
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Catherine Bennetau-Pelissero | -- | 11890 | 2023-02-04 15:40:52 | | | |
| 2 | Catherine Bennetau-Pelissero | + 11 word(s) | 11901 | 2023-02-05 14:46:17 | | | | |
| 3 | Camila Xu | -4395 word(s) | 7506 | 2023-02-06 04:04:11 | | | | |
| 4 | Camila Xu | Meta information modification | 7506 | 2023-02-08 07:19:35 | | |
Video Upload Options
Estrogenic isoflavones are essentially considered to be the hydroxylated compounds i.e. genistein, daidzein and glycitein, the methoxylated substances i.e. biochanin A and formononetin as well as the isoflavane metabolite: equol. Their estrogenic effects have been shown in many occasions and many models and they can be either beneficial or adverse depending on the physiological status of the consummers.
1. Human Exposure and Bioavailability
1.1. Exposure According to Diet
Isoflavones are currently the most present phytoestrogens in the human environment due to the adoption of vegan diets and the use of soybean for both soy-based foodstuffs and transformed foodstuffs. While the exposure in China was recently evaluated on more than 53,000 people to range from 0.8 to 78.0 mg/day (median: 13.5 mg/day; IQR: 7.7, 21.4 mg/day) [1], in Japan [2], the isoflavones exposure, estimated on more than 30,000 people, ranged from 14 to 75 mg/day. In Western countries, the isoflavones intake is currently considered to be lower: a few mg/day in the general population. However, in some subpopulations like vegan consumers or the Adventists of the 7th day in the USA, the exposure can be higher, reaching 17.9 mg/day as a median [3]. Recently, an estimation of the French population exposure to soy isoflavones was published [4]. It showed that soy non-eaters consuming hidden soy through transformed foodstuffs were exposed to a mean intake of 1.9 mg/day of isoflavones while the mean exposure among soy consumers was about 6.9 mg/day. These figures hide a great variability as in the same recent French exposure estimation, soy isoflavone intake varied from 0 to 213 mg/day [4]. In [5], the authors gave an extensive set of isoflavones measurements in French foodstuffs. These data were obtained by specific ELISAs after a water extraction of the isoflavone glucosides. It can be seen that some foods can bring between 40 and 50 mg of isoflavones per usual portion. From the literature, it seems like there is no reproductive effects in adults below 20 mg/day but an increase in hypothyroidism symptoms was noticed in women at 16 mg/day [6]. The bioavailability of soy isoflavones in men and women has been studied extensively [7] and the Tmax is known to occur usually around 8 hours for the main isoflavones [8] (except equol for which Tmax = 16 hours). Such Tmax allows isoflavones to reach a steady state level when ingested regularly at a rhythm of twice a day [9]. In addition, according to [10] about 85% of the ingested isoflavones are excreted in the human urine within 48 hours after ingestion showing that isoflavones are some of the most bioavailable polyphenols ever known. However, they are not available in native form in plasma. Indeed, in soy, isoflavones are in glycosidic forms including glucoside, acetyl and malonyl mono- or di-conjugates. On the enterocyte barrier the lactase-phloridzine hydrolase is able to de-conjugate all polyphenols and also isoflavones. Mainly as aglycone, isoflavones enter enterocytes were they can be re-conjugated to either glucuronide or sulfate moieties. Via the enterohepatic blood system, isoflavones, either aglucone or conjugated, are transported to the liver where metabolic transformations are further performed. Finally, only less than 5% of isoflavones are usually found as aglucone forms (active forms) in the blood stream [11] and are directly active at target-cells. It should be noted, that the free fraction of the native estradiol, also represents a tiny proportion (<10%) of the total circulating estradiol that is usually measured. The elimination half-life of isoflavones ranges between 12 and 30 hours depending on the compound considered and recirculation phenomena between liver – gall-bladder on the one hand and duodenum on the other hand [8]. As mentioned earlier, the ingestion of clover-based dietary supplements containing formononetin and biochanin A in humans, leads to an increase of daidzein and genistein in plasma due to the conversion of the methoxylated isoflavones into hydroxylated parents by CYP450 hepatic enzymes [12].
1.2. Gut Flora Involvement
In human, between 40 and 60% of people have a gut flora able to produce equol [13] from daidzein. Equol was first discovered by Marrian and Haslewood [14] in the urine of mare. The new polyphenol was named equol for this reason and was first considered as an inactive compound. It was shown that the gut flora was responsible for equol production as early as in the 60’s [15]. Figure 1 shows the global metabolic transformations of isoflavones in animals and human beings. In humans, these conversions were recently shown to be caused by some bacterial genera [16]. Indeed, the latter were shown to be present only in equol producing women. These are Collinsella, Faecalibacterium and members of the Clostridium clusters IV and XIVa. In parallel, in [17] it was considered that the microbiological conversion of daidzein into S-equol is performed in three successive enzymatic reactions via dihydrodaidzein and tetra-hydrodaidzein. The study reported that several equol-producing bacteria belong to diverse genera i.e. Eggerthella sp. YY7918, Lactococcus sp. strain 20-92, Slackia sp. strain NATTS, as well as Slackia isoflavoniconvertens. Unlike humans, all horses and rodents (including rats and mice models), harbor an equol-producing gut-flora. This means that comparing the effects of daidzein alone or daidzein in soy extracts, between rodents and humans, may lead to effects misinterpretation at least for non-equol-producers. This is why, so far, the only isoflavone tested for toxicological effects in rat is genistein. Testing daidzein in rat may, indeed, lead to situations that are not easily extrapolated to humans, thus inducing potential over-protective decisions.
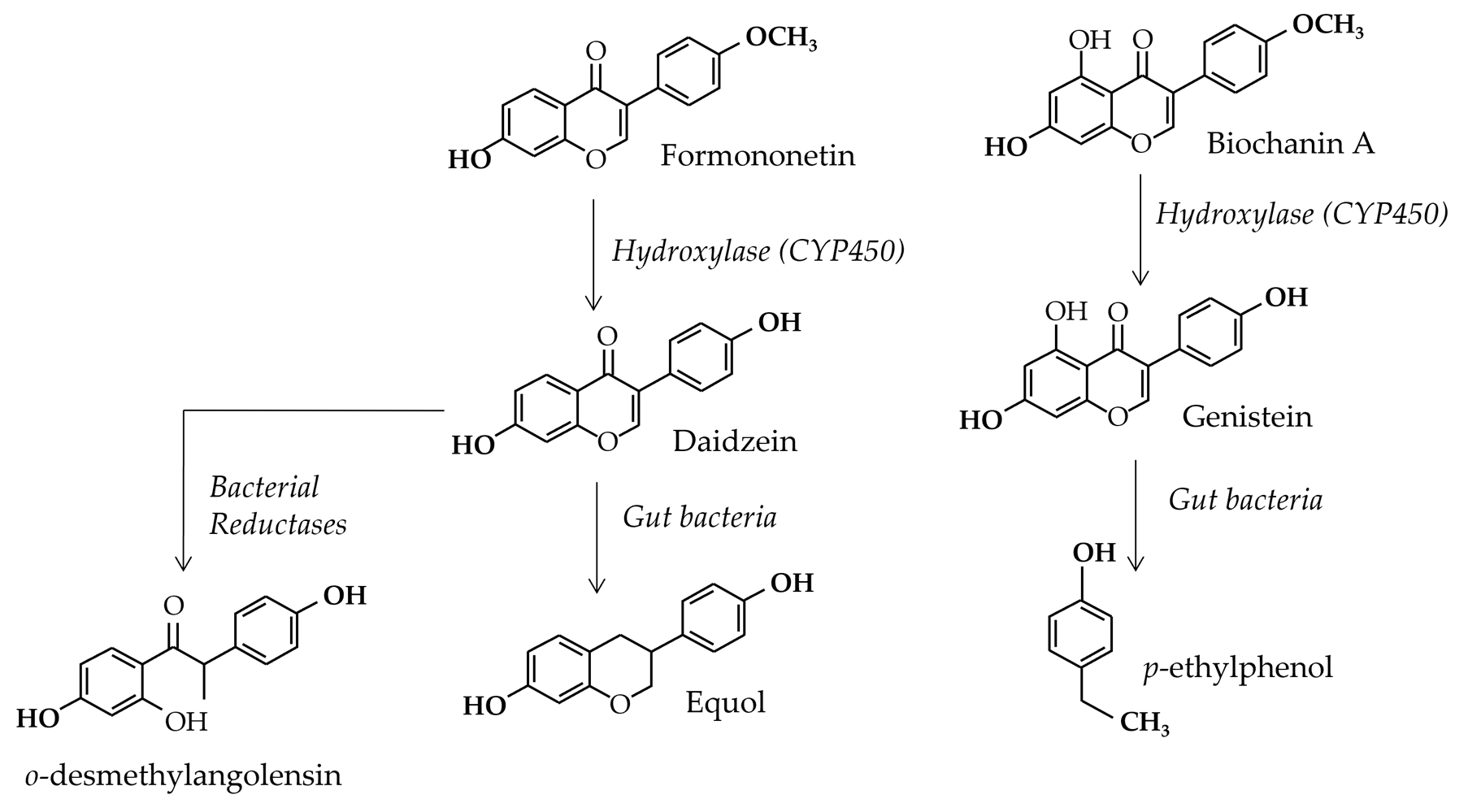
Figure 1. Global feature of isoflavones metabolism in animals and human beings.
Nowadays, investigations on these bacterial metabolisms aim at finding ways to influence the gut ecosystem to promote the production of these compounds of health interest [18].
1.3. Blood Concentrations
1.3.1. Hydroxylated isoflavones
Blood concentrations of isoflavones have been extensively studied and it is known that a twice daily intake can lead to a steady-state level [9]. In this study where 60 menopausal women were exposed to 100 mg/day of soy isoflavones in food supplements at a rhythm of two 50 mg-daily-intakes (in the morning and in the evening), a steady state concentration was obtained after 5 days with a mean level of 4.56 µM eq aglycone (range: 1.61 µM – 9.96 µM). The study involved a rather high isoflavone daily intake and could be used as an upper exposure reference. It showed that a great variability could be observed in blood concentrations even under rigorously controlled conditions. This inter-individual variability, reaching a factor 10, was in accordance with the toxicological safety factor that considers such a variability in human bioavailability of xenobiotics [19]. In the study by Mathey et al. [9], the plasma isoflavone-conjugates were hydrolyzed prior to extraction and analysis. Because the aglycone forms tend to represent only 5% of the total circulating compounds [11] in humans, if no conversion to aglycone forms is admitted at tissue levels, the aglycone isoflavone concentrations tested in vitro should not exceed 0.5-1 µM. If higher concentrations are tested, the effects observed can involve cell signaling pathways that are currently not activated in vivo. Higher concentrations can only be obtained if the conversion of conjugated-isoflavones into aglucone forms is demonstrated locally at tissue level. Nevertheless, the distribution of isoflavones in tissue is generally considered to be low, confirmed by a low distribution volume. To support this property, [20] showed that in women under soy milk, the genistein concentration in breast glandular tissue was 3 times lower than in serum (797.04 ± 237.27 pmol/mL of serum vs 283.71 ± 35.88 pmol/g in breast glandular tissue). The study also showed that genistein in breast glandular tissue is essentially under conjugated form: genistein-7-O-glucuronide 268 ± 179 pmol/g; genistein-4’-O-glucuronide 86 ± 46 pmol/g and free genistein 12 ± 2 pmol/g.
1.3.2. Methoxylated isoflavones
Finally, as already mentioned the methoxylated isoflavones formononetin and biochanin A can be absorbed with some pulses i.e. lentils, chick peas, beans, broad beans, mungo beans, etc... They can either be in aglucone or in glucoside forms, and their glycosidic conjugates are named ononine and sissotrine, respectively. As other glycosylated isoflavones, they can be hydrolyzed by the b-glucosidase of the enterocytes (lactase phloridzine hydrolase) and transformed into biochanin A and formononetin. Biochanin A seems to have a greater interaction with the food bowl proteins [21], and consequently, has a lower absorption rate than its hydroxylated parent compound, genistein. Nevertheless, once the enterocyte barrier crossed, the compounds enter the entero-hepatic blood stream and are directed to the liver. There, both methoxylated compounds– biochanin A and formononetin– are metabolized by phase 1 enzymes, the so called Cytochrome P450, which transform them into genistein and daidzein, respectively [22] (see Figure 1). Therefore, the plasma concentrations of the methoxylated isoflavones are generally below the detection limit in humans [23]. The study by Muchiri and van Breenen showed that even with a clover formula balanced in genistein, daidzein, formononetin and biochanin A, the resulting methoxylated isoflavone concentrations in blood are 2 to 10 times lower than that of the hydroxylated parent compounds [24]. In the same study [24], the Cmax for formononetin in a woman ingesting 120 mg of red clover extract was 33.3 ± 7.7 ng/mL, 5 hours after ingestion, while under the same conditions, it was 8.92 ± 0.79 ng/mL for biochanin A. These concentrations correspond to 0.12 µM for formononetin and 0.03 µM for biochanin A. Considering the poor estrogenic potencies of the two methoxylated isoflavones: formononetin and biochanin A, the estrogenic effects of a clover extract in humans should essentially rely on their hydroxylated metabolites i.e. daidzein and genistein, respectively.
2. Beneficial Effects
2.1. Hormonal Effects
2.1.1. Menopausal symptoms
Among all phytoestrogens health effects that were studied so far in humans, menopausal symptoms are those most documented. Coumestrol and resorcylic acid lactones being considered as toxic compounds, no data were found on their potential effects on climacteric symptoms of menopausal and peri-menopausal women in interventional studies. Beside, many studies were undertaken using dietary supplements containing various classes of phytoestrogens to check for their efficiency. Currently, the market of food supplements offers preparations based on soy, clover, alfalfa, kudzu, linseed and hop. Other plants are used for menopausal symptoms including black cohosh, chasteberry or yam but their action modes are not strictly estrogenic. Although effects are reported and confirmed by meta-analyses, the effects of phytoestrogens on menopausal symptoms are still debated. The reasons are: a great interindividual variability, different effects according to the peri- or post-menopausal status, strong placebo effects and studies based on self-declarations.
Several meta-analyses can be cited to assess these effects. According to one meta-analysis [25] which compared several drugs and natural treatments on menopausal vasomotor symptoms, the most efficient natural treatments were those involving isoflavones and black cohosh. For isoflavones, the odd ratio and CI 95% was 0.62 (0.44 – 0.67) while it was 0.4 (0.16 – 0.90) for black cohosh.
Soy isoflavones are the most popular for menopausal symptoms relief and another meta-analysis [26] analyzed specifically the trials involving these compounds, based on 16 articles meeting the inclusion criteria. It compared efficient values in placebo and treated groups and examined about 1710 subjects in total. According to this study, with soy isoflavones, a 25.2% hot flashes reduction was reported after elimination of the placebo effect. It should be noted that this placebo effect counted for 57% of the symptom reduction by the reference treatment i.e. estradiol. The study also showed that a time of treatment of 13.4 weeks was required for soy isoflavones to achieve half of its maximal effects. In addition to that, the study stated that at least 48 weeks were needed to achieve 80% of soy isoflavones’ maximum effects.
Clover treatment was not always efficient as reported in the meta-analysis by Hanna and coworkers [27]. Such variable efficacy is observed at doses up to 120 mg/day.
The effect of kudzu on menopausal symptoms was studied in another meta-analysis [28]. The authors based their analysis on 8 RCTs and concluded that the efficacy of kudzu on menopausal symptoms was inconclusive. The authors pointed out methodological shortcomings including the placebo effects due to self-assessment/recall questionnaires and the lack of standardization of the kudzu extracts. They advised to improve the trials via a better knowledge of concomitant plant usages and a better assessment of the menopausal symptoms in comparison with estradiol treatments. They also recommended a pharmacovigilance assessment.
2.1.2. Bone health
The main results reported on bone health concerned soy isoflavones. Two meta-analysis converged in saying that isoflavones from soy may preserve bone density in menopausal women. However, the efficiency was only observed at high doses i.e. over 80 mg/day. The first meta-analysis [29] only considered the effect of soy isoflavone extracts supplementation (not soy proteins) on bone mineral density (BMD) in menopausal women. It only assessed RCTs published in English, Japanese, or Chinese and reporting the effects of soy isoflavone extracts on lumbar spine or hip BMD in menopausal women. Eleven studies matched the fixed criteria and the analysis included 1,240 menopausal women in total. It showed that a daily intake of 82 mg of soy isoflavones (aglycone) on average, for 6 to 12 months, were significantly associated with higher spine BMD. Treatment duration, geographic origin and basal BMD were major influencing factors. There were no significant effects on either femoral neck, hip total, or trochanter BMD and the positive effects of soy isoflavone extract supplements on BMD was restricted to lumbar spine in menopausal women. The second meta-analysis [30], included RCTs which examined the effects of soy isoflavone supplementations in women for at least one year. The main outcomes were BMD changes from baseline at the lumbar spine, total hip and femoral neck. 10 RCTs gathering 896 women were found to be eligible according to the retained criteria. A mean dose of 87 mg of soy isoflavones for at least one year did not significantly affect BMD. However, when doses were stratified, it was shown that only large dose over 80 mg/day of isoflavones tended to weakly preserve BMD at lumbar spine and hip. Note that in human or in rodent, isoflavones have never been able to re-build an osteoporotic skeleton.
2.1.3. Estrogen responsive tissues
As far as isoflavones are concerned, a controversy remains on their beneficial and / or deleterious effects on breast cancers in women. This is due to opposite effects when cell [31] or animal [32] studies serving as toxicological references are examined, compared to epidemiological population studies [33]. If there is a clear reduction of breast cancer risk in Asian women ingesting soy from childhood [34], the preventive effect in Western women is not so obvious [35]. When metanalysis of RCT are considered in women, the main problem was that none of them was clearly designed to see a direct effect on breast cancer. In addition, volunteers were selected on health criteria which excluded breast cancer occurrence. Because breast cancer can occur in the general population, these studies cannot be considered as representative of the total population. In addition, exposing women to phytoestrogens to check for an aggravation of their breast cancer is not considered ethical and rightfully not approved by ethical comities. Because there are data arguing for both breast cancer aggravation and prevention, the best interpretation has to take them all, trying to make them fit within the same mechanistical hypothesis. Doing that, it came out that genistein may be protective during the early promotion phase, i.e. when cells were healthy, while it acted as a growth factor on estrogen-dependent tumour cells. This was in accordance with [36] which showed a reduced breast cancer risk after one year of phytoestrogen intakes suggesting that during the first year, the tumours already present may have been boosted by phytoestrogen intakes. Then once all women with undetected breast cancers had revealed their pathology, the remaining women still maintained under phytoestrogen treatments had no tumours and were protected. The protective action may be obtained via epigenetic effects, with a certain degree of transgenerational transmission. This could explain the protection observed in Asian populations exposed from childhood to modern soy-food rich in isoflavones [34]. Besides, isoflavones are growth factors of breast cancer cells in vitro when they express canonical estrogen receptors or GPER [37][38]. In vitro, isoflavones doses compatible with human soy consumption, exerted a growing effect. The doses of isoflavones which induced anti-proliferative effects on estrogen-dependent breast cancer cells, were over 20 µM [39]. Such doses cannot be achieved physiologically. Soy isoflavones also act as growth factors in nude mice models implanted with human breast cancer cells and in that case, the plasma levels and metabolic forms are closer to the in vivo situation in women [40]. Additionally, in the only RCT existing so far on breast cancer growth in women, plasma genistein was associated with the expression of genes controlling cell proliferation [41]. Ancient studies also showed that soy-food had estrogenic and proliferative effects on healthy breast cells in premenopausal women [42]. Equally, soy isoflavones have been shown to increase the mammary density in Western post-menopausal women [43]. To help interpreting such controversial results, it should be noted that existing studies most probably underestimated soy and isoflavones intakes, not considering hidden isoflavones from manufactured foodstuffs. These isoflavone levels ranged from 1.55 to 20.35 mg in Western foodstuffs containing soy [4]. Besides, in Western countries, soybean consumers tend to have healthier behaviours which can reduce the analyses statistical power. Finally, when breast cancer prevalence in the West and Asia are compared, global Asian dietary habits, which include tea or fish intake, beside soybean products may also be protective [44][45].
Regarding vaginal and endometrial health, it should be reminded that both vaginal mucosa and endometrium develops under estradiol stimulation and thus bears estradiol receptors: ERa, ERb, GPER as well as the estradiol related receptors ERRs [46][47][48]. The first isoflavones estrogenic effect ever shown was uterotrophy in New Zealand ewes grazing on clover pasture. These effects were reported either in reproductive females or in females freshly ovariectomized. In all cases, estradiol receptors were available to mediate an estrogenic effect of isoflavones. Conversely, in humans, most of the studies which were designed to show an effect of isoflavones on the genital tract were performed in menopausal women. In most studies the time distance to menopause was largely variable and the availability of ERs in the vagina and endometrium, known to decrease after ovarian function arrest [48], was not checked. Thus, it appeared that isoflavones were globally ineffective on Western post-menopausal women vagina and endometrium [49]. However, in [50], a vaginal gel containing isoflavones exhibited a significant estrogenic effect of on vaginal dryness, dyspareunia and maturing index of vaginal cells in menopausal women. In addition, in [51] which reported results obtained on Japanese students on classical Japanese diet, an isoflavone supplementation (20 or 40 mg of isoflavones daily) lengthened the menstrual cycle by two days and this lengthening in both menstruations and bleedings was dose dependent. Considering the mean urinary levels at base line, the usual isoflavone exposure due to Japanese diet was close to 25 mg/day. The effect appeared to vary individually and no significant modifications of either steroid nor gonadotrophin hormones were noted. This suggested a direct effect of isoflavones on the endometrium mucosa. Additionally, several studies reported a potential beneficial effect of isoflavones and particularly equol, on the prevention of premenstrual syndrome in Japanese women [52].
Considering uterine cancer, although soy isoflavones at over 20 mg/day have been shown to increase endometrium thickness [49] via endometrial cell proliferation, there is no convincing data showing a deleterious effect on endometrial cancer [53]. Indeed, it is known that hormonal replacement therapies can have opposite effects on breast and uterus cancer [54]. In the case of isoflavones, one explanation could be that uterus, ovary and vagina are rich in ERb subtypes for which isoflavones have a greater affinity than for ERa. Moreover, ERb main variants are thought to be involved in cell differentiation which counteracts cell proliferation that is rather induced by ERa [55]. To conclude, isoflavones effects on vagina and uterus of premenopausal women are not fully demonstrated and additional investigation is required. In post-menopausal women, a vaginal effect of isoflavones may be achieved while an endometrial effect has never been clearly shown despite a large number on trials.
Concerning prostate cancer, the effects of isoflavones on the incidence of the pathology differed in Western and Asian populations [56]. The difference observed seemed to concern cancer progression, since the occurrence of cancer, as analysed by post-mortem diagnosis, showed similar frequencies between both populations [57]. Here, the type of estradiol receptors (ER) involved is crucial. Indeed, isoflavones, can be protective on ERb bearing tumours, and harmful on tumours bearing the ERb2 variant [58][59]. They can also be anti-androgenic [60].
2.2. Metabolic Beneficial Effects
2.2.1. Effect on cholesterol
The effect of soybean on cholesterol has long been studied. Soy intake was confirmed to reduce slightly both LDL and Total Cholesterol (TC) in a recent meta-analysis gathering data from 46 clinical trials [61]. The effect of soybean in cholesterol lowering was always shown to be modest overall: LDL cholesterol was decreased by ∼3.2% and TC by 2.8% when consuming ∼25 g soy protein/day. However, this lipid reduction was significant even if heterogeneous. It is thought that soybean effect on blood lipids is due to the substitution of meat-proteins containing cholesterol, by pulse-proteins devoid of cholesterol. To sustain this hypothesis, similar TC lowering effect was shown with other pulses including peas [62]. Besides, several studies performed on animal models [62] showed that this lipid lowering effect could occur without phytoestrogens, as peas only contains very low levels of estrogenic isoflavones. Therefore, the role of estrogenic isoflavones in this cholesterol lowering effect is still subjected to debate. In 1999, a RCT [63] reported that the LDL-cholesterol was isoflavones-dose-dependently reduced in subjects eating soybean compared to a casein control. However, the subgroups which were analysed counted less than 20 subjects and the overall reduction was not mentioned. Nevertheless, the highest decrease recorded in this study was 10% and was observed in the highest isoflavone-intake group (i.e. 37 mg/day). In addition, another study [64] compared the effect of soy-milk proteins and water-washed soy-proteins with a reduced concentration of isoflavones. No difference was observed between the groups which counted 79 or 80 subjects, exposed to the diets for 3 weeks. However, water-washes can remove other constituents alongside isoflavones.
2.2.2. Effect on metabolic syndrome
Several reviews addressed the link between isoflavones and metabolic syndrome. They included in vitro mechanistic demonstrations based on PPAR interactions [65] that have not always been obtained with relevant concentrations of isoflavones. Indeed, the EC50 of isoflavone for PPAR are usually above 20 µM while they are below 0.1 µM for ERa and ERb [66]. Despite this high affinity for the nuclear estradiol receptors, some authors still contest the estrogenic effects of isoflavones in vivo in humans. Therefore, considering the isoflavone plasma levels in humans, a significant interaction of isoflavones with PPAR receptors, in dietary conditions, must be questioned. The following reviews can be consulted with interest [65][67][68]. However, it can be difficult to assess the specific effects of isoflavones on metabolic syndrome, as these substances are ingested at higher rate in a vegetable-based diet which is known to reduce the risk of metabolic syndrome per se. Many studies [69][70] correlated isoflavone intake with reduced risk of metabolic symptoms. However, the effect was usually depending on sex and such a correlation is not a definitive proof. Besides, studies were performed based on genistein supplementation at doses of about 50 mg/day which can correspond to a dietary intake. The study [71] tested a 54 mg/day genistein supplementation on post-menopausal women with metabolic syndrome for one year. The study design was a multicentric double blind RCT. After 1 year of treatment the treated subjects exhibited a reduced fasting glucose, fasting insulin, and HOMA-IR while these parameters were unchanged in the placebo group. Genistein statistically increased HDL-Chol, while TC, LDL-Chol, triglycerides, visfatin, and homocysteine blood levels were decreased. Systolic and diastolic blood pressure were also reduced in the treated group. Similarly, in [72], a treatment of Italian postmenopausal women with or without metabolic syndrome was performed using 54 mg genistein per day for 6 months. At the end of the treatment, the Flow Mediated Dilation (FMD) at 50s and peak FMD were slightly, although significantly, increased in the treated group compared with placebo. In addition, TC, triglycerides, homocysteine and visfatin were significantly decreased in the genistein treated group compared with placebo, while blood adiponectin levels were increased. Finally, in [73] 10 mg/day of S-Equol, a natural metabolite of daidzein, was tested on over-weight and obese Japanese subjects in a double blind, randomized, cross-over trial. There was no wash-out period and the treatments lasted for 12 weeks. It was first shown that equol production was less frequent in the selected group (over-weight and obese subjects) than in the general population, and that a supplementation with S-equol was more efficient in equol-non-producers than in equol producers. Several parameters were followed during this trial, including HbA1c, serum LDL-Chol and cardio-ankle vascular index. These parameters evolved favorably and significantly although their modification was tiny. The study indicated that soy effect on the metabolic syndrome may act in the sense of a reduction, and that the active compounds to look for, in the diet, would include genistein and also daidzein, the precursor of S-Equol.
2.2.3. Effects on diabetes
Many studies including epidemiological prospective cohort studies, cross sectional studies, cases-control studies or RCTs have been published in order to establish a definitive link between estrogenic isoflavones and Type 2 Diabetes Mellitus (T2DM). Till now, the picture remains unclear, with on one hand, data clearly showing a beneficial effect of soy isoflavones, per se or in soy-food, on the reduction of risk of T2DM; and on the other hand, more cautious studies indicating a possible link with only some isoflavones or some soy-food or in some populations, but not in others. Briefly, Tang et al. [74] analyzed 15 unique cohorts including 565,810 individuals and 32,093 incident cases. The relative risks of developing a T2DM associated with legumes or soy intake was not significant. Besides, a risk reduction was observed with soy-milk, soy-proteins, tofu and soy isoflavones. However, the authors considered that the heterogeneity between the studies was sometimes high, and that further work was needed to ascertain the risk reduction of T2DM by soy and its isoflavones. To sustain this conclusion, the study by Barańska et al. [75] showed a significant effect of soy isoflavones on lipid blood criteria but not on blood glucose biomarkers including: fasting glucose, fasting insulin, HbA1c, and HOMA-IR. This meta-analysis gathered 12 randomized controlled trials, 7 parallel randomized design, 5 case-crossover randomized designs and overall 691 subjects. Cautious conclusions were also drawn from the study by Glisic et al. [76]. It was performed on RCTs and 9 prospective population-based studies gathering 1687 and 212,796 subjects, respectively. The authors showed that phytoestrogen supplementation could improve fasting glucose and HOMA-IR without significant decrease of insulin plasma concentrations. They also showed that the results of RCTs varied with the phytoestrogen considered. Thereby, soy-derived isoflavones and genistein improved glucose homeostasis, while isoflavones mix and daidzein had no effect or were associated with an adverse glycemic profile. The highest phytoestrogen intake was associated with a 10% risk reduction of T2DM in observational studies. The authors also mentioned that adverse glycemic profiles could be induced by soy and isoflavones in women. Conversely, in the study by Li et al. [77] gathering 8 studies, a significant inverse association was observed between soy intake and T2DM risk with a high heterogeneity. However, the relationship was obvious between soy protein and isoflavones intake and decreased risk of T2DM. This time there was no heterogeneity. The protection was observed in women, in cross-sectional studies and in Asian populations. Finally, and to highlight the complexity of the putative effects of phytoestrogens, the study by Guevara-Cruz et al., [78] showed that genistein, as pure supplemented compound (50 mg/day), was able to improve glucose tolerance and insulin resistance in obese subjects. In this study, all subjects exhibited strong insulin-resistance (HOMA >2.5) and BMI >30. Nevertheless after 2 months of genistein supplementation, the glucose tolerance was improved in the genistein treated subjects but not in the control group. The authors showed that genistein was able to modify the gut flora increasing, for instance, the presence of Akkermansia muciniphila. This bacteria has been shown to reduce obesity and insulin resistance in rodent models. The study also reported a modification of lipid metabolism that could be responsible for a change in the muscles lipid profile. Overall, a beneficial effect of isoflavones on T2DM seems modest and the quality of evidence remains low. The heterogeneity of the studies would indicate that isoflavones may not be the main substances responsible for the observed effects and that confounding factors still exist in these studies. All authors agreed in saying that more high-quality evidence from prospective studies was required.
3. Adverse Effects
3.1. Reference Doses
When toxic effects are considered, they are usually tested in toxicological studies which are led according to validated protocols, in the most appropriate model. For reproduction or cancer issues, rats or rabbits are usually used, and multigenerational exposure at low doses is usually more informative than acute exposure. Indeed, the effect of a define compound can be different according to the period of exposure. For instance, neonatal exposure can be much more deleterious than adult exposure. However, a life-long exposure may induce physiological disorders that will be difficult to link to a define life period especially if epigenetic effects are involved. Chronic exposure can lead to define a lowest observable adverse effect level (LOAEL) and a non-observable adverse effect level (NOAEL). These notions are crucial since they are used to build reference doses (RfD) that will be used as limit of exposure for humans. Discrepancies can arise from toxicological studies, and they essentially reflect the notion of adverse effect. When the animal model used in toxicity studies shows a modification in a physiological parameter, the question is to determine if this modification is beneficial, neutral or adverse. For estrogenic endocrine disruption, the physiological criteria examined in males and/or females are usually: pituitary morphology, anogenital distance, age at vaginal opening, uterotrophy, cyclicity, penis length, bulbourethral gland morphology, sperm production, fertility in first and subsequent generation… etc. In addition to that, an effect may be interpreted differently if observed on pups, on pregnant females or in adults. Usually, the effects of toxic compounds are recorded and compared to each other to allow a better transposition for human safety decision. This is the case for endocrine disruptors, particularly, as their effects are usually complex and not always easy to determine. RfD are classically derived from NOAEL applying safety factors to transpose dose-effects from rodent model to human. The first safety factor accounts for the difference between animal and human. It has been fixed at a value of 10 [19, [79]. The second safety factor has also a value of 10 and accounts for the interindividual variation in the human species [19]. As mentioned previously, this factor has been observed for isoflavones in human bioavailability studies [9]. Then, when a NOAEL is not available and that the only dose available is LOAEL a third factor is introduced as a protection for the human subjects. This factor equals 3 most often, but according to some authors, it can be optimized at a lower level after appropriate statistical analysis [80]. Table 1 gives the theoretical RfD derived from LOAEL or NOAEL available for the phytoestrogens studied here. These RfD are compared to that of diethylstilboestrol, a synthetic estrogen considered as endocrine disruptor, and to an estimation of the exposure evaluated in France. For lignans, the exposure is underestimated since it is only related to matairesinol intake [81]. The only compounds showing in France a potential intake superior to the deduced RfD are genistein and daidzein.
Table 1. Reference doses for the phytoestrogens studied, compared to Diethylstilbestrol as reference and to potential intake as recorded in France.
|
Compounds |
Chemical family |
NOAEL or LOAEL* in animal |
Model |
Theoretical Reference dose |
Potential intake |
References |
|
Diethylstilbestrol |
E2 analogue |
NOAEL |
Rat |
0.05 |
Drug |
[82] |
|
Genistein |
Phytoestrogen |
LOAEL |
Rat |
0.12 |
0 - 1.5 |
|
|
Daidzein |
Phytoestrogen |
NOAEL |
Hen |
0.5 |
0 - 0.8 |
|
|
Biochanin A |
Phytoestrogen |
LOAEL |
Rat |
0.083 |
0.0003 |
|
|
Formononetin |
Phytoestrogen |
NOAEL |
Mouse |
0.05 |
0.0013 |
* the value is the limit fixed for human adults by the Belgian health authorities. ** Safety factor from LOAEL to NOAEL = 3 in this table.
3.2. Hormonal Based Effects
3.2.1. Pituitary interactions
Estrogens are known to regulate pituitary reproductive hormones, namely Follicle-Stimulating Hormone (FSH) and Luteinising Hormone (LH). Such an effect is due to the regulation of the hypothalamic Gonadotrophin Releasing Hormone (GnRH) [88]. Depending on the cycle period, estradiol can either stimulate or repress pituitary hormone release. Using this property, contraceptive drugs have been developed essentially based on the synthetic ethynyl-estradiol which pharmacokinetic is longer than that of estradiol conferring it a higher potency. Therefore, expecting an effect of phytoestrogens on pituitary hormones release seems sensible. If such an effect is recorded it should induce menstrual cycle impairment and steroid synthesis modifications. Doing that phytoestrogens can act as endocrine disruptors and affect male and female fertility. As will be seen below, such effects are sometimes recorded but other studies failed to identify any endocrine disruption. This may be due to low dosages of phytoestrogens, to short treatments or too few tested subjects.
The effects of methoxylated and hydroxylated isoflavones have been studied on animal reproduction since the discovery of the clover infertility syndrome or clover disease in the late ‘40s [89]. Their effects on gonadotrophins were first reported in ewes by Findlay and co-workers [90]. Such effects were then reported in women [91] and in men [92] affecting FSH, LH and progesterone levels in pre-menopausal women [91] and affecting sperm production in men [92]. The doses required in premenopausal women were shown to be 45 mg/day in a rigorously controlled diet while in men, the effect was reported using 120 mg/day. The disruption on menstrual cycles in women was further confirmed by other authors [51][93] and always obtained with dosages between 40 and 50 mg/day. Such dosages can easily be achieved with 2 soy-based foodstuffs per day [5]. Note that gonadotrophin hormone levels were also altered in trout fed semi-synthetic diet in fish farms, and enriched with 500 ppm genistein [94]. Such a result showed that the gonadotrophin regulation by estrogens was a particularly well conserved process among vertebrates.
3.2.2. Estrogen based toxic effects
Many data exist on the effects of isoflavones on reproductive parameters on humans. All studies did not provide consistent or significant results, and this was essentially due to: experimental bias, too short time of exposure, too small population observed or to exposures that were below the effective dose. Indeed, unlike other endocrine disruptors which can exhibit effects at very low doses, isoflavones are substances showing a threshold effect, at least in vivo. Looking at the literature, it can be seen that no adverse effects were ever reported so far in humans, for an exposure below 0.3mg/kg bodyweight/day. This is 20 mg/day of aglycone hydroxylated isoflavones for an adult weighing 60 kg. Nevertheless, when compared to the phytoestrogens’ reproductive effects described for coumestrol or mycotoxins, the effects on reproduction are undoubtable based on animal model studies. Indeed, a multigenerational reprotoxic study was published by the National Toxicology Program in the USA in 2008 [95]. It showed that females exposed to 51 mg/kg bodyweight/day of genistein gave birth to pups with: lower pre- and post-weaning weights in F0, F1, F2, F3, F4; a reduction in anogenital distance in F1, F2, F3, a reduction of the age at vaginal opening in F1, F2, F3, a cycle alteration in F1, F2, F3. For males on 35mg/kg bodyweight/day, the pre- and post-weaning weights were decreased in several generations. The anogenital distance was reduced in F1 and the rate of mammary gland hyperplasia was increased in F0, F1, F2. Renal tubules showed calcifications in F1 and F2. As far as the fertility was concerned, a reduction of the litter size was observed in the F2 generation. All these effects sign an estrogenic and anti-androgenic effect as was shown for other endocrine disruptors.
In humans, breast milk from Chinese mothers was shown to contain only low levels of isoflavones compared to soy-based infant formulas. Indeed according to Zhou et al., [96] the woman breast milk contained average concentrations of daidzein and genistein ranging from 0.52 to 202.87 μg/kg. Besides, according to [97] American soy-based infant formulas contained between 32,000 and 47,000 µg/kg and infants exclusively fed these soy-based formulas were the human subjects most exposed to these substances. Thus, the neonatal exposure through soy-based infant formulas was studied in various occasions even if in the US, safety authorities consider that the adverse effects were currently overcome by the beneficial ones [98]. From this high exposure (5 to 11 mg/kg/day according to formulas and infant age [98]) potentially leading to LH secretion impairment, it is expected to observe a reduction of testis development at the time of exposure and a subsequent alteration of sperm production in men adulthood. In addition, the neonatal exposure to estrogen of baby girls should result in a masculinized behaviour and an alteration of female pituitary secretions and of the genital function and physiology in women adulthood. Such an impairment is suspected to lead to a decreased fertility in adult women.
When differences were observed in human subjects on soy-based infant formula, it was generally by comparison to breast feeding, not to cow milk formula [99]. Likewise, in [100] it was shown that neonatal use of soy-based infant formula reduced testis diameter in 4 month-old-babies. The exposure lasted for less than 4 months, and the cohort was only of 15 babies in each group. However, to date, there is no investigation on the potential effect of such a neonatal exposure on sperm production and fertility in men adulthood. Such a retrospective study would require large cohorts of volunteers since many endocrine disruption events could occur between early life and adulthood potentially reducing the significance of the observations. In young girls, Adgent and co-workers showed [101] that the playing behaviour could be transiently masculinized (42 months old) when they had been fed soy-based infant formula during their first 6 months of life. In addition, several studies showed significant increase of impaired menstrual cycles, increased menstrual bleeding and pain, greater incidence of uterine fibrosis in women fed soy-based infant formula during the 4-6 first months of their life [102][103][104][105]. Such traces of early exposure to isoflavones suggest epigenetic alterations that were now shown in girls’ vaginas [106].
Early soy exposure i.e. in prepubertal period was associated by some authors to precocious puberty. In some studies, the precocious puberty incidence was increased with soy consumption in girls [107][108] and in boys [109], while in others it was decreased [110]. Although, the puberty was not always assessed on the same criteria (age at menarche, breast development, facial hairs or pubarche in boys…), in [111] which was performed on the Sister Study cohort, both effects were recorded. The puberty acceleration was observed on women fed infant formula between 1960–1974 and mainly in low outcome families. This suggests that the isoflavone levels that were higher in these formulas (delivering from 9 to 11 mg isoflavones/kg bodyweight/day) is important for the resulting effect. In [112] the puberty was delayed in Chinese children that were essentially breast-fed during infancy but received substantial amounts of isoflavones daily during childhood. This indicates that the physiological modifications due to an exposure to significant levels of isoflavones are different according to the time of exposure (infancy or childhood).
When isoflavones are consumed by adults, high dosages can induce deleterious effects on the reproductive tract and function. Several cases of soy over-consumption were reported in men showing secondary hypogonadism, gynecomastia and libido impairment [113][114]. In [113] the isoflavone intake was estimated at 200 mg/day (aglycone form) via 1.2 L of soy-drink/day for 4 years. Such a consumption led to persistent dramatic drop in LH and testosterone levels explaining male behaviour impairment. Consequences of over-consumption of soy-food, have also been reported in women [105][115]. In [115], the high isoflavone intake was responsible for menstrual cycles impairment under norethisterone contraception, endometrial fibrosis, uterine myomas, endometriosis features and secondary infertility. All these pathological signs vanished when soy was stopped.
Nowadays in men, 5 population studies linked a decrease in sperm count and quality, to high isoflavones intakes (>40 mg/day) and high isoflavones levels in biological fluids [116][117][118][119][120]. Conversely, intervention studies did not show any effect but either the time of exposure was too short [121] or the dose of exposure was below the efficient intake [122]. In American women, high isoflavone exposure (>50 mg/day) was shown to increase the occurrence of luteal phase deficiencies that can delay conception [123] and shown to increase the risk to be nulliparous at the age of 26 or at menopause [124]. Such results lead to conclude in favour of a fertility impairment in women for soy isoflavone dosages over 50 mg/day. In Asia, where these exposures can usually be achieved, reproductive effects of soy isoflavones are difficult to demonstrate due to the absence of control populations. Considering the epigenetic effects of isoflavones, soy arrest may not immediately lead to substantial modifications in reproductive issues when populations were exposed to high levels for 2 to 3 generations. Nevertheless, total fertility rate figures are globally lower in Asian countries than in other countries with similar contraceptive rates and similar GDP per capita [125]. It should be noted that at doses below 10 mg/day, isoflavones seemed to improve in vitro fertilization [126]. This sustains the threshold effect previously described.
3.2.3. Thyroid based toxic effects
As far as researchers know there are no toxicology data nor reference values for isoflavones on the thyroid function. However, interactions of soy and isoflavones on the thyroid function have been reported in hypothyroid babies since the development of soy based infant formula in the USA in the 1960s. At that time was define the “soy goiter” [127], which was efficiently reduced by iodine supplementation. The article by Doerge and Sheenan [128] gives an extensive list of the existing data at the time of 2002. Other observation studies and clinical cases were reported later like [129][130], for instance, showing that the effect is major in hypothyroid patients, that it prevents from an efficient regulation by levothyroxine and can induce mental retardation in young children. The question remained for a while about the role of soy proteins or soy isoflavones in this adverse effect. Recently the RCT by Sathyapalan and co-workers answered the question testing a soy diet bringing either 2 mg/day or 16 mg/day to lightly hypothyroid patients [6]. Although they were no statistical differences on thyroid hormone parameters between the groups exposed to the two isoflavone levels, 6 women evolved toward deeper hypothyroidism when fed the high-isoflavone-diet. The mechanisms by which isoflavones are involved in such a deleterious effect relies on their interactions at different steps of the thyroid endocrine system. Indeed, isoflavones decrease T3 and T4 synthesis by reducing the activity of the thyroxine peroxidase enzyme [131] and capturing iodate ions [132]. They bind to transthyretin a thyroid hormone blood transporter [133] and bind to the thyroid hormone receptor, being able to induce thyroid dependent gene transcription [134] at relevant concentrations.
3.2.4. Androgen based toxic effects
As mentioned earlier, phytoestrogens are able to decrease the anogenital distance in rats and this feature is a biomarker of feminization or anti-androgenic effect occurring in utero [135]. As far as phytoestrogens are concerned, this anti-androgenic effects most probably results from the alteration of hypothalamus and pituitary secretions of gonadotrophins during foetal development [92]. If there are many studies showing such an effect in rodent models using dietary doses of phytoestrogens like [83][136], for instance, very few data seems to be available on the effect of phytoestrogens on the male in utero exposure to isoflavones although, isoflavones can be identified in cord blood [137]. Rather, studies were performed looking at the effects of isoflavones on neonatal development. When such an issue was studied in humans it essentially delt with high neonatal exposure to soy phytoestrogens through soy-based infant formula. Finally, the results are unclear with cases showing anti-androgenic effects [100], cases showing androgenic effects [138] and cases where no effect could be reported on androgenic biomarkers [139]. In adult men the over consumption of soybean and isoflavones decreases sperm production via LH and testosterone dramatic drop [92]. In vitro, isoflavones only marginally bind to the androgen receptors and in that case do not induce gene transcription and play as anti-androgens [140]. However, it is known that aromatase which is able to convert testosterone into estradiol as well as GPER and ERb for which isoflavones have a great affinity are involved in the male genital tract development in humans [141]. Therefore, although the effect of isoflavones on the increased penis length in infant fed soy-based formula as reported in [138] is not explained so far, the interaction of phytoestrogens with the androgen axis in male humans requires attention. Moreover, early heavy phytoestrogen exposure through infant formulas can definitively have an endocrine effect in male and female babies.
4. Conclusions
Isoflavones that exhibit an intermediate estrogenic activity, compared to 8-prenylnaringenin or coumestrol nowadays reach active concentrations in biological tissues and fluids. This was not the case in the past neither in Western nor in Asian populations. Deciphering their beneficial and adverse effects, it appears that the most plausible effects are estrogenic and anti-thyroid actions in human subjects. Beneficial effects on metabolic syndrome and diabetes are still controversial and sometimes based on unrealistic mechanisms considering physiological blood concentrations. Beneficial effects on bone preservation and menopausal symptoms are most plausible when sufficient intake doses are involved. Meanwhile, adverse effects on reproduction, which were demonstrated on many occasions in animals, can also be observed in humans over a defined threshold.
Finally, estrogens and estrogenic isoflavones have both beneficial and adverse effects according to the consumers’ physiological status and they should be used with discernment. Such a wise view cannot be achieved by consumers who usually do not know their exposure magnitude. Therefore, to benefit from phytoestrogens, it would be better to consider their intake via food-supplements or drugs and to reduce them in conventional diets, as it was the case in earlier times.
References
- Zhu, J.; Qi Zhao, Q.; Qiu Y.; et al. Soy Isoflavones Intake and Obesity in Chinese Adults: A Cross-Sectional Study in Shanghai, China. Nutrients 2021, 13(8), 2715. doi: 10.3390/nu13082715.
- Murai, U. Sawada, N.; Charvat, H.; Inoue, M.; Yasuda, N.; Yamagishi, K.; Tsugane, S. JPHC Study Group. Soy product intake and risk of incident disabling dementia: the JPHC Disabling Dementia Study. Eur J Nutr. 2022, 61, 4045–4057. doi: 10.1007/s00394-022-02937-5.
- Jacobsen, B.K.; Jaceldo-Siegl, K.; Knutsen, S.F.; Fan, J.; Oda, K.; Fraser, G.E. Soy isoflavone intake and the likelihood of ever becoming a mother: the Adventist Health Study-2. Int J Womens Health. 2014, 6, 377-384. doi: 10.2147/IJWH.S57137.
- Lee, A.; Beaubernard, L.; Lamothe, V.; Bennetau-Pelissero C. New Evaluation of Isoflavone Exposure in the French Population. Nutrients. 2019, 11(10), 2308. doi: 10.3390/nu11102308.
- Lee, A.; Bensaada, S.; Lamothe, V.; Lacoste, M.; Bennetau-Pelissero C. French population exposure to Endocrine disruptors in and on plant foodstuffs”, Mendeley Data. 2021, V9, doi: 10.17632/dk8gsx2r4j.9
- Sathyapalan, T.; Manuchehri, A.M.; Thatcher, N.J.; Rigby, A.S.; Chapman, T.; Kilpatrick, E.S.; Atkin, S.L. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2011, 96(5), 1442-1449. doi: 10.1210/jc.2010-2255.
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr. 2017, 57(6), 1280-1293. doi: 10.1080/10408398.2014.989958.
- Shinkaruk, S.; Durand, M.; Lamothe, V.; Carpaye, A.; et al. Bioavailability of glycitein relatively to other soy isoflavones in healthy young Caucasian men. Food Chem. 2012, 135(3), 1104-1111. doi: 10.1016/j.foodchem.2012.03.135.
- Mathey, J.; Lamothe, V.; Coxam, V.; Potier, M.; Sauvant, P.; Bennetau-Pelissero C. Concentrations of isoflavones in plasma and urine of post-menopausal women chronically ingesting high quantities of soy isoflavones. J Pharm Biomed Anal. 2006, 41(3), 957-965. doi: 10.1016/j.jpba.2006.01.051.
- Vergne, S.; Bennetau-Pelissero, C.; Lamothe, V.; et al. Higher bioavailability of isoflavones after a single ingestion of a soya-based supplement than a soya-based food in young healthy males. Br J Nutr. 2008, 99(2), 333-344. doi: 10.1017/S0007114507803953.
- Rüfer, C.E.; Bub, A.; Möseneder, J.; Winterhalter, P.; Stürtz, M.; Kulling, S.E. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: a randomized, double-blind, crossover study. Am J Clin Nutr. 2008, 87(5), 1314-1323. doi: 10.1093/ajcn/87.5.1314.
- Setchell, K.D.; Brown, N.M.; Desai, P.; et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001, 131(4 Suppl), 1362S-75S. doi: 10.1093/jn/131.4.1362S.
- Schröder, C.; Matthies, A.; Engst, W.; Blaut, M.; Braune, A. Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens. Appl Environ Microbiol. 2013, 79(11), 3494-3502. doi: 10.1128/AEM.03693-12.
- Marrian, G.F.; Haslewood G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares' urine Biochem J. 1932, 26(4), 1227-1232. doi: 10.1042/bj0261227.
- Shutt, D.A.; Braden, A.W.H. The significance of equol in relation to the estrogenic responses in sheep ingesting clover with a high formononetin content. Aust J Agric Res. 1968, 19, 545-553.
- Guadamuro, L.; Dohrmann, A.B.; Tebbe, C.C.; Mayo, B.; Delgado, S. Bacterial communities and metabolic activity of faecal cultures from equol producer and non-producer menopausal women under treatment with soy isoflavones. BMC Microbiol. 2017, 17(1), 93. doi: 10.1186/s12866-017-1001-y.
- Kawada, Y.; Yokoyama, S.; Yanase, E.; Niwa, T.; Suzuki, T. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Biosci Microbiota Food Health. 2016, 35(3), 113-121. doi: 10.12938/bmfh.2015-023.
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.;, Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017, 106(3), 909-920. doi: 10.3945/ajcn.117.153353.
- Gaylor, D.W.; Kodell, R.L. Dose-response trend tests for tumorigenesis, adjusted for body weight. Toxicol Sci. 1999, 49(2), 318-323. doi: 10.1093/toxsci/49.2.318.
- Bolca, S.; Urpi-Sarda, M.; Blondeel, P.; Roche, N.; et al. Disposition of soy isoflavones in normal human breast tissue. Am J Clin Nutr. 2010, 91(4), 976-984. doi: 10.3945/ajcn.2009.28854.
- Cao, H.; Jing, X.; Wu, D.; Shi, Y. Methylation of genistein and kaempferol improves their affinities for proteins. Int J Food Sci Nutr. 2013, 64(4), 437-443. doi: 10.3109/09637486.2012.759186.
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Wolfe, B.; Jha, P.; Heubi, J.E. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014, 5(3), 491-501. doi: 10.1039/c3fo60402k.
- Rowland, I.; Faughnan, M.; Hoey, L.; Wähälä, K.; Williamson, G.; Cassidy, A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003, 89(Suppl 1), S45-58. doi: 10.1079/BJN2002796.
- Muchiri, R.N.; van Breemen, R.B. Single-Laboratory Validation of UHPLC-MS/MS Assays for Red Clover Isoflavones in Human Serum and Dietary Supplements. J AOAC Int. 2020, 103(4), 1160-1166. doi: 10.1093/jaoacint/qsaa033.
- Sarri, G.; Pedder, H.; Dias, S.; Y Guo, Y.; Lumsden, M.A. Vasomotor symptoms resulting from natural menopause: a systematic review and network meta-analysis of treatment effects from the National Institute for Health and Care Excellence guideline on menopause. BJOG 2017, 124, 1514–1523. doi: 10.1111/1471-0528.14619
- Li, L.; Lv, Y.; Xu, L.; Zheng, Q. Quantitative efficacy of soy isoflavones on menopausal hot flashes. Br J Clin Pharmacol. 2014, 79(4), 593–604. doi: 10.1111/bcp.12533
- Hanna, K.; Day, A.; O’Neill, S.; Patterson, C.; Lyons-Wall, P. Does scientific evidence support the use of nonprescription supplements for treatment of acute menopausal symptoms such as hot flushes? Nutr Diet 2005, 62, 138–151.
- Kongkaewa, C.; Scholfielda, N.C.; Dhippayoma, T.; Dilokthornsakul P.; Saokaew, S.; Chaiyakunapruk, N. Efficacy and safety of Pueraria candollei var. mirifica (Airy Shaw & Suvat.) Niyomdham for menopausal women: A systematic review of clinical trials and the way forward. J Ethnopharmacol 2018, 216, 162–174 doi: 10.1016/j.jep.2018.01.028
- Taku, K.; Melby, M.K.; Takebayashi, J.; Mizuno, S.; Ishimi, Y.; Omori, T.; Watanabe, S. Effect of soy isoflavone extract supplements on bone mineral density in menopausal women: meta-analysis of randomized controlled trials Asia Pac J Clin Nutr. 2010, 19(1), 33-42.
- Liu, J.; Ho, S.C.; Su, Y.X.; Chen, W.Q.; Zhang, C.X.; Chen, Y.M. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone. 2009, 44(5), 948-953. doi: 10.1016/j.bone.2008.12.020
- Gutendorf, B.; Westendorf, J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicol. 2001, 166, 79-89.
- National Toxicology Program. Toxicology and Carinogenesis study of Genistein (Cas N° 446-72-0) in Sprague-Dawley rats (Feed study). NTP TR 545, 2007. NIH Publication N°. 08-4430.
- Woo, H.D.; Park, S.; Oh, K.; Kim, H.J.; Shin, H.R.; Moon, H.K.; Kim, J. Diet and cancer risk in the Korean population: a meta- analysis. Asian Pac J Cancer Prev. 2014, 15(19), 8509-8519. doi: 10.7314/apjcp.2014.15.19.8509
- Nagata, C.; Mizoue, T.; Tanaka, K.; et al. Soy intake and breast cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2014, 44(3), 282-295. doi: 10.1093/jjco/hyt203
- Grosso, G.; Godos, J.; Lamuela- Raventos, R.; et al. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61(4), 1600930 doi: 10.1002/mnfr.201600930
- Boucher, B.A.; Cotterchio, M.; Anderson, L.N.; Nancy Kreiger, N.; Kirsh, V.A.; Thompson, L.U. Use of Isoflavone supplements is associated with reduced postmenopausal breast cancer risk. Int. J. Cancer 2013, 132, 1439-1450. doi: 10.1002/ijc.27769
- Lucki, N.C.; Sewer, M.B. Genistein Stimulates MCF-7 Breast Cancer Cell Growth by Inducing Acid Ceramidase (ASAH1) Gene Expression. J Biol Chem. 2011, 286(22), 19399–19409. doi: 10.1074/jbc.M110.195826
- Ariyani, W.; Miyazaki, W.; Amano, I.; Hanamura, K.; Shirao, T.; Koibuchi, N. Soy Isoflavones Accelerate Glial Cell Migration via GPER-Mediated Signal Transduction Pathway. Front. Endocrinol. 2020, 11, 554941. doi: 10.3389/fendo.2020.554941
- Uifalean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C.A. Soy Isoflavones and Breast Cancer Cell Lines: Molecular Mechanisms and Future Perspectives. Molecules 2016, 21, 13; doi:10.3390/molecules21010013
- Wu, Q.; Yang, Y.; Yu, J.; Jin, N. Soy isoflavone extracts stimulate the growth of nude mouse xenografts bearing estrogen-dependent human breast cancer cells (MCF-7). J Biomed Res. 2012, 26(1), 44-52. doi: 10.1016/S1674-8301(12)60006-2
- Shike, M.; Doane, A.S.; Russo, L.; et al. The effects of soy supplementation on gene expression in breast cancer: a randomized placebo-controlled study. J Natl Cancer Inst. 2014, 106(9), Dju 189 doi: 10.1093/jnci/dju189.
- McMichael-Phillips, D.F.; Harding, C.; Morton, M.; et al. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998; 68(6 Suppl), 1431S-1435S. doi: 10.1093/ajcn/68.6.1431S
- Isidoro, B.; Lope, V.; Whelan, D.; et al. Use of hormone therapy and isoflavones and mammographic density in Spain. Menopause. 2016, 23(5), 556-564. doi: 10.1097/GME.0000000000000569
- Liu, J.; Ma, D.W. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients. 2014, 6(11), 5184-5223. doi: 10.3390/nu6115184
- Li, M.J.; Yin, Y.C.; Wang, J.; Jiang, Y.F. Green tea compounds in breast cancer prevention and treatment. World J Clin Oncol. 2014, 5(3), 520-528. doi: 10.5306/wjco.v5.i3.520
- Mylonas, I.; Jeschke, U.; Shabani, N.; Kuhn, C.; Kriegel, S.; Kupka, M.S.; Friese, K. Normal and malignant human endometrium express immunohistochemically estrogen receptor alpha (ER-alpha), estrogen receptor beta (ER-beta) and progesterone receptor (PR). Anticancer Res 2005, 25(3A), 1679-1686
- Tica, A.A.; Tica, O.S.; Georgescu, C.V.; et al. GPER and ERα expression in abnormal endometrial proliferations. Rom J Morphol Embryol. 2016, 57(2), 413-418
- Cavallini, A.; Dinaro, E.; Giocolano, A.; Caringella, A.M.; Ferreri, R.; Tutino, V.; Loverro, G. Estrogen receptor (ER) and ER-related receptor expression in normal and atrophic human vagina. Maturitas. 2008, 59(3), 219-225
- Liu, J.; Yuan, F.; Gao, J.; Shan, B.; Ren, Y.; Wang, H.; Gao, Y. Oral isoflavone supplementation on endometrial thickness: a meta-analysis of randomized placebo-controlled trials. Oncotarget. 2016, 7(14), 17369-17379
- Lima, S.M.; Yamada, S.S.; Reis, B.F.; Postigo, S.; Galvão da Silva, M.A.; Aoki, T. Effective treatment of vaginal atrophy with isoflavone vaginal gel. Maturitas 2013, 74(3), 252-258
- Watanabe, S.; Terashima, K.; Sato, Y.; Arai, S.; Eboshida, A. Effects of isoflavone supplement on healthy women. Biofactors. 2000, 12(1-4), 233-241
- Takeda, T.; Chiba, Y. Evaluation of a natural S-equol supplement in treating premenstrual symptoms and the effect of the gut microbiota: An open-label pilot study. Neuropsychopharmacol Rep. 2022, 42(2), 127-134. doi: 10.1002/npr2.12234.
- Zhong, X-S.; Ge, J.; Chen, S-W.; Xiong, Y-Q.; Ma, S-J.; Chen, Q. Association between Dietary Isoflavones in Soy and Legumes and Endometrial Cancer: A Systematic Review and Meta-Analysis. J Acad Nutr Diet. 2018, 118(4), 637-651. doi: 10.1016/j.jand.2016.09.036.
- Zhang, G.Q.; Chen, J.L.; Luo, Y.; et al. Menopausal hormone therapy and women's health: An umbrella review. PLoS Med. 2021, 18(8), e1003731. doi: 10.1371/journal.pmed.1003731.
- Shanle, E.K.; Xu, W. Selectively targeting estrogen receptors for cancer treatment. Adv Drug Deliv Rev. 2010, 62(13), 1265-1276. doi: 10.1016/j.addr.2010.08.001.
- Allemani, C.; Weir, H.K.; Carreira, H.; et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015, 385(9972), 977-1010. doi: 10.1016/S0140-6736(14)62038-62039
- Zlotta, A.R. Kuk, C. Prevalence of prostate cancer across the globe: what can autopsy studies teach us about this peculiar disease? Arch Esp Urol. 2014, 67(5), 400-408.
- Dey, P.; Barros, R.P.; Warner, M.; et al. Insight into the mechanisms of action of estrogen receptor β in the breast, prostate, colon, and CNS. J Mol Endocrinol. 2013, 51(3), T61-74. doi: 10.1530/JME-13-0150
- Rizzardi, A.E.; Zhang, X.; Vogel, R.I.; et al. Quantitative comparison and reproducibility of pathologist scoring and digital image analysis of estrogen receptor β2 immunohistochemistry in prostate cancer. Diagn Pathol. 2016, 11(1), 63-73. doi: 10.1186/s13000-016-0511-5
- Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014; 140, 116-132. doi:10.1371/journal.pone.0078479
- Blanco Mejia, S.; Messina M.; Li, S.S; et al.; A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults J Nutr 2019, 149(6), 968-981. doi: 10.1093/jn/nxz020.
- Hermsdorff, H.H.M.; Ángeles Zulet, M.; Abete, I.; Martínez, J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011, 50(1), 61-69. doi: 10.1007/s00394-010-0115-x.
- Crouse, J.R. 3rd; Morgan, T.; Terry, J.G.; Ellis, J.; Vitolins, M.; Burke, G.L. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch Intern Med. 1999, 159(17), 2070-2076. doi: 10.1001/archinte.159.17.2070.
- Ma, Y.; Chiriboga, D.; Olendzki, B.C.; Nicolosi, R.; Merriam, P.A.; Ockene, I.S. Effect of soy protein containing isoflavones on blood lipids in moderately hypercholesterolemic adults: a randomized controlled trial. J Am Coll Nutr. 2005, 24(4), 275-285. doi: 10.1080/07315724.2005.10719475.
- Jungbauer A, Medjakovic S. Phytoestrogens and the metabolic syndrome. J Steroid Biochem Mol Biol. 2014, 139, 277-89. doi: 10.1016/j.jsbmb.2012.12.009.
- Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998, 139(10), 4252-4263. doi: 10.1210/endo.139.10.6216.
- Yamagata, K.; Yamori, Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules. 2021, 26(19), 5863. doi: 10.3390/molecules26195863.
- Chen, L.R.; Chen, K.H. Utilization of Isoflavones in Soybeans for Women with Menopausal Syndrome: An Overview. Int J Mol Sci. 2021, 22(6), 3212. doi: 10.3390/ijms22063212.
- Liu, J.; Mi, S.; Du, L.; et al. The associations between plasma phytoestrogens concentration and metabolic syndrome risks in Chinese population. PLoS One. 2018, 13(3):e0194639. doi: 10.1371/journal.pone.0194639.
- Woo, H.W.; Kim, M.K.; Lee, Y.H.; Shin, D.H.; Shin, M.H.; Choi, B.Y. Habitual consumption of soy protein and isoflavones and risk of metabolic syndrome in adults ≥ 40 years old: a prospective analysis of the Korean Multi-Rural Communities Cohort Study (MRCohort). Eur J Nutr. 2019; 58(7), 2835-2850. doi: 10.1007/s00394-018-1833-8.
- Squadrito, F.; Marini, H.; Bitto, A.; al. Genistein in the metabolic syndrome: results of a randomized clinical trial. J Clin Endocrinol Metab. 2013, 98(8), 3366-3374. doi: 10.1210/jc.2013-1180.
- Irace, C.; Marini, H.; Bitto, A.; et al. Genistein and endothelial function in postmenopausal women with metabolic syndrome. Eur J Clin Invest. 2013, 43(10), 1025-1031. doi: 10.1111/eci.12139.
- Usui, T.; Tochiya, M.; Sasaki, Y.; et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin Endocrinol (Oxf). 2013, 78(3), 365-372. doi: 10.1111/j.1365-2265.2012.04400.x.
- Tang, J.; Wan, Y.; Zhao, M.; Zhong, H.; Zheng, J.S.; Feng, F. Legume and soy intake and risk of type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr. 2020, 111(3), 677-688. doi: 10.1093/ajcn/nqz338.
- Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Kanadys, W.; Malm, M.; Janiszewska, M.; Jędrych, M. Effects of Soy Isoflavones on Glycemic Control and Lipid Profile in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021, 13(6), 1886. doi: 10.3390/nu13061886.
- Glisic, M.; Kastrati, N.; Gonzalez-Jaramillo, V.; et al. Associations between Phytoestrogens, Glucose Homeostasis, and Risk of Diabetes in Women: A Systematic Review and Meta-Analysis. Adv Nutr. 2018, 9(6), 726-740. doi: 10.1093/advances/nmy048.
- Li, W.; Ruan, W.; Peng, Y.; Wang, D. Soy and the risk of type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Diabetes Res Clin Pract. 2018, 137, 190-199. doi: 10.1016/j.diabres.2018.01.010.
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle AMPK activation in obese subjects. BMJ Open Diabetes Res Care. 2020, 8(1), e000948. doi: 10.1136/bmjdrc-2019-000948.
- Dourson, M.L.; Stara, J.F. Regulatory history and experimental support of uncertainty (safety) factors. Regul Toxicol Pharmacol. 1983, 3(3), 224-238. doi: 10.1016/0273-2300(83)90030-2
- Pieters, M.N.; Kramer, H.J.; Slob, W. Evaluation of the uncertainty factor for subchronic-to-chronic extrapolation: statistical analysis of toxicity data. Regul Toxicol Pharmacol. 1998, 27(2), 108-111. doi: 10.1006/rtph.1997.1196.
- ANSES, 2011. Étude de l’alimentation totale française 2 (EAT 2) Tome 1. Contaminants inorganiques, minéraux, polluants organiques persistants, mycotoxines, phyto-estrogènes. Avis de l’Anses. Rapport d’expertise. Juin 2011. Edition scientifique.
- NIH / PubChem. Diethylstilbestrol. https://pubchem.ncbi.nlm.nih.gov/compound/Diethylstilbestrol
- NTP, 2008. Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). Natl Toxicol Program Tech Rep Ser 539, 1-266. PMID: 18685713
- Lee, A.; Bensaada, S.; Lamothe, V.; Lacoste, M.; Bennetau-Pelissero, C. Endocrine disruptors on and in fruits and vegetables: Estimation of the potential exposure of the French population. Food Chem. 2022, 373(Pt B), 131513. doi: 10.1016/j.foodchem.2021.131513.
- Shi, S.R.; Gua, H.; Chang, L.L.; Wang, Z.Y.; Tong, H.B.; Zou, J.M. Safety evaluation of daidzein in laying hens: Part I. Effects on laying performance, clinical blood parameters, and organs development. Food Chem Toxicol. 2013, 55, 684–688. doi: 10.1016/j.fct.2013.01.009
- Soujanya, M.G.S.; Prathap Reddy, K.; Sridevi1, V.; Ramachandra Reddy, P.; Sreenivasula Reddy, P. In Utero Exposure of Biochanin-A Alters Female Reproduction in Rat. J Clin Mol Endocrinol. 2016, 1(2), 08. doi: 10.21767/2572-5432.10008
- Parandin, R.; Mohammadi, L. The effects of Formononetin Derived from Red Clover During Pregnancy on Puberty, Some Reproductive Parameters and Lordosis Behavior of Female Mice. Armaghan Danesh. 2019, 24(2-133), 199-213.
- Nedresky, D.; Singh, G. Physiology, Luteinizing Hormone. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Shier, F.L.; Rossiter, R.C. Clover disease. Practical findings and recommendations for control. J Agri West Aust. 1949, 26, 111-116
- Findlay, J.K.; Buckmaster, J.M.; Chamley, W.A.; Cumming, I.A.; Hearnshaw, H.; Goding, J.R. Release of luteinising hormone by œstradiol 17b and a gonadotrophin-releasing hormone in ewes affected with clover disease. Neuroendocrinol. 1973, 11, 57-66.
- Cassidy, A.; Bingham, S.; Setchell, K.D. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994, 60(3), 333-340. doi: 10.1093/ajcn/60.3.333.
- Gardner-Thorpe, D.; O'Hagen, C.; Young, I.; Lewis, S.J. Dietary supplements of soya flour lower serum testosterone concentrations and improve markers of oxidative stress in men. Eur J Clin Nutr. 2003, 57(1), 100-106. doi: 10.1038/sj.ejcn.1601495.
- Hooper, L.; Ryder, J.J.; Kurzer, M.S.; Lampe, J.W.; Messina, M.J.; Phipps, W.R.; Cassidy, A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009, 15(4), 423-440. doi: 10.1093/humupd/dmp010.
- Bennetau-Pelissero, C.; Breton, B.; Bennetau, B.; Corraze, G. Effect of genistein-enriched diets on the endocrine process of gametogenesis and on reproduction efficiency of the rainbow trout Oncorhynchus mykiss. Gen Comp Endocrinol. 2001, 121(2), 173-187. doi: 10.1006/gcen.2000.7585.
- National Toxicology Program. Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). Natl Toxicol Program Tech Rep Ser. 2008, 539, 1-266. PMID: 18685713
- Zhou, W.; Wu, H.; Wang, Q.; et al. Simultaneous determination of formononetin, biochanin A and their active metabolites in human breast milk, saliva and urine using salting-out assisted liquid-liquid extraction and ultra-high performance liquid chromatographyelectrospray ionization tandem mass spectrum. J Chromatogr B Analyt Technol Biomed Life Sci. 2020, 1145, 122108. doi: 10.1016/j.jchromb.2020.122108 2020
- Setchell, K.D.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998, 68(6 Suppl), 1453S-1461S. doi: 10.1093/ajcn/68.6.1453
- McCarver, G.; Bhatia, J.; Chambers, C.; et al. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res B Dev Reprod Toxicol. 2011, 92(5), 421-468. doi: 10.1002/bdrb.20314.
- Chin H.B.; Kelly, A.; Adgent, M.A.; et al. Reproductive Hormone Concentrations and Associated Anatomical Responses: Does Soy Formula Affect Minipuberty in Boys? J Clin Endocrinol Metab. 2021, 106(9), 2635-2645. doi: 10.1210/clinem/dgab354.
- Gilchrist, J.M.; Moore, M.B.; Andres, A.; Estroff, J.A.; Badger, T.M. Ultrasonographic patterns of reproductive organs in infants fed soy formula: comparisons to infants fed breast milk and milk formula. J Pediatr. 2010, 156(2), 215-220. doi: 10.1016/j.jpeds.2009.08.043.
- Adgent, M.A.; Daniels, J.L.; Edwards, L.J.; Siega-Riz, A.M.; Rogan, W.J. Early-life soy exposure and gender-role play behavior in children. Environ Health Perspect. 2011, 119(12), 1811-1816. doi: 10.1289/ehp.1103579.
- Strom, B.L.; Schinnar, R.; Ziegler, E.E.; Barnhart, K.T.; et al. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood JAMA. 2001, 286(7), 807-814. doi: 10.1001/jama.286.7.807.
- Upson, K.; Harmon, Q.E.; Laughlin-Tommaso, S.K.; Umbach, D.M.; Baird, D.D. Soy-based Infant Formula Feeding and Heavy Menstrual Bleeding Among Young African American Women. Epidemiology. 2016, 27(5), 716-725. doi: 10.1097/EDE.0000000000000508.
- Upson, K.; Adgent, M.A.; Wegienka, G.; Baird, D.D. Soy-based infant formula feeding and menstrual pain in a cohort of women aged 23-35 years. Hum Reprod. 2019, 34(1), 148-154. doi: 10.1093/humrep/dey303.
- Qin, H.; Lin, Z.; Vásquez, E.; Luan, X.; Guo, F.; Xu, L. High soy isoflavone or soy-based food intake during infancy and in adulthood is associated with an increased risk of uterine fibroids in premenopausal women: a meta-analysis. Nutr Res. 2019, 71, 30-42. doi: 10.1016/j.nutres.2019.06.002.
- Harlid, S.; Adgent, M.; Jefferson, W.N.; et al. Soy Formula and Epigenetic Modifications: Analysis of Vaginal Epithelial Cells from Infant Girls in the IFED Study. Environ Health Perspect. 2017, 125(3), 447-452. doi: 10.1289/EHP428
- Felício, J.S.; Leite de Alcântara A.; Janaú, L.C. , et al. Association of Soy and Exclusive Breastfeeding With Central Precocious Puberty: A Case-Control Study. Front Endocrinol (Lausanne). 2021, 12, 667029. doi: 10.3389/fendo.2021.667029
- Oliveira F.R.K.; Gustavo, A.F.S.E.; Gonçalves, R.B.; Bolfi, F.; Mendes, A.L.; Dos Santos Nunes-Nogueira, V. Association between a soy-based infant diet and the onset of puberty: A systematic review and meta-analysis. PLoS One. 2021, 16(5), e0251241. doi: 10.1371/journal.pone.0251241
- Segovia-Siapco, G.; Pribis, P.; Oda, K.; Sabaté, J. Soy isoflavone consumption and age at pubarche in adolescent males. Eur J Nutr. 2018, 57(6), 2287-2294. doi: 10.1007/s00394-017-1504-1
- Cheng, G.; Remer, T.; Prinz-Langenohl, R.; Blaszkewicz, M.; Degen, G.H.; Buyken, A.E. Relation of isoflavones and fiber intake in childhood to the timing of puberty Am J Clin Nutr. 2010, 92(3), 556-564. doi: 10.3945/ajcn.2010.29394.
- Goldberg, M.; D'Aloisio, A.A.; O'Brien, K.M.; Zhao, S.; Sandler, D.P. Early-life exposures and age at thelarche in the Sister Study cohort. Breast Cancer Res. 2021, 23(1), 111. doi: 10.1186/s13058-021-01490-z
- Xiong, J.; Xu, Y.; Liu, X.; et al. Prospective association of dietary soy and fibre intake with puberty timing: a cohort study among Chinese children. BMC Med. 2022, 20(1), 145. doi: 10.1186/s12916-022-02320-5.
- Imai, H.; Nishikawa, H.; Suzuki, A.; Kodama, E. Secondary Hypogonadism due to Excessive Ingestion of Isoflavone in a Man. Intern Med. 2022, 26. doi: 10.2169/internalmedicine.8578-21.
- Gardner-Thorpe, D.;O'Hagen, C.; Young, I.; Lewis, S.J. Dietary supplements of soya flour lower serum testosterone concentrations and improve markers of oxidative stress in men Eur J Clin Nutr. 2003, 57(1), 100-106. doi: 10.1038/sj.ejcn.1601495.
- Chandrareddy, A.; Muneyyirci-Delale, O.; McFarlane, S.I.; Murad, O.M. Adverse effects of phytoestrogens on reproductive health: a report of three cases. Complement Ther Clin Pract. 2008, 14(2), 132-135. doi: 10.1016/j.ctcp.2008.01.002.
- Chavarro, J.E.; Toth, T.L.; Sadio, S.M.; Hauser, R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod. 2008, 23(11), 2584-2590. doi: 10.1093/humrep/den243.
- Toshima, H.; Suzuki, Y.; Imai, K.; Yoshinaga, J. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: a pilot study on exposure and semen quality. Int J Hyg Environ Health. 2012, 215(5),502-506. doi: 10.1016/j.ijheh.2011.09.005.
- Xia Y.; Chen, M.; Zhu, P.; Lu, C. Urinary phytoestrogen levels related to idiopathic male infertility in Chinese men. Environ Int. 2013, 59, 161-167. doi: 10.1016/j.envint.2013.06.009.
- Mumford, S.L.; Kim, S.; Chen, Z.; Barr, D.B.; Buck-Louis, G.M. Urinary Phytoestrogens Are Associated with Subtle Indicators of Semen Quality among Male Partners of Couples Desiring Pregnancy. J Nutr. 2015, 145(11), 2535-2541. doi: 10.3945/jn.115.214973
- Yuan, G.; Liu, Y.; Liu, G.; et al. Associations between semen phytoestrogens concentrations and semen quality in Chinese men. Environ Int. 2019, 129, 136-144. doi: 10.1016/j.envint.2019.04.076.
- Beaton, L.K.; McVeigh, B.L.; Dillingham, B.L.; Lampe, J.W.; Duncan, A.M. Soy protein isolates of varying isoflavone content do not adversely affect semen quality in healthy young men Fertil Steril. 2010, 94(5), 1717-22. doi: 10.1016/j.fertnstert.2009.08.055.
- Povey, A.C.; Clyma, J-A.; McNamee, R.; Moore, H.D. Phytoestrogen intake and other dietary risk factors for low motile sperm count and poor sperm morphology. Andrology. 2020, 8(6), 1805-1814. doi: 10.1111/andr.12858.
- Andrews, M.A.; Schliep, K.C.; Wactawski-Wende, J.; et al. Dietary factors and luteal phase deficiency in healthy eumenorrheic women. Hum Reprod. 2015, 30(8), 1942-1951. doi: 10.1093/humrep/dev133.
- Jacobsen, B.K.; Jaceldo-Siegl, K.; Knutsen, S.F.; Fan, J.; Oda, K.; Fraser, G.E. Soy isoflavone intake and the likelihood of ever becoming a mother: the Adventist Health Study-2 Int J Womens Health. 2014, 6, 377-384. doi: 10.2147/IJWH.S57137
- Index Mundi, Total fertility rate, country comparison : https://www.indexmundi.com/g/r.aspx?v=31
- Vanegas, J.C.; Afeiche, M.C.; Gaskins, A.J.; et al. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil Steril. 2015, 103(3), 749-755.e2. doi: 10.1016/j.fertnstert.2014.12.104.
- Ripp, J.A. Soybean-induced goiter. Am J Dis Child. 1961, 102, 106-109. doi: 10.1001/archpedi.1961.02080010108017.
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002, 110 (Suppl 3), 349-353. doi: 10.1289/ehp.02110s3349
- Conrad, S.C.; Chiu, H.; Silverman, B.L. Soy formula complicates management of congenital hypothyroidism. Arch Dis Child. 2004, 89(1), 37-40. doi: 10.1136/adc.2002.009365.
- Fruzza A.G.; Demeterco-Berggren, C.; Lee Jones, K. Unawareness of the effects of soy intake on the management of congenital hypothyroidism. Pediatrics. 2012, 130(3), e699-702. doi: 10.1542/peds.2011-3350
- Divi, R.L.; Chang, H.C.; Doerge, D.R. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997, 54(10), 1087-1096. doi: 10.1016/s0006-2952(97)00301-8.
- Sosvorová, L.; Mikšátková, P.; Bičíková, M.; Kaňová, N.; Lapčík, O. The presence of monoiodinated derivates of daidzein and genistein in human urine and its effect on thyroid gland function. Food Chem Toxicol. 2012, 50(8), 2774-2779. doi: 10.1016/j.fct.2012.05.037.
- Radović, B.; Mentrup, B.; Köhrle, J.Genistein and other soya isoflavones are potent ligands for transthyretin in serum and cerebrospinal fluid Br J Nutr. 2006, 95(6), 1171-1176. doi: 10.1079/bjn20061779.
- Ariyani, W.; Iwasaki, T.; Miyazaki, W.; Yu, L.; Takeda, S.; Koibuchi, N.A Possible Novel Mechanism of Action of Genistein and Daidzein for Activating Thyroid Hormone Receptor-Mediated Transcription. Toxicol Sci. 2018, 164(2), 417-427. doi: 10.1093/toxsci/kfy097
- Thankamony, A.; Pasterski, V.; Ong, K.K.; Acerini, C.L.; Hughes, I.A. Anogenital distance as a marker of androgen exposure in humans. Andrology. 2016, 4(4), 616-625. doi: 10.1111/andr.12156.
- Becker, L.A.; Kunkel, A.J.; Brown, M.R.; Ball, E.E.; Williams, M.T. Effects of dietary phytoestrogen exposure during perinatal period. Neurotoxicol Teratol. 2005, 27(6), 825-834. doi: 10.1016/j.ntt.2005.05.007.
- Nagata, C.; Iwasa, S.; Shiraki, M.; et al. Associations among maternal soy intake, isoflavone levels in urine and blood samples, and maternal and umbilical hormone concentrations (Japan). Cancer Causes Control. 2006, 17(9), 1107-1113. doi: 10.1007/s10552-006-0044-4.
- Chin, H.B.; Kelly, A.; Adgent, M.A.; Patchel, S.A. Reproductive Hormone Concentrations and Associated Anatomical Responses: Does Soy Formula Affect Minipuberty in Boys? J Clin Endocrinol Metab. 2021, 106(9), 2635-2645. doi: 10.1210/clinem/dgab354.
- Adgent, M.A.; Umbach, D.M.; Zemel, B.S.; et al. A Longitudinal Study of Estrogen-Responsive Tissues and Hormone Concentrations in Infants Fed Soy Formula. J Clin Endocrinol Metab. 2018, 103(5), 1899-1909. doi: 10.1210/jc.2017-02249.
- Pfitscher, A.; Reiter, E.; Jungbauer, A. Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J Steroid Biochem Mol Biol. 2008, 112(1-3), 87-94. doi: 10.1016/j.jsbmb.2008.08.007
- Berensztein, E.B.; Baquedano, M.S.; Gonzalez, C.R.; Saraco, N.I. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr Res. 2006, 60(6), 740-744. doi: 10.1203/01.pdr.0000246072.04663.bb.




