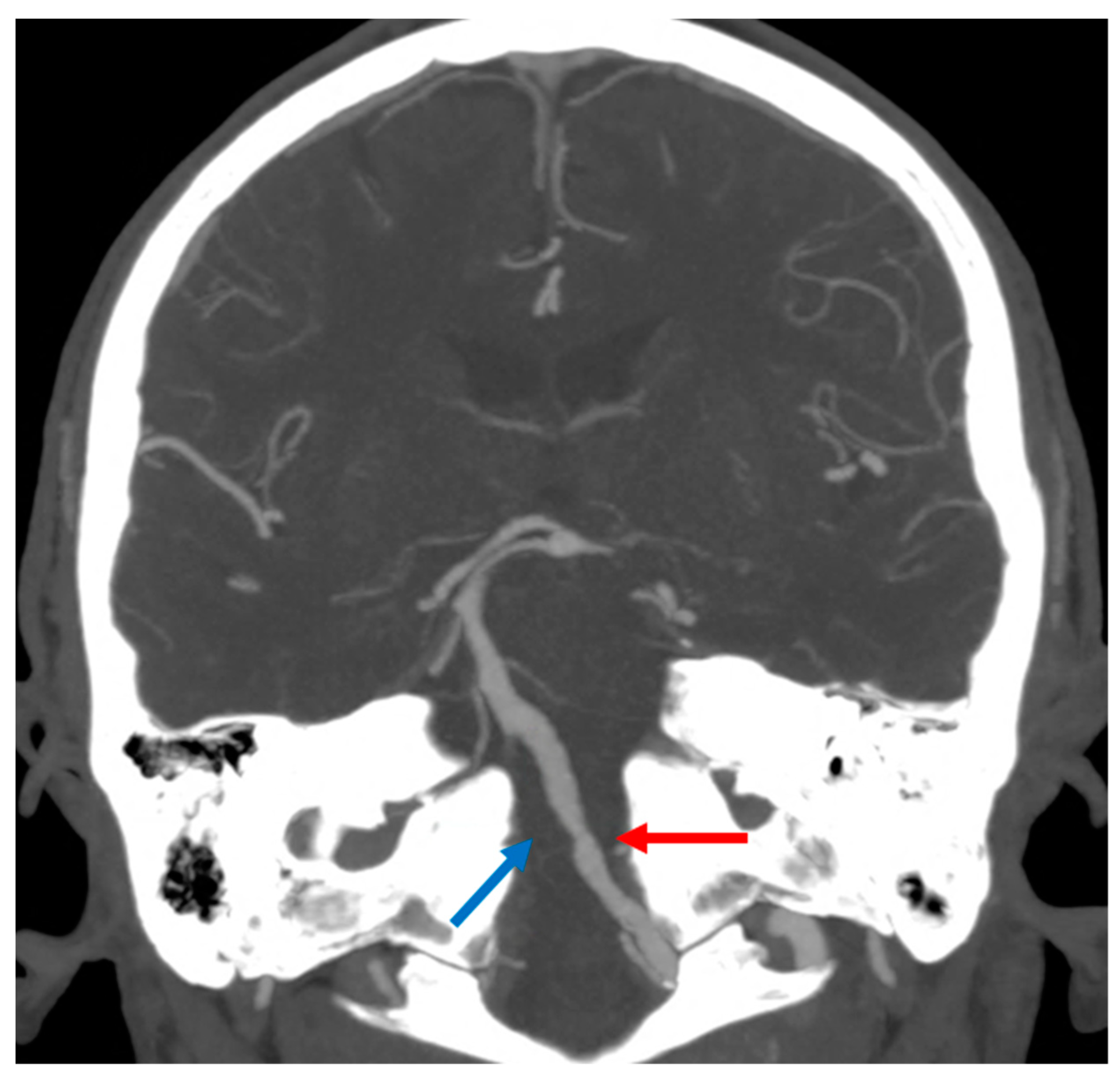
Figure 3. Intracranial Computed Tomography Angiography (CTA) with Maximum Intensity Projections (MIP) reconstruction in coronal plane, showing atheromatous changes in left V4 VA (red arrow) and occlusion of the right V4 VA (blue arrow).
The treatment strategy in the acute phase was a double antiplatelets regimen with high dosage of statins associated with low-molecular weight heparin (LMWH) for the prevention of venous thromboembolism.
Another even rarer subtype of medullary infarction is the infarction of the pyramidal decussation, which is scarcely described in the literature [11][12][13][14][15]. The expected clinical syndrome involves quadriplegia with the sparing of the face and sensory fibers. The proposed mechanism is the occlusion of the perforators off of the ASA, because at the level of the pyramidal decussation, the ASA does not supply the sensory tracts, which are instead usually fed by the VA and PSAs, thus explaining the absence of sensory deficits. Moreover, anarthria and dysphagia might be a sign of the involvement of the hypoglossal nucleus and fibers, with perhaps some involvement of the cranial nerves IX and X.
2. Pontine Infarction
Pontine infarctions are relatively rare, accounting for only about 7% of all ischemic strokes of the brain [16][17][18][19]. Considering the brainstem strokes, the pons, either in isolation or as part of multifocal ischemia, is involved much more often than the other brainstem structures are. Isolated pontine infarcts account for 12–27% of posterior circulation ischemia [2][20].
Based on clinical, anatomical and neuroradiological correlations, the following constant vascular territories have been defined in pontine ischemia: ventromedial (anteromedial), anterolateral, lateral (tegmental), dorsal and bilateral infarcts. The relative size and extension of each vascular territory may show a variability both in the causal, middle and rostral pons and in the axial distribution.
On the longitudinal axis, the infarction can occur in the caudal (lower) pons, middle or rostral (upper) pons or in two or three of these portions. In the axial plane, the paramedian, anterolateral, lateral or posterior region and their various combinations may be affected [16][17][18][19][20][21][22][23][24][25][26][27][28][29][30].
Ventral (paramedian) infarcts are the most common location (two out of three isolated pontine infarctions), and they are caused by the occlusion of the anteromedial or anterolateral-perforating arteries coming off from the BA through the BA plaques; conversely, small deep pontine infarctions are more frequently to be associated with small vessel disease neuroimaging signs [21][22].
Perforating arteries are on average, unilaterally, 5.8 mm in size (range: 4–10), and they have a mean diameter of 0.39 mm (range: 0.09–0.81 mm) [22]. They can be divided into the caudal group, entering the foramen caecum, the middle group, penetrating the edges of the basilar groove, and the rostral group, which entered the most caudal part of the interpeduncular fossa. Some of them always arise from the BA, either separately or by their own common trunks, or by common stems with some of the BA leptomeningeal vessels. Sixty-two point-five percent of these pontine-perforating arteries give off 1–3 anterolateral twigs [22], and a branch coming off unilaterally may supply both the ipsilateral and contralateral paramedian region of the pons. Similarly, the caudal pontine branches may nourish the upper part of the medullary pyramid and the olive. The BA gives origin to the leptomeningeal branches too, including the short pontomedullary (PMA) and anterolateral arteries (ALAs), the long inferolateral pontine artery (ILPA), the superolateral (SLPA) and posterolateral ones (PLPA), as well as some branches of the cerebellar arteries (AICA, SCA and more rarely PICA). The short ALAs have multiple (mean, 5.2) vessels on each side, arising directly from the BA or from the perforating arteries and less frequently from the long leptomeningeal or cerebellar arteries [22][23]. The PMA is a short BA branch which ends superiorly to the medullary olive. The ILPA, a single vessel, runs inferiorly and laterally, and it ends just below the level of the trigeminal nerve root, but it can give rise to the ALAs, rarely to a single perforating artery, and always to the lateral intrapontine branches.
The SLPA usually gives off one or two ALAs, rarely a perforating artery and very often lateral twigs, ending at the level of the trigeminal nerve root. The PLPA arises just below the origin site of the SCA, and it ends above the trigeminal nerve root.
The cerebellar arteries may contribute to the arterial supply of the pons: the AICA mainly gives off twigs to the lateral part of the lower pons, and the SCA to the most posterior (dorsal) part of the rostral tegmentum. Some perforating arteries and ALAs also can arise from the cerebellar arteries.
Several anastomoses have been described both between the neighboring perforating branches (25% of the cases) and among the SLPA and ILPA branches (56.3% of the cases) [22].
The medial basis pontis (corticospinal tract) is supplied by the short midline perforators branching directly off the BA, from which the long midline perforators supply the medial tegmentum (including the medial part of the medial lemniscus, the abducens nucleus, medial longitudinal fasciculus and paramedian pontine reticular formation).
Paramedian infarcts often extend to the ventral surface, whereas an ischemia restricted to the paramedian tegmentum is less common [20]. The clinical hallmark of a ventral pontine infarction is contralateral hemiparesis with a moderate-to-severe severity, and it is more marked in the upper extremity and in the distal part of the limbs. It may be an isolated neurological deficit such as pure motor hemiparesis in about one half of the ventral pontine infarctions [20]. Distinct lingual, contralateral palatal–lingual or palatal–lingual–laryngeal hemiparesis are uncommon patterns of motor deficit and resemble those observed in capsular genu syndrome. Moderate or marked dysarthria is almost a constant finding in large paramedian infarcts, particularly of the upper pons, and it is often accompanied by hemiparesis, brachial monoparesis, supranuclear facial palsy and hemiataxia, while an isolated dysarthria is a rare occurrence [24].
3. Midbrain Infarction
Midbrain infarcts account for 2% of all cerebral infarcts, whereas their frequency increases up to 8% in the infarcts limited to the posterior circulation [27][28][29]. Isolated midbrain infarcts are infrequent because of the midbrain’s common vascular supply with other infra- or supratentorial anatomical sites, and the incidence of isolated midbrain infarction varies only from 0.7% to 2.3% [29]. Midbrain infarcts more often coexist with infarcts in neighboring structures (diencephalon and pons) or the temporo-occipital cortex and superior cerebellum [27][29].
Bilateral midbrain infarcts are an extremely rare vascular syndrome, and they are located in the anteromedial (paramedian) vascular territory, which is supplied by the penetrating perforators from the BA, SCA and P1 PCA. The paramedian territories of the midbrain and thalamus are supplied by interpeduncular perforating branches. In previous anatomical studies, the interpeduncular branches have been described as three groups of vessels that originate from the last 5 mm of the basilar artery from the initial 7 mm of both the SCAs and from the P1 PCA [30].
SPMA and PThA frequently originate as a single or ‘common’ trunk, known as the type IIb variant of the artery of Percheron [31]. The interpeduncular perforating branches have great variability with respect to their number, size, origin and territorial contribution to the midbrain and thalamus [30]. This can result in a variable range of imaging appearances in stroke patients when the vascular disease involves the interpeduncular perforating branches.
The anteromedial segment of the midbrain contains critical structures for the vertical gaze (posterior commissure, periaqueductal region and the rostral interstitial nucleus of the medial longitudinal fasciculus) at the upper midbrain tegmentum. Therefore, infarcts involving the territories of the paramedian perforators of the BA, perforating branches of the SCA or the posterior thalamo-subthalamic paramedian artery (branch of the P1 PCA) usually produce vertical gaze palsies. Bilateral upper midbrain infarcts are characterized by a wide range of conjugate or disconjugate supranuclear vertical gaze palsies (rarely in isolation) [27][28][29][32]. A complete bilateral ophthalmoplegia (bilateral ptosis with loss of all extraocular movements) is an unusual sign of bilateral infarcts at the meso-diencephalic junction. Midbrain paramedian rostral infarcts can also manifest without gaze palsies, including pure motor hemiparesis or isolated gait ataxia, but they have been described only in unilateral lesions. When the ischemia is located around the red nucleus, the predominant feature may be body lateropulsion (contraversive falls to the side of lesion) due to interruption of ascending fibers of the crossed dentate-rubro-thalamic pathway.
Nuclear third nerve palsy (isolated or associated with hemiparesis or ataxia) is a localizing sign of the paramedian territory infarct at the middle level of the midbrain. The most common pattern of the nuclear oculomotor disorder is ipsilateral third nerve palsy with contralateral superior rectus paresis, which often presents with bilateral ptosis and mydriasis. Dysarthria, which is often associated with other signs, can be found in half of the patients with a pure midbrain infarction, whereas dysarthria (hypokinetic and palilalia) as the prominent clinical manifestation can be due to a single ischemic lesion involving the medial ventral part of the substantia nigra. Acquired stuttering can, in rare instances, be the outstanding manifestation of a small midbrain ischemia.
At the lower paramedian midbrain level, tetraataxia—which is often associated with hemiparesis, delayed tremor or palatal myoclonus—is a rare occurrence following bilateral, or less commonly, unilateral infarcts involving the crossing efferent dentato-rubral fibers [27]. Uni- or bilateral internuclear ophthalmoplegia (INO) due to the involvement of the medial longitudinal fasciculus together with limb or gait ataxia, dysarthria and tremor can reveal a caudal paramedian infarct, whereas an isolated bilateral INO is less common. Pathological laughter, although very rare, can herald a paramedian lower midbrain infarct (associated with dysarthria and hemiparesis) that disrupts the brainstem basal ganglia–forebrain circuitry which participates in laughter control. The caudal paramedian midbrain includes small, extremely medial portions of the cerebral peduncles (fronto-pontine tract), extremely medial portions of the substantia nigra, the superior cerebellar peduncles and their decussation, the central tegmental tract (the component of Mollaret’s triangle) and its decussation, the medial longitudinal fasciculi (MLF), the nuclei of the trochlear nerve (CN 4), the short segment of the CN4 fibers and the reticular structure [1]. Anatomically, the decussation of the superior cerebellar peduncles and the central tegmental tract constitute the horseshoe-shaped Wernekink’s commissure that is named after German anatomist Friedrich Wernekink [33]. A caudal paramedian midbrain infarction (CMPI) is an extremely rare form of an ischemic stroke (representing 12 out of 6820 cerebral infarction patients in the biggest published series) [34], and bilateral infarcts are even rarer (5/12 of them in the same case series). Neuroimaging features are a backward oblique sign in the lower level of the midbrain in unilateral sagittal MRI images, and they have a characteristic “V-shaped” appearance in the axial MRI in bilateral infarcts. In all of the patients, the neurological signs are bilateral cerebellar dysfunction (dysarthric speech, truncal or gait ataxia and four-limb ataxia), diplopia and INO, but not hemiparesis. CPMI is a rare cerebrovascular disease that destroys the Wernekink’s commissure, medial longitudinal fasciculi and other adjacent structures, but it usually does not involve the corticospinal tracts. The primary mechanisms of unilateral CPMI involve small vessel disease. The underlying stroke mechanisms of bilateral CPMI are either large artery atherosclerosis disease or a cardiac embolism. In bilateral CPMI cases, a “heart sign” has been reported in diffusion-weighted imaging (DWI) and fluid attenuated inversion recovery (FLAIR) images [35], which is smaller than the corresponding hearts in the medulla and pons. The clinical pattern of the infarct corresponds to the above described Wernekink’s syndrome (bilateral cerebellar dysfunction, wall-eyed bilateral internuclear ophthalmoplegia or WEBINO) without delayed palatal myoclonus. The Wernekink’s commissure consists of crossed dentate-rubro-thalamic tracts and dento-rubro-olivary bundles [33]. Bilateral cerebellar dysfunction is attributed to the interruption of the dentate-rubro-thalamic pathways prior to and after decussation. WEBINO is ascribed to the destruction of the medial longitudinal fasciculus (MLF) near the Wernekink’s commissure. Delayed palatal myoclonus and hypertrophy olivary degeneration in the medulla oblongata caused by primary lesions in the dento-rubro-olivary pathway have been reported in a few cases.
The “heart appearance” sign in the medulla oblongata or pons was considered to appear when the infarct occurred in the anterior-medial and anterior-lateral territories [36]. However, for a “heart appearance” in the caudal midbrain infarction, resulting in Wernekink’s commissure syndrome, it should involve the medial and lateral paramedian branches on both sides, sometimes with a stepwise timing.
Hemiparesis is not the typical feature of midbrain paramedian infarcts, so even bilateral paramedian infarcts are not associated with double hemiparesis and even less so to quadriplegia. Bilateral infarcts are uncommon, occurring mainly when both of the paramedian territories at the upper level are affected. In such cases, the ischemia may extend to the neighboring thalamus. Different patterns of vertical gaze palsy are the main clinical findings. Locked-in syndrome has been found to be associated with restricted ischemia, affecting both the cerebral peduncles. Oculomotor signs predominate along with hemiparesis and hemiataxia when combined anteromedial and anterolateral infarcts occur [27][29]. In the case series of forty patients with Asian ancestry described by Kim et al. [29], pure midbrain anteromedian infarcts are not associated with hemiparesis, but the anterolateral infarctions showed definite hemiparesis in 30% of the cases. A similar clinical picture has been provided by a case series in a different population sample [28].
A more complex and anecdotal vascular syndrome of the midbrain, which is associated with bilateral pyramidal tract damage, is the bilateral cerebral peduncular infarction (BCPI) [37][38][39]. Data from one medical center show that BCPI accounts for 0.26% of all of the admitted patients with an ischemic stroke [40], but the main limitation is that they were not pure midbrain infractions, and the other sites of infarction were found in the thalamus, pons and cerebellum. Moreover, the case described by Asakawa et al. [41] proposing the Mickey Mouse ears sign as a feature of this infarct the midbrain involvement was accompanied by a cerebellar infarction. Interestingly, two reported cases [37][38] had normal muscular strength. The most common symptoms of isolated BCPI included ataxia, dysarthria, sensory disturbance and mild paresis of the extremities, with a few disorders of eye movement or light reflex appearing upon a neurologic examination (rare involvement of the oculomotor nerve if the paramedian area is interested). The nerve fibers of the corticospinal tract (CST) run in the pedunculus cerebri, reaching the cerebellum via the cortico-ponto-cerebellar tract (CPCT). The damage to the CST and CPCT in the pedunculus cerebri is the cause of the mild paresis of the extremities, dysarthria and ataxia in BCPI. The reason why only mild hemiparesis was present even when the pyramidal tract at the crus cerebri was heavily involved has not been convincingly explained. One possible explanation for this is that the patients with hemiparesis also consistently showed the representation of the lower limb in the lateral part of cerebral peduncle, with the face and upper limb being represented more medially [27].
The vascularization of the midbrain is complex because there is a significant contribution by the perforating branches of the posterior communicating arteries and the peduncular perforating arteries and circumflex branches of the P1 or P2 segment of the PCA in addition to the supply through the BA and cerebellar arteries. The blood supply on the cerebral peduncle is achieved through multiple branches of the PCA, the SCA or the interpeduncular perforating branches originating from the tip of the BA. These arteries are known as the medial mesencephalic branches (MMB). [42]. Therefore, the blood supply on the cerebral peduncle mostly comes from the PCA and SCA, which are located at the distal segments of the BA. The perforating branches of the P1 PCA may play an important role in isolated cerebral peduncular infarction [40].
References
- Burger, K.M.; Tuhrim, S.; Naidich, T.P. Brainstem Vascular Stroke Anatomy. Neuroimaging Clin. N. Am. 2005, 15, 297–324.
- Bassetti, C.; Bogousslavsky, J.; Mattle, H.; Bernasconi, A. Medial medullary stroke: Report of seven patients and review of the literature. Neurology 1997, 48, 882–890.
- Kameda, W.; Kawanami, T.; Kurita, K.; Daimon, M.; Kayama, T.; Hosoya, T.; Kato, T. Lateral and Medial Medullary Infarction. A comparative analysis of 214 patients. Stroke 2004, 35, 694–699.
- Kim, J.S.; Kim, H.G.; Chung, C.S. Medial medullary syndrome. Report of 18 new patients and a review of the literature. Stroke 1995, 26, 1548–1552.
- Urban, P.P.; Wicht, S.; Vucorevic, G.; Fitzek, S.; Marx, J.; Thömke, F.; Mika-Grüttner, A.; Fitzek, C.; Stoeter, P.; Hopf, H.C. The course of corticofacial projections in the human brainstem. Brain 2001, 124 Pt 9, 1866–1876.
- Deshpande, A.; Chandran, V.; Pai, A.; Rao, S.; Shetty, R. Bilateral medial medullary syndrome secondary to Takayasu arteritis. BMJ Case Rep. 2013, 2013, bcr0120125600.
- Pongmoragot, J.; Parthasarathy, S.; Selchen, D.; Saposnik, G. Bilateral Medial Medullary Infarction: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2013, 22, 775–780.
- Tokuoka, K.; Yuasa, N.; Ishikawa, T.; Takahashi, M.; Mandokoro, H.; Kitagawa, Y.; Takagi, S. A case of bilateral medial medullary infarction presenting with “heart appearance” sign. Tokai J. Exp. Clin. Med. 2007, 32, 99–102.
- Maeda, M.; Shimono, T.; Tsukahara, H.; Maier, S.E.; Takeda, K. Acute Bilateral Medial Medullary Infarction: A Unique ‘Heart Appearance’ Sign by Diffusion-Weighted Imaging. Eur. Neurol. 2004, 51, 236–237.
- Thijs, R.D.; Wijman, C.A.C.; van Dijk, G.W.; van Gijn, J. A case of bilateral medial medullary infarction demonstrated by magnetic res-onance imaging with diffusion-weighted imaging. J. Neurol. 2001, 248, 339–340.
- Wilkins, E.G.; Kamel, H.; Johnson, E.C.; Shalev, S.M.; Josephson, S.A. Ischemic Stroke of the Pyramidal Decussation Causing Quadriplegia and Anarthria. J. Stroke Cerebrovasc. Dis. 2012, 21, 620.e1–620.e2.
- Meyer, J.S.; Herndon, R.M. Bilateral infarction of the pyramidal tracts in man. Neurology 1962, 12, 637.
- Jagiella, W.M.; Sung, J.H. Bilateral infarction of the medullary pyramids in humans. Neurology 1989, 39, 21–24.
- Kobayashi, Z.; Hino, T.; Kanazawa, T.; Yokote, H.; Yokota, T.; Kanda, T.; Mizusawa, H. Bilateral medial medullary infarction presented with monoplegia of the lower limb, followed by paraplegia and finally by tetraplegia. Rinsho Shinkeigaku 1993, 43, 195–198. (In Japanese)
- Stopford, J.S. The arteries of the pons and medulla oblongata. Part II. J. Anat Physiol. 1916, 50 Pt 3, 255–280.
- Oh, S.; Bang, O.Y.; Chung, C.S.; Lee, K.H.; Chang, W.H.; Kim, G.M. Topographic Location of Acute Pontine Infarction Is Associated with the Development of Progressive Motor Deficits. Stroke 2012, 43, 708–713.
- Kobayashi, J.; Ohara, T.; Minematsu, K.; Nagatsuka, K.; Toyoda, K. Etiological mechanisms of isolated pontine infarcts based on arterial territory involvement. J. Neurol. Sci. 2014, 339, 113–117.
- Fisher, C.M. Lacunar strokes and infarcts: A review. Neurology 1982, 32, 871.
- Kataoka, S.; Miaki, M.; Saiki, M.; Saiki, S.; Yamaya, Y.; Hori, A.; Hirose, G. Rostral lateral pontine infarction: Neurological/topographical correlations. Neurology 2003, 61, 114–117.
- Kumral, E.; Bayulkem, G.; Evyapan, D. Clinical spectrum of pontine infarction: Clinical—MRI correlations. J. Neurol. 2002, 249, 1659–1670.
- Xia, C.; Chen, H.S.; Wu, S.W.; Xu, W.H. Etiology of isolated pontine infarctions: A study based on high-resolution MRI and brain small vessel disease scores. BMC Neurol. 2017, 17, 216.
- Vlašković, T.; Brkić, B.G.; Stević, Z.; Vukićević, M.; Đurović, O.; Kostić, D.; Stanisavljević, N.; Marinković, I.; Kapor, S.; Marinković, S. Anatomic and MRI Bases for Pontine Infarctions with Patients Presentation. J. Stroke Cerebrovasc. Dis. 2022, 31, 106613.
- Marinković, S.; Gibo, H.; Milisavljević, M. The Surgical Anatomy of the Relationships between the Perforating and the Leptomeningeal Arteries. Neurosurgery 1996, 39, 72–83.
- Kataoka, S.; Hori, A.; Shirakawa, T.; Hirose, G. Paramedian pontine infarction: Neurological/topographical correlation. Stroke 1997, 28, 809–815.
- Venkatesan, P.; Balakrishnan, R.; Ramadoss, K.; Iyer, R.S. Heart appearance sign in pontine stroke: A result of bilateral infarction due to small vessel disease. Neurol. India 2014, 62, 115–116.
- Sen, D.; Arora, V.; Adlakha, S.; Gulati, Y.S.; Doppaladudi, A.; Tiwary, S. The “Heart Appearance” Sign in Bilateral Pontine Infarction. J. Stroke Cerebrovasc. Dis. 2015, 24, e21–e24.
- Bogousslavsky, J.; Maeder, P.; Regli, F.; Meuli, R. Pure midbrain infarction: Clinical syndromes, MRI, and etiologic patterns. Neurology 1994, 44, 2032–2040.
- Kumral, E.; Bayulkem, G.; Akyol, A.; Yunten, N.; Sirin, H.; Sagduyu, A. Mesencephalic and associated posterior circulation infarcts. Stroke 2002, 33, 2224–2231.
- Kim, J.S.; Kim, J. Pure midbrain infarction. Clinical, radiologic, and pathophysiologic findings. Neurology 2005, 64, 1227–1232.
- Pedroza, A.; Dujovny, M.; Ausman, J.I.; Diaz, F.G.; Artero, J.C.; Berman, S.K.; Mirchandani, H.G.; Umansky, F. Microvascular anatomy of the interpeduncular fossa. J. Neurosurg. 1986, 64, 484–493.
- Lazzaro, N.; Wright, B.; Castillo, M.; Fischbein, N.J.; Glastonbury, C.M.; Hildenbrand, P.G.; Wiggins, R.; Quigley, E.; Osborn, A. Artery of Percheron Infarction: Imaging Patterns and Clinical Spectrum. Am. J. Neuroradiol. 2010, 31, 1283–1289.
- Moncayo, J.; Bogousslavsky, J. Eye movement disorders in posterior circulation stroke. Expert Rev. Ophthalmol. 2009, 4, 259–281.
- Voogd, J.; van Baarsen, K. The Horseshoe-Shaped Commissure of Wernekinck or the Decussation of the Brachium Conjunctivum Methodological Changes in the 1840s. Cerebellum 2013, 13, 113–120.
- Zhou, C.; Xu, Z.; Huang, B.; He, Y.; Zhu, Y.; Zhao, Y.; Wang, P. Caudal paramedian midbrain infarction: A clinical study of imaging, clinical features and stroke mechanisms. Acta Neurol. Belg. 2019, 121, 443–450.
- Zhou, C.; He, Y.; Chao, Z.; Zhu, Y.; Wang, P.; Gao, X. The “heart appearance” sign on MRI of Wernekink’s commissure syndrome caused by bilateral caudal paramedian midbrain infarction. Neurol. Sci. 2017, 39, 587–589.
- Krishnan, M.; Rajan, P.; Kesavadas, C.; Iyer, R.S. The ‘heart appearance’ sign in MRI in bilateral medial medullary infarction. Postgrad. Med. J. 2011, 87, 156.
- Zhou, C.; He, Y.; Tian, X.; Chao, Z.; Zhu, Y.; Cheng, D.; Li, K. A Case Report of Isolated Bilateral Cerebral Peduncular Infarction. Case Rep. Neurol. Med. 2017, 2017, 9845917.
- Fu, X.; Li, H.; Tian, X.; Wang, W.; Liu, H. Rare presentation of an isolated bilateral cerebral peduncular infarction. Medicine 2019, 98, e17665.
- Ye, Q.; Xiang, T. A clinical characteristic analysis of five cases of rare bilateral cerebral peduncular infarction (BCPI) with the ‘Mickey Mouse ears’ sign. Brain Inj. 2021, 35, 363–367.
- Chen, W.; Yi, T.; Chen, Y.; Zhang, M.; Wu, Z.; Wu, Y.; Chen, B.; Guo, T.; Wu, C.; Yang, M.; et al. Assessment of bilateral cerebral peduncular infarction: Magnetic resonance imaging, clinical features, and prognosis. J. Neurol. Sci. 2015, 357, 131–135.
- Asakawa, Y.; Suzuki, K.; Takekawa, H.; Okamura, M.; Komagamine, T.; Kawasaki, A.; Yamamoto, M.; Sada, T.; Hirata, K. The ‘Mickey Mouse ears’ sign: A bilateral cerebral peduncular infarction. Eur. J. Neurol. 2013, 20, e37–e39.
- Ogawa, K.; Suzuki, Y.; Oishi, M.; Kamei, S. Clinical Study of Twenty-One Patients with Pure Midbrain Infarction. Eur. Neurol. 2011, 67, 81–89.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
06 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




