Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mokgadi Miranda Hlongwane | -- | 3357 | 2023-02-04 18:53:59 | | | |
| 2 | Sirius Huang | Meta information modification | 3357 | 2023-02-06 02:00:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hlongwane, M.M.; Mohammed, M.; Mokgalaka, N.S.; Dakora, F.D. Phytochemical Composition of Lessertia frutescens. Encyclopedia. Available online: https://encyclopedia.pub/entry/40831 (accessed on 07 February 2026).
Hlongwane MM, Mohammed M, Mokgalaka NS, Dakora FD. Phytochemical Composition of Lessertia frutescens. Encyclopedia. Available at: https://encyclopedia.pub/entry/40831. Accessed February 07, 2026.
Hlongwane, Mokgadi M., Mustapha Mohammed, Ntebogeng S. Mokgalaka, Felix D. Dakora. "Phytochemical Composition of Lessertia frutescens" Encyclopedia, https://encyclopedia.pub/entry/40831 (accessed February 07, 2026).
Hlongwane, M.M., Mohammed, M., Mokgalaka, N.S., & Dakora, F.D. (2023, February 04). Phytochemical Composition of Lessertia frutescens. In Encyclopedia. https://encyclopedia.pub/entry/40831
Hlongwane, Mokgadi M., et al. "Phytochemical Composition of Lessertia frutescens." Encyclopedia. Web. 04 February, 2023.
Copy Citation
Lessertia frutescens is a multipurpose medicinal plant indigenous to South Africa. The curative ability of the medicinal plant is attributed to its rich phytochemical composition, including amino acids, triterpenoids, and flavonoids.
Lessertia frutescens
phytochemicals
stress
1. Introduction
Increased interest regarding the health benefits of L. frutescens has encouraged researchers to look into the phytochemical composition of the plant. To date, a variety of compounds has been isolated from mainly the aerial part of the plant and are reported to provide the basis for the plant’s multipurpose medicinal benefits [1]. So far, the phytochemicals isolated include, but not limited to amino acids, flavonoids (including flavonol glycosides), pinitol and triterpenoids (including triterpenoids glycosides) [1][2][3][4][5][6][7][8][9].
2. Amino Acids
Apart from being the building blocks of proteins, amino acids play a key role in the growth, development and well-being of humans [10]. The molecules consist of two broad categories, essential and non-essential. The term non-essential amino acids does not mean the amino acids are not crucial, but it refers to amino acids that are mainly synthesized by body cells, which then need to be provided for through diet. Meanwhile, the essential amino acids are not synthesized by body cells and should be compensated for through diet [10]. The study conducted by Mncwangi and Viljoen [11] on wild and cultivated populations of L. frutescens from various locations revealed that amino acid content ranged from 10 to 15%, and 60% of the total amino acids, comprising asparagine, alanine and proline. In this study, about 100 mg of dried homogenized leaves was extracted with 50% acetonitrile and 0.1% formic acid mixture prior to analysis. The findings identified asparagine, alanine, and proline as major amino acid constituents of L. frutescens. Although not part of the major amino acids of L. frutescens, canavanine and gamma aminobutyric acid (GABA) enjoy scientists’ attention, due to their notable health benefits [12][13][14].
A comparison of the concentration of amino acids in natural populations of L. frutescens is depicted in Table 1. The two studies by Mncwangi and Viljoen [11] and Moshe [15] were conducted 14 years apart. The results highlight a significant depreciation in overall amino acid concentrations over the years. The authors are cognizant that the evaluation should be considered with caution, since the samples were not collected from the same location. Although both authors used leaves for analysis, Mncwangi and Viljoen [11] used an acetonitrile: formic acid mixture, while Moshe [15] used ethanol to prepare extracts. In another study, Shaik and colleagues [7] reported GABA and canavanine content to be 3.48 and 0.08 mg/g, respectively, in the methanolic extracts of leaf samples, confirming the depreciation of amino acid content as noted above. These results were consistent with the 3 mg/g GABA and 0.4 mg/g canavanine content, wherein methanolic extracts of pulverized leaves were used for an analysis [3].
2.1. Alanine
Alanine (Figure 1a), commonly known as L-alanine, is a non-essential amino acid [16] with benefits for humans. According to Araujo and colleagues [17], alanine is instrumental in regulating pancreatic β-cell physiology to minimize lifestyle-induced obesity. For example, it is used in the regulation of healthy blood sugar levels through gluconeogenesis. Gluconeogenesis is the process of producing glucose molecules in the liver [18]. Thus, avid exercisers benefit greatly from the involvement of alanine in gluconeogenesis. Mncwangi and Viljoen [11] reported alanine concentrations in L. frutescens ranging from 0.37 to 1.67 mg/g, while Moshe [15] observed concentrations of up to 10.7 mg/g. A significant depreciation in the phytochemical is evident in the results.
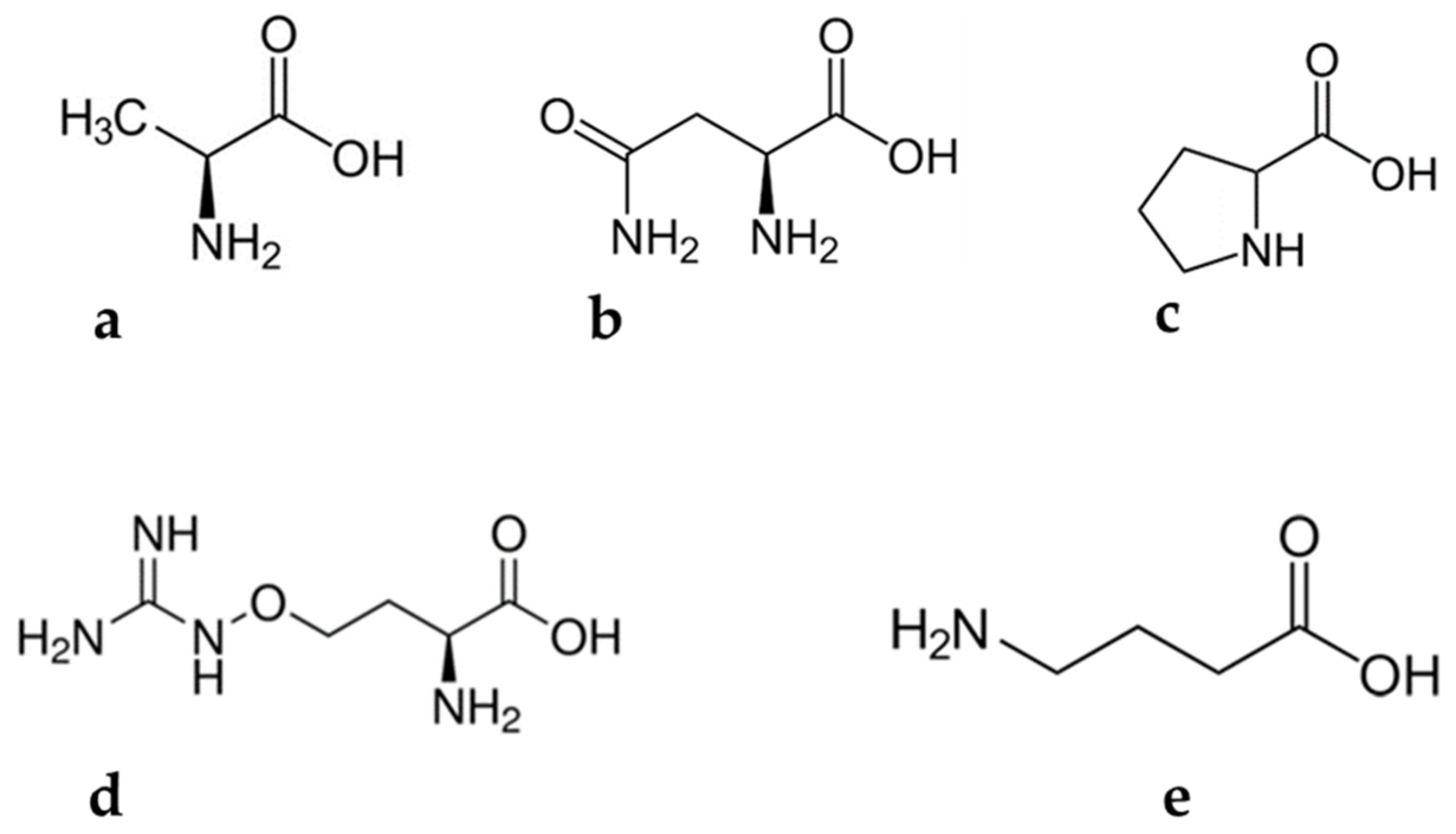
Figure 1. Amino acids found in Lessertia frutescens: (a) alanine, (b) asparagine, (c) proline, (d) canavanine and (e) gamma aminobutyric acid.
2.2. Asparagine
Asparagine (Figure 1b) is an amide of amino succinic acid which is partially soluble in water. Asparagine comprises a standard nitrogen: carbon ratio of 2:4. This special trait makes the molecule a suitable candidate for the storage and transportation of N2 in living organisms [19]. In a very recent study, it was suggested that asparagine reduces viral replication in human cells. This discovery makes the phytochemical a potential agent for antiviral development [20]. Soluble asparagine accumulates in almost all parts of plants. It has also been noted that the accumulation of this compound may occur as a result of abiotic stress [19]. However, Dell’Aversana and co-workers [21] reported contrasting observations. They observed a significant decrease in the asparagine content of Hordeum vulgare, which was subjected to salt stress. So far, the influence of abiotic stress on the accumulation of asparagine has not been explored in the case of L. frutescens. The available literature suggests that the asparagine concentration in L. frutescens ranges between 3.00 and 61.0 mg/g [11][15]. The contradicting reports on the content of asparagine in plants subjected to abiotic stress highlights the importance of seeking to study specific plants before making conclusions, in order to avoid inaccurate claims.
2.3. Canavanine
Canavanine (Figure 1c) is a non-protein amino acid commonly present in leguminous plants. The metabolite exhibits antagonistic characteristics against arginine and may inadvertently substitute arginine molecules during protein synthesis [22]. This phenomenon leads to abnormal three-dimensional protein molecules, also called canavanyl proteins [22][23]. The substitution of arginine by canavanine is not a favorable biosynthesis pathway as it may interrupt enzyme activities and subsequently obliterate the conformation of proteins [22]. Relatively high concentrations of canavanine are detected in the seeds of leguminous plants, while moderate levels are found in their aerial parts [22][23]. For example, canavanine constitutes a major proportion of the amino acids in alfalfa seeds.
Canavanine is known for its superior antitumor properties [24]. Research into this claim began decades ago, with the in vivo experiments on mammals taking place in the early 1980s. The study by Green et al. [25] assessed the anticancer properties of canavanine in leukemic mice infected with L1210. Their findings indicated that high doses of canavanine injections to the mice deactivate cancerous cell proliferation. In addition, the treatment increased the life span of the mice by 44%. The anticancer activity and mechanism of the phytochemical was reviewed by Bence and Crooks [26]. In addition to the antitumor properties, canavanine is reported to provide protection for plants against pathogens and predators [22][24].
Canavanine is among the most-studied amino acids in L. frutescens. Of interest is that Mncwangi and Viljoen [11] observed a huge variation in the concentration of some amino acids, including canavanine, in propagated L. frutescens plants. To date, the concentration of canavanine in the aerial part of L. frutescens ranges from 1.00 to 18.0 mg/g [3][7][11][15]. Such discrepancies in the phytochemical composition of medicinal plants are unfavorable, lest they result in challenges for formulation of standardization protocols. Therefore, practical and sustainable interventions on the cultivation level to optimize phytochemical compounds in the plant are necessary.
2.4. Gamma Aminobutyric Acid
Gamma aminobutyric acid (Figure 1d) is a four carbon non-protein amino acid essential biochemical, commonly found in microorganisms and plants [27][28][29] Various types of foodstuffs contain low levels of GABA, with fermentation causing an escalation in its concentration [30]. It is suspected that lactic acid is a precursor for the phytochemical [31]. Gamma aminobutyric acid was initially regarded as just a phytochemical. However, decades after its discovery, speculations that the compound could be a signaling molecule surfaced. Extensive research to investigate the suggestion commenced and still continues [29][32]. Unlike proline, GABA is a flexible compound and can assume various structural conformations, including a cyclic shape. Gamma aminobutyric acid induces anti-abiotic stress defenses in plants by preventing reactive oxygen species (ROS) formation, redox imbalance and cell death [23].
Of all the amino acids reviewed here, GABA is the second-most beneficial to humans, after canavanine. It is highly esteemed for its inhibitory neurotransmitter properties in the central nervous system [31]. Furthermore, Inoue et al. [33] reported that blood pressure reduction was witnessed in humans who consumed GABA. In addition to these benefits, GABA can induce relaxation and reduce anxiety. These benefits may be the reasons why GABA is used as a fortifier for various foodstuffs in Japan [31].
Lessertia frutescens natural populations contain GABA content, which is relatively close to the minimum range. For instance, Moshe [15] and Tai and partners [3] reported GABA concentrations of 0.045 and 0.029 μmol·g−1, respectively. According to [29] the concentration of GABA in plants shoots up under extreme environmental conditions. Therefore, under extreme environmental conditions, the increase in GABA may be accompanied by a reduction in the concentration of L-glutamate, because it is a precursor molecule for GABA. Again, this claim needs to be ascertained in the case of L. frutescens. As a result, GABA is often used to circumvent adverse effects of extreme environmental conditions. Priya and co-authors [30] demonstrated how GABA application can benefit the crop industry through alleviation of heat in mung beans. The authors observed a decline in endogenous GABA contents of the plants subjected to heat stress. However, the application of exogenous GABA to the plants resulted in improved plant parameters when compared with just the heat-stressed mung beans. Meanwhile, Yang et al. [34] soaked peach fruits in GABA solution to develop resistance to chilling injury due to the activation of antioxidant enzymes and the maintenance of higher energy level status in the fruits. In the most recent study, GABA was used as a drought stress suppressant in two variants of black pepper plants [35]. Physiological parameters of pretreated pepper plants were better than untreated plants, when both were subjected to drought stress.
2.5. Proline
Proline (Figure 1e) is a unique proteogenic amino acid with its γ-amino acid group positioned as a secondary amine. The compound is characterized by a rigid, cyclic structure, which restricts conformational flexibility [36][37]. The fact that proline is considered a non-essential amino acid in humans unless they are injured has almost disguised its indispensable value in neonates [38]. Interesting discoveries were made in studies that involved pig neonates, which could be extrapolated to human neonates, since similarities in the two were validated [38]. In one study, a remarkable demand for proline, used in protein synthesis, was noted during pre- and postnatal periods [39]. Meanwhile, Brunton et al. [40] indicated a huge dependability of protein synthesis in neonates, on parenteral proline supply.
Proline plays a vital role in plant primary metabolism [41]. Its compatible solute characteristics are remarkable in dehydrated seeds and pollen, where it combats cellular structure degradation [36]. It has also been reported that the metabolite plays a critical role in plant pathogen interactions, programmed death and development [37]. Proline accumulation in plants is attributed to their exposure to stressful environmental conditions such as salt, drought, ultraviolet (UV) radiation, extreme temperatures, and toxic metals [41]. This accumulation is apparently at a pace that is relatively faster than any other amino acids. Hence, other researchers proposed its exploitation as a tool to develop irrigation protocols for plants and select drought resistant species. However, in the case of L. frutescens there is no data available to support or refute whether the metabolite accumulates under extreme environmental conditions. The concentration of proline in L. frutescens natural population aerial parts ranged between 0.32 and 36.8 mg/g [11][15]. High concentrations of proline are purportedly found in the reproductive organs of plants when compared to other plant parts. The variability extends to plant leaves, where young ones contain relatively high concentrations when compared to the mature counterparts [36].
3. Pinitol
Pinitol (Figure 2) is a cyclitol which was first isolated from Pinus monticola, from which the name was derived. It occurs naturally in plants, especially of the Leguminosae family [42]. The concentration of pinitol in plants may vary in response to abiotic stress. Considering that pinitol is among the phytochemicals that are responsible for L. frutescens’ medicinal properties, it does not come as a surprise that several studies [7][11][15] have documented its valuable health benefits in humans. These include antidiabetic [43][44], antioxidant [45] and anticancer [46][47] capabilities. A significant improvement in the condition of a liver was noted in the patients of non-alcoholic fatty liver disease, when pinitol was administered. Pinitol induced the reduction of oxidative stress and accumulation of fatty acids, in order to effect the improvement [45]. Meanwhile, it induced cytotoxicity in human leukemia cells, MOLT-4. In this case, the dose-dependent cell apoptosis was achieved through the generation of reactive oxygen species [46]. Thus, a reduction in the level of this phytochemical in plants may lead to compromised efficacy.
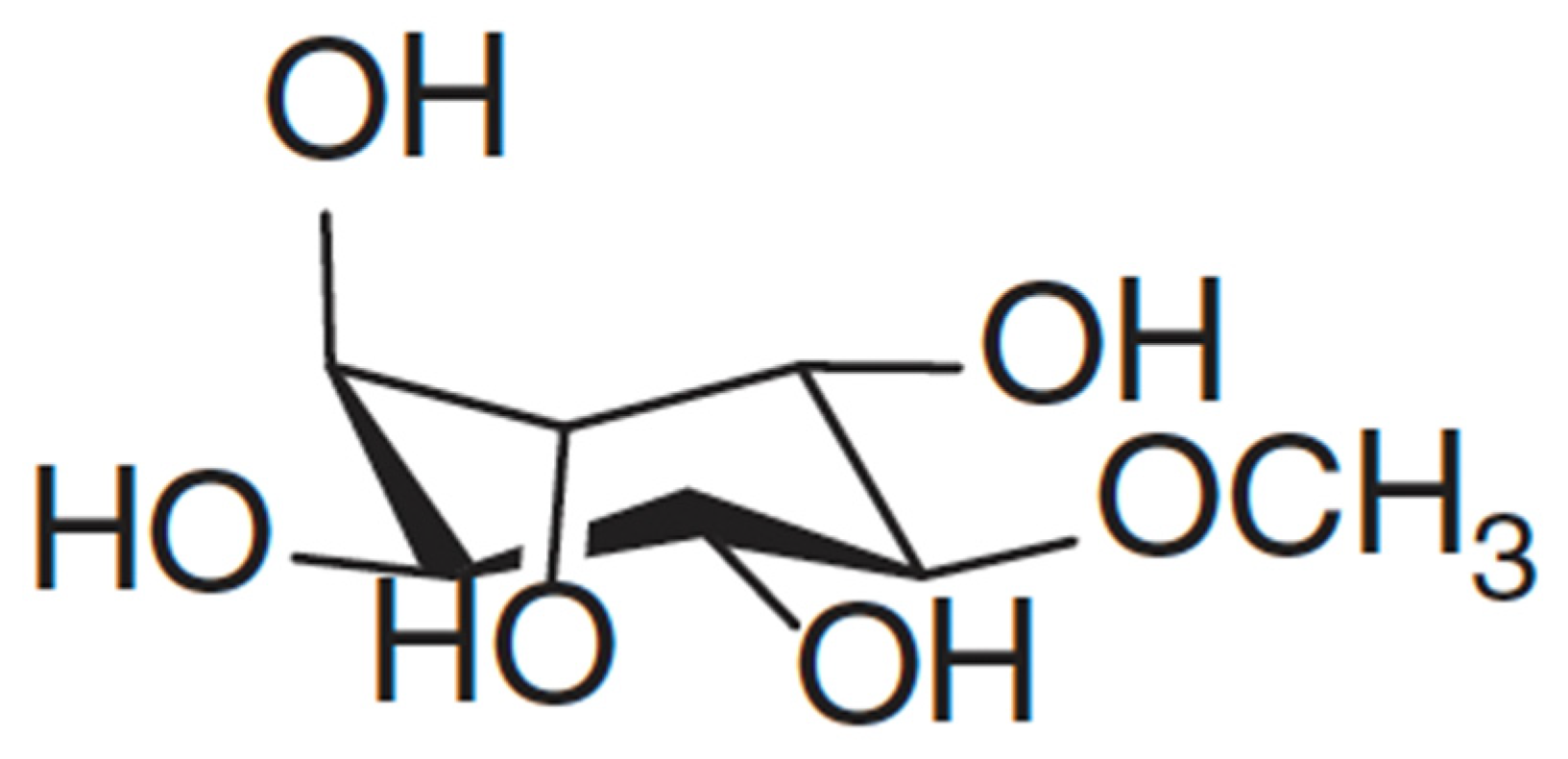
Figure 2. Structure of D-Pinitol adapted from Mncwangi and Viljoen [11]. CC BY license (https://creativecommons.org/licenses/by/4.0/).
Lessertia consists of several subspecies that are very similar, and that may be difficult for laymen to distinguish [12]. Moshe [15] determined the concentration of pinitol in Lessertia frutescens in subspecies microphylla only, and found it to be 14.4 mg/g. Meanwhile, Shaik, Singh and Nicholas [7] found pinitol to be the most abundant in the field-grown leaves of L. frutescens at a concentration of 18.17 mg/g, while Mncwangi and Viljoen (2012) reported concentrations ranging between 0.02 and 1.32 mg/g. The field leaves that Shaik and colleagues [7] used in their study were sourced commercially, rendering it difficult to find more details about their origin. Knowledge of the exact source or location of the samples would provide insight into how the environment may affect pinitol content and allow informed deliberations on the variation of pinitol that is evident. Previously, it was reported that variable geographical locations may result in different metabolite profiles [48]. These results also demonstrate that the concentration of pinitol fluctuates vastly due to factors such as storage, cultivating and harvesting season and abiotic stress [7].
4. Triterpenoids
Triterpenes are naturally occurring C30-terpene compounds characterized by isoprene subunits and diverse structures. Terpene compounds are classified into monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterpenes (C25), triterpenes (C30), and tetraterpenes (C40), depending on the number of carbons in their structure [49][50][51]. Although often interchanged with triterpenes, triterpenoids are slightly different from triterpenes. Triterpenoids are a collective name for all triterpenes, including natural degradation products, natural and synthetic derivatives, and hydrogenation products [49][50][52]. There are more than 20,000 identified triterpenoids and the number continues to grow due to the heightened interests in the compounds because of their bioactive capability [49][51]. Moreover, there are two main groups of triterpenoids, tetracyclic and pentacyclic triterpenoids, with the latter group being the largest, while monocyclic, bicyclic, tricyclic, hexacyclic and acyclic triterpenoids also exist [51][52]. The structural diversity of triterpenoid compounds is attributed to the enzyme oxidosqualene cyclase process which facilitates the rearrangement of triterpenoids scaffolds to form distinct structures [51].
Triterpenoid Glycosides
Triterpenoid glycoside is a general term for a natural product, a triterpenoid in this case, attached to glycone compounds [53]. Researchers continue to isolate and identify novel triterpenoid glycosides from plant extracts [54][55]. Triterpenoid glycosides act as self-defense agents for plants. For example, Kubanek et al. [56] established that triterpenoid glycosides served as deterrents for predators of marine sponges. The compounds also exhibit anti-proliferative properties on cancer cells [57]. Fu and co-workers [58] isolated and identified four novel triterpenoid/cycloartane glycosides, Sutherlandiosides A–D, from L. frutescens extracts. The group also established that Sutherlandioside B was the major compound of the four. Furthermore, Fu and co-workers [58] proposed for Sutherlandioside B to be used as a biomarker for L. frutescens, a proposal which was refuted by Albrecht and partners [4] on the basis that the compound is not detectable in all populations and it has not been certified as the main active ingredient. In addition, Brownstein and co-workers [9] proposed that Sutherlandioside B may have caused a reduction of corticosterone in rats that had chronic immobilization stress, validating it as a potential anti-stress agent in humans.
To date, there has not been a conclusive investigation on the biological activities of the individual phytochemicals of Sutherlandiosides A–D, partly due to complications associated with attaining their pure compounds. Should pure isolates of these compounds be accessible, accurate speculations into their individual biological activities would be achieved. A successful attempt was however made to isolate and purify Sutherlandioside B [9]. When an antiviral activity assay was conducted for the isolated Sutherlandioside B against Epstein–Barr virus (EBV), very little activity was evident. However, the detected antiviral activity was lower than that of the positive control employed in the study. Moreover, unpublished data from Fu [59] indicated no antimicrobial activity on the four triterpenoid glycosides, despite increasing their concentration up to 20 µg/mL. The results for the little to no antiviral activity of Sutherlandiosides found in L. frutescens should however not deter the interests in their health benefits. Rather, it should spur researchers to look into other biological activities of the compounds, because their presence in L. frutescens is associated with superior anticancer activities [60].
Albrecht and partners [4] discovered two types of L. frutescens ecotypes in samples collected from the Cape, those that contain detectable amounts of Sutherlandiosides B (NC), and others without the compound (WC). The samples for this study were prepared by extracting the whole plant with an acetonitrile: formic acid mixture. The authors speculated that the two types of plants (with and without Sutherlandioside B) were incana and microphylla subspecies. However, they do discount the possibility of variation due to different origins and environmental conditions. According to Zonyane and partners [48] Sutherlandiosides B and C were detected in the ethanolic extracts prepared from the leaves of L. frutescens collected in the NC, while WC samples did not contain any of these phytochemicals. These findings corroborate what Albrecht and partners [4] speculated, insinuating that plants from the two provinces are of different subspecies. Studies that explored the variation in the Sutherlandiosides A–D profile to see if they can be linked to specific subspecies concluded that subspecies L. microphylla is characterized by the presence of Sutherlandiosides B and D [61].
5. Flavonoids
Flavonoids are diverse polyphenolic class of compounds and secondary metabolites, occurring naturally in plants. These compounds consist of 15-carbon atoms fashioned in three rings as C6-C3-C6 [8][62]. To date, the highest number of flavonoids isolated is 9000 [62], with other studies citing 6500 [63] and 4000 [64]. Thus, the numbers continue to grow due to increasing interest in the health benefits conferred by these compounds when present in human and animal diets. The biological activities possessed by the phenolic compounds include antioxidant activity, weight management, protection against cardiovascular diseases, anti-allergy properties, antibacterial activity, anti-inflammatory effects, anticancer properties and age-related neurodegenerative disease prevention [62][65]. Flavonoids cannot be synthesized by animals. However, plants, particularly higher plants, synthesize them from phenylamine through the shikimic acid pathway [65].
Flavonoids are reported to give plants their distinct color, flowers special aroma, and fruit characteristics that attract pollinators [63]. They also play a vital role in seed germination, growth and development of seedlings [63]. Pourcel et al. [66] highlighted the ability of flavonoids to alleviate biotic and abiotic stress in plants. It is reported that the compounds act as UV-radiation filters, thereby minimizing the impact of abiotic stress [66].
Flavonoids are categorized based on the substrate that attaches to the C3 ring of the fundamental C6-C3-C6 skeletal structure. Some of the classes of flavonoids include chalcone, flavones, flavonols, flavanones, anthocyanins, and isoflavonoids [62][65]. Most of these groups have established significant commercial value in the market [65]. They exist commonly in a glycosylated form [62][63], and four of them were discovered by Fu and co-authors [8] in L. frutescens.
Flavonol Glycosides
Flavonol glycosides are flavonoids with a glycoside substrate attached to the C3 ring of the flavonol compounds. To date, of the six novel flavonol glycosides in L. frutescens discovered and discussed by Van Wyk and Albrecht [12], only four were successfully isolated and identified. Fu and colleagues [8] established the structures of the four compounds and named them Sutherlandins A, B, C and D. These compounds are quercetin and kaempferol derivatives [4]. Although they are not major compounds of the medicinal plant, they may be used as its biomarker or to distinguish origins of L. frutescens raw materials. The findings of Zonyane and co-workers [48] provide a basis for their use to predict the origin of raw materials, since only samples from the WC province of SA contained Sutherlandin B, while samples from the EC and NC provinces contained both Sutherlandin A and D. Meanwhile, Acharya and colleagues [61] used the presence of these glycosides to differentiate between subspecies. For example, Sutherlandin B’s presence was noted in L. frutescens, while Sutherlandin A was found in L. microphylla.
Although flavonol glycosides may exhibit some of the general health benefits of flavonoids, it is imperative to investigate compound-specific activities. This would only be possible if pure compounds of these glycosides were readily accessible. Thus, the work of Chen et al. [67] could have an application, provided the yields are improved. Chen and co-workers (2017) reported a yield of 110.4 mg per 0.90 g plant extract, which the authors regard as low and wish to enhance.
References
- Mavimbela, T.; Vermaak, I.; Chen, W.; Viljoen, A. Rapid quality control of Sutherlandia frutescens leaf material through the quantification of SU1 using vibrational spectroscopy in conjunction with chemometric data analysis. Phytochem. Lett. 2018, 25, 184–190.
- Aboyade, O.M.; Styger, G.; Gibson, D.; Hughes, G. Sutherlandia frutescens: The meeting of science and traditional knowledge. J. Altern. Complement. Med. 2014, 20, 71–76.
- Tai, J.; Cheung, S.; Chan, E.; Hasman, D. In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J. Ethnopharmacol. 2004, 93, 9–19.
- Albrecht, C.; Stander, M.; Grobbelaar, M.; Colling, J.; Kossmann, J.; Hills, P.; Makunga, N. LC–MS-based metabolomics assists with quality assessment and traceability of wild and cultivated plants of Sutherlandia frutescens (Fabaceae). S. Afr. J. Bot. 2012, 82, 33–45.
- Colling, J.; Stander, M.A.; Makunga, N.P. Nitrogen supply and abiotic stress influence canavanine synthesis and the productivity of in vitro regenerated Sutherlandia frutescens microshoots. J. Plant Physiol. 2010, 167, 1521–1524.
- Sia, C. Spotlight on ethnomedicine: Usability of Sutherlandia frutescens in the treatment of diabetes. Rev. Diabet. Stud. 2004, 1, 145.
- Shaik, S.; Singh, N.; Nicholas, A. HPLC and GC analyses of in vitro-grown leaves of the cancer bush Lessertia (Sutherlandia) frutescens L. reveal higher yields of bioactive compounds. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 105, 431–438.
- Fu, X.; Li, X.-C.; Wang, Y.-H.; Avula, B.; Smillie, T.J.; Mabusela, W.; Syce, J.; Johnson, Q.; Folk, W.; Khan, I.A. Flavonol glycosides from the South African medicinal plant Sutherlandia frutescens. Planta Med. 2010, 76, 178–181.
- Brownstein, K.J.; Knight, M.; Ito, Y.; Rottinghaus, G.E.; Folk, W.R. Isolation of the predominant cycloartane glycoside, sutherlandioside B, from Sutherlandia frutescens (L.) R.Br. by spiral countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 423–429.
- Wu, G. Functional Amino Acids in Growth, Reproduction, and Health. Adv. Nutr. 2010, 1, 31–37.
- Mncwangi, N.P.; Viljoen, A.M. Quantitative variation of amino acids in Sutherlandia frutescens (Cancer bush)—Towards setting parameters for quality control. S. Afr. J. Bot. 2012, 82, 46–52.
- Van Wyk, B.; Albrecht, C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae). J. Ethnopharmacol. 2008, 119, 620–629.
- Gibson, D. Ambiguities in the making of an African Medicine: Clinical trials of Sutherlandia frutescens (L.) R. Br (Lessertia frutescens). Afr. Sociol. Rev./Rev. Afr. Sociol. 2011, 15, 124–137.
- Johnson, Q.; Syce, J.; Nell, H.; Rudeen, K.; Folk, W. A Randomized, Double-Blind, Placebo-Controlled Trial of Lessertia frutescens in Healthy Adults. PLoS Clin. Trials 2007, 2, e16.
- Moshe, D. A Biosystematic Study of the Genus Sutherlandia Br. R. (Fabaceae, Galegeae). Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 1998.
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential distribution of amino acids in plants. Amino Acids 2017, 49, 821–869.
- Araujo, T.R.; Freitas, I.N.; Vettorazzi, J.F.; Batista, T.M.; Santos-Silva, J.C.; Bonfleur, M.L.; Balbo, S.L.; Boschero, A.C.; Carneiro, E.M.; Ribeiro, R.A. Benefits of L-alanine or L-arginine supplementation against adiposity and glucose intolerance in monosodium glutamate-induced obesity. Eur. J. Nutr. 2017, 56, 2069–2080.
- Schroer, A.B. Cycling Time Trial Performance Is Not Enhanced by Either Whey Protein or L-Alanine Intake during Prolonged Exercise. Master’s Thesis, James Madison University, Harrisonburg, VA, USA, 2013.
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26.
- Pant, A.; Yang, Z. Asparagine: An Achilles Heel of Virus Replication? ACS Infect. Dis. 2020, 6, 2301–2303.
- Dell’Aversana, E.; Hessini, K.; Ferchichi, S.; Fusco, G.M.; Woodrow, P.; Ciarmiello, L.F.; Abdelly, C.; Carillo, P. Salinity Duration Differently Modulates Physiological Parameters and Metabolites Profile in Roots of Two Contrasting Barley Genotypes. Plants 2021, 10, 307.
- Akaogi, J.; Barker, T.; Kuroda, Y.; Nacionales, D.C.; Yamasaki, Y.; Stevens, B.R.; Reeves, W.H.; Satoh, M. Role of non-protein amino acid L-canavanine in autoimmunity. Autoimmun. Rev. 2006, 5, 429–435.
- Rodrigues-Corrêa, K.C.d.S.; Fett-Neto, A.G. Abiotic stresses and non-protein amino acids in plants. Crit. Rev. Plant Sci. 2019, 38, 411–430.
- Rosenthal, G.A.; Nkomo, P. The Natural Abundance Of L-Canavanine, An Active Anticancer Agent, in Alfalfa, Medicago sativa (L.). Pharm. Biol. 2000, 38, 1–6.
- Green, M.H.; Brooks, T.L.; Mendelsohn, J.; Howell, S.B. Antitumor activity of L-canavanine against L1210 murine leukemia. Cancer Res. 1980, 40, 535–537.
- Bence, A.K.; Crooks, P.A. The mechanism of L-canavanine cytotoxicity: Arginyl tRNA synthetase as a novel target for anticancer drug discovery. J. Enzym. Inhib. Med. Chem. 2003, 18, 383–394.
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678.
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420.
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603.
- Priya, M.; Sharma, L.; Kaur, R.; Bindumadhava, H.; Nair, R.M.; Siddique, K.H.M.; Nayyar, H. GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci. Rep. 2019, 9, 7788.
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of γ-aminobutyric acid (GABA) administration in humans. BioFactors 2006, 26, 201–208.
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115.
- Inoue, K.; Shirai, T.; Ochiai, H.; Kasao, M.; Hayakawa, K.; Kimura, M.; Sansawa, H. Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 2003, 57, 490–495.
- Yang, A.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 2011, 129, 1619–1622.
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2016, 78, 57–67.
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962.
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arab. Book/Am. Soc. Plant Biol. 2010, 8, e0140.
- Köhler, E.S.; Sankaranarayanan, S.; van Ginneken, C.J.; van Dijk, P.; Vermeulen, J.L.; Ruijter, J.M.; Lamers, W.H.; Bruder, E. The human neonatal small intestine has the potential for arginine synthesis; developmental changes in the expression of arginine-synthesizing and-catabolizing enzymes. BMC Dev. Biol. 2008, 8, 107.
- Wu, G.; Flynn, N.E.; Knabe, D.A. Enhanced intestinal synthesis of polyamines from proline in cortisol-treated piglets. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E395–E402.
- Brunton, J.A.; Baldwin, M.P.; Hanna, R.A.; Bertolo, R.F. Proline supplementation to parenteral nutrition results in greater rates of protein synthesis in the muscle, skin, and small intestine in neonatal Yucatan miniature piglets. J. Nutr. 2012, 142, 1004–1008.
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97.
- Sanchez-Hidalgo, M.; Leon-Gonzalez, A.J.; Gálvez-Peralta, M.; Gonzalez-Mauraza, N.H.; Martin-Cordero, C. d-Pinitol: A cyclitol with versatile biological and pharmacological activities. Phytochem. Rev. 2020, 20, 211–224.
- Sharma, N.; Verma, M.K.; Gupta, D.K.; Satti, N.K.; Khajuria, R.K. Isolation and quantification of pinitol in Argyrolobium roseum plant, by 1H-NMR. J. Saudi Chem. Soc. 2016, 20, 81–87.
- Bates, S.H.; Jones, R.B.; Bailey, C.J. Insulin-like effect of pinitol. Br. J. Pharmacol. 2000, 130, 1944–1948.
- Lee, E.; Lim, Y.; Kwon, S.W.; Kwon, O. Pinitol consumption improves liver health status by reducing oxidative stress and fatty acid accumulation in subjects with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Nutr. Biochem. 2019, 68, 33–41.
- Yao, X.; Shi, K.; Yang, Y.; Gu, X.; Tan, W.; Wang, Q.; Gao, X.; Veeraraghavan, V.P.; Mohan, S.K.; Jin, S. D-Pinitol treatment induced the apoptosis in human leukemia MOLT-4 cells by improved apoptotic signaling pathway. Saudi J. Biol. Sci. 2020, 27, 2134–2138.
- Lin, T.-H.; Tan, T.-W.; Tsai, T.-H.; Chen, C.-C.; Hsieh, T.-F.; Lee, S.-S.; Liu, H.-H.; Chen, W.-C.; Tang, C.-H. D-pinitol inhibits prostate cancer metastasis through inhibition of αVβ3 integrin by modulating FAK, c-Src and NF-κB pathways. Int. J. Mol. Sci. 2013, 14, 9790–9802.
- Zonyane, S.; Chen, L.; Xu, M.-J.; Gong, Z.-N.; Xu, S.; Makunga, N.P. Geographic-based metabolomic variation and toxicity analysis of Sutherlandia frutescens (L.) R.Br.—An emerging medicinal crop in South Africa. Ind. Crops Prod. 2019, 133, 414–423.
- Muffler, K.; Leipold, D.; Scheller, M.-C.; Haas, C.; Steingroewer, J.; Bley, T.; Neuhaus, H.E.; Mirata, M.A.; Schrader, J.; Ulber, R. Biotransformation of triterpenes. Process Biochem. 2011, 46, 1–15.
- Parmar, S.K.; Sharma, T.P.; Airao, V.B.; Bhatt, R.; Aghara, R.; Chavda, S.; Rabadiya, S.O.; Gangwal, A.P. Neuropharmacological effects of triterpenoids. Phytopharmacology 2013, 4, 354–372.
- Sandeep; Ghosh, S. Chapter 12—Triterpenoids: Structural diversity, biosynthetic pathway, and bioactivity. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 411–461.
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291.
- Whisgary, D. Effects of Environmental Growth Conditions on the Levels of Sutherlandins 3 and 4 and Sutherlandiosides B and D, in Sutherlandia frutescens (L.) R. Br. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2011.
- Kumar, R.; Chaturvedi, A.K.; Shukla, P.K.; Lakshmi, V. Antifungal activity in triterpene glycosides from the sea cucumber Actinopyga lecanora. Bioorg. Med. Chem. Lett. 2007, 17, 4387–4391.
- Shao, Y.; Harris, A.; Wang, M.; Zhang, H.; Cordell, G.A.; Bowman, M.; Lemmo, E. Triterpene Glycosides from Cimicifuga racemosa. J. Nat. Prod. 2000, 63, 905–910.
- Kubanek, J.; Whalen, K.E.; Engel, S.; Kelly, S.R.; Henkel, T.P.; Fenical, W.; Pawlik, J.R. Multiple defensive roles for triterpene glycosides from two Caribbean sponges. Oecologia 2002, 131, 125–136.
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer Activity of Sea Cucumber Triterpene Glycosides. Mar. Drugs 2015, 13, 1202–1223.
- Fu, X.; Li, X.-C.; Smillie, T.J.; Carvalho, P.; Mabusela, W.; Syce, J.; Johnson, Q.; Folk, W.; Avery, M.A.; Khan, I.A. Cycloartane glycosides from Sutherlandia frutescens. J. Nat. Prod. 2008, 71, 1749–1753.
- Fu, X. Phytochemical Studies on the Medicinal Plant Sutherlandia frutescens. Ph.D. Thesis, University of Mississippi, Oxford, MS, USA, 2012.
- Zonyane, S.; Fawole, O.A.; Grange, C.L.; Stander, M.A.; Opara, U.L.; Makunga, N.P. The implication of chemotypic variation on the anti-oxidant and anti-cancer activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from different geographic locations. Antioxidants 2020, 9, 152.
- Acharya, D.; Enslin, G.; Chen, W.; Sandasi, M.; Mavimbela, T.; Viljoen, A. A chemometric approach to the quality control of Sutherlandia (cancer bush). Biochem. Syst. Ecol. 2014, 56, 221–230.
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23.
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35.
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287.
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228.
- Pourcel, L.; Routaboul, J.-M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36.
- Chen, C.; Folk, W.R.; Lazo-Portugal, R.; Finn, T.M.; Knight, M. Isolation of Sutherlandins A, B, C and D from Sutherlandia frutescens (L.) R. Br. by counter-current chromatography using spiral tubing support rotors. J. Chromatogr. A 2017, 1508, 7–15.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
06 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




