Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Landi | -- | 3971 | 2023-02-03 07:34:06 | | | |
| 2 | Rita Xu | Meta information modification | 3971 | 2023-02-03 09:41:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fedenko, V.S.; Landi, M.; Shemet, S.A. Plant Phenolics as Ligands for Metal(loid)s. Encyclopedia. Available online: https://encyclopedia.pub/entry/40796 (accessed on 07 February 2026).
Fedenko VS, Landi M, Shemet SA. Plant Phenolics as Ligands for Metal(loid)s. Encyclopedia. Available at: https://encyclopedia.pub/entry/40796. Accessed February 07, 2026.
Fedenko, Volodymyr S., Marco Landi, Sergiy A. Shemet. "Plant Phenolics as Ligands for Metal(loid)s" Encyclopedia, https://encyclopedia.pub/entry/40796 (accessed February 07, 2026).
Fedenko, V.S., Landi, M., & Shemet, S.A. (2023, February 03). Plant Phenolics as Ligands for Metal(loid)s. In Encyclopedia. https://encyclopedia.pub/entry/40796
Fedenko, Volodymyr S., et al. "Plant Phenolics as Ligands for Metal(loid)s." Encyclopedia. Web. 03 February, 2023.
Copy Citation
Plant adaptive strategies have been shaped during evolutionary development in the constant interaction with a plethora of environmental factors, including the presence of metals/metalloids in the environment. Among adaptive reactions against either the excess of trace elements or toxic doses of non-essential elements, their complexation with molecular endogenous ligands, including phenolics, has received increasing attention.
anthocyanins
binding sites
complexation
flavonoids
1. Introduction
The life processes of plants have evolved in coordination with environmental factors. In addition, intensified anthropogenic load on ecosystems has led to increasing levels of chemical contamination and resulted in the emergence of new pollutants, namely xenobiotics. To understand the peculiarities of plant–environment interactions, it is essential to take into account the environmental and ecological aspects of the problem [1]. Hazardous metals and metalloids are among the major and widespread pollutants due to their high toxicity to the biosphere and the amplitude of their contamination in the natural environment [2]. Mining and metal extraction, fossil fuel combustion, agricultural application of fertilizers, sewage sludge, metal-containing pesticides, wastewater irrigation, and atmospheric deposition are the main anthropic sources of metal(loid)s [3].
A special feature of plants regarding their relations to metal(loid)s is that a certain amount of trace metals is necessary for a number of biologically essential processes (metalloenzymes, mineral nutrition, photosynthesis, prooxidant/antioxidant systems, etc.), whilst both the overdoses of essential and toxic doses of non-essential elements negatively affect plant metabolism. Therefore, to optimize those processes, plants have evolved multiple regulatory and defence mechanisms to counteract metal(loid) toxicity [4][5][6].
Among others, plants detoxify metal(loid)s via their biotransformation into metabolically inactive compounds [7][8]. Among biotransformation reactions, the in vivo chelating of metal ions is pivotal, which is accomplished by endogenous chelators: glutathione (GSH), phytochelatins (PCs), metallothioneins (MTs), organic acids, nicotinamine, amino acids [6][9][10]. An important feature of those bioligands is their capacity to bind various metals [10]. Such a property of binding both essential and non-essential metal(loid)s has been confirmed in vivo for flavonoid pigments of anthocyanins (ACNs) [11][12][13]. Those results allow postulating a hypothesis about the participation of phenolic compounds (PCs) in metal(loid) detoxification in plants [12]. However, a comprehensive systemic analysis of the role of PCs as endogenous chelators in plant metal tolerance has not yet been performed.
It is noteworthy that the current level of metal(loid) tolerance in plants is characterized by the extensive use of the integrated “omics” approach [14][15][16][17][18]. The “omics” approach is aimed at the studying of the organism as a holistic system, based on the integrative analysis of and interrelations among major biological processes [19]. The “omics” research object related to the behaviour of metals in living organism is referred to as the “metallome” [20] and the corresponding research field as “metallomics” [21].
In recent years, the key significance of PCs was confirmed as pivotal and versatile plant defensive compounds against abiotic stresses, including metal(loid) tolerance [5][22][23][24]. Systematizing extensive experimental data resulted in the hypothesis of a universal dominant tendency to increased accumulation of PCs as components of the antioxidant defence system, which ensures the balance between the production and detoxification of reactive oxygen species (ROSs) under metal(loid) exposure [25]. Beside their ROS scavenging prerogative, other possible roles of those secondary metabolites in the metallomics context remain a poorly investigated issue.
2. Plant Phenolics as Ligands for Metal(loid)s
The systematization of the available information on the chelating capacity of plant PCs should be performed in two consecutive stages. In the first stage, it is necessary to analyse the binding of PCs with metal(loid)s’ ions in vitro to establish the structure of the metallocomplexes formed and the key criteria of such a binding. In the second stage, using the identified criteria of binding, it is possible to systematize the experimental results about the PC’s chelation with metal(loid) ions (Men+) in plants in vivo.
2.1. Complexing In Vitro
In the studies of PC–Men+ chelation, two main directions can be distinguished:
- (1)
-
Evaluation of the complexation of individual PCs with Men+, based on the features of the ligands, which are modified due to chelation;
- (2)
-
Assessment of metal chelating ability toward PCs and plant extracts based on the alterations in the absorption of metallochromic indicators.
2.1.1. Individual Phenolic Compounds
Various aspects related to the synthesis, identification of the structure, biological activity, and application of PC–Men+ complexes have been systematized in numerous reviews [26][27][28][29][30][31][32][33]. However, some aspects of this problem remain unclear due to the scarce attention given to the involvement of PCs in the modulation of the metallome
In this regard, researchers analysed the available data on the ability of natural phenolic metabolites to form metallocomplexes or identifying the binding of individual compounds to Men+ in vitro in order to answer the following questions:
- (1)
-
How are metal binding properties manifested for the natural compounds from different PC subclasses, which are formed in the process of plant phenolic metabolism?
- (2)
-
Which structural fragments of PCs are crucial for the complexation?
- (3)
-
Can PCs be considered as universal ligands for multiple Men+?
Researchers systematized available experimental data, and the main results are presented in Table 1 for individual representatives of various plant PC subgroups. The structural formulas of the ligands are shown in Figure 1 with their division into separate subgroups (phenolic acids 1–12, coumarins 14–16, chalcones 17, dihydrochalcones 18, flavanones 19–23, flavanonols 24, 25, flavonols 26–37, flavan-3-ols 38–43, flavones 44–51, isoflavones 52–54, anthocyanidins 55–57, xanthonoids 58, stilbenes 59, curcuminoids 60, lignans 61, flavonolignans 62, lignins, tannins 63–65). For some flavonoids, the data on complexation are combined with their derivatives. The number of Men+ ions, for which the formation of metal complexes is confirmed, is presented regardless of the compounds with different stoichiometric ligand:Men+ ratios, or for one ligand with different Men+ ions (heterometallic complexes), or for one Men+ with different ligands (mixed complexes). For some ligands, radiolabelled complexes are included. Chemical elements, for which the complexation with PC ligands has been established, are provided in Figure 2. It should be noted that researchers analysed only the data on metal complexes with natural PCs; currently, however, numerous studies are being performed on synthetic ligands of a phenolic nature, obtained by structural modification of the binding sites of natural PCs in order to create new effective biologically active substances.
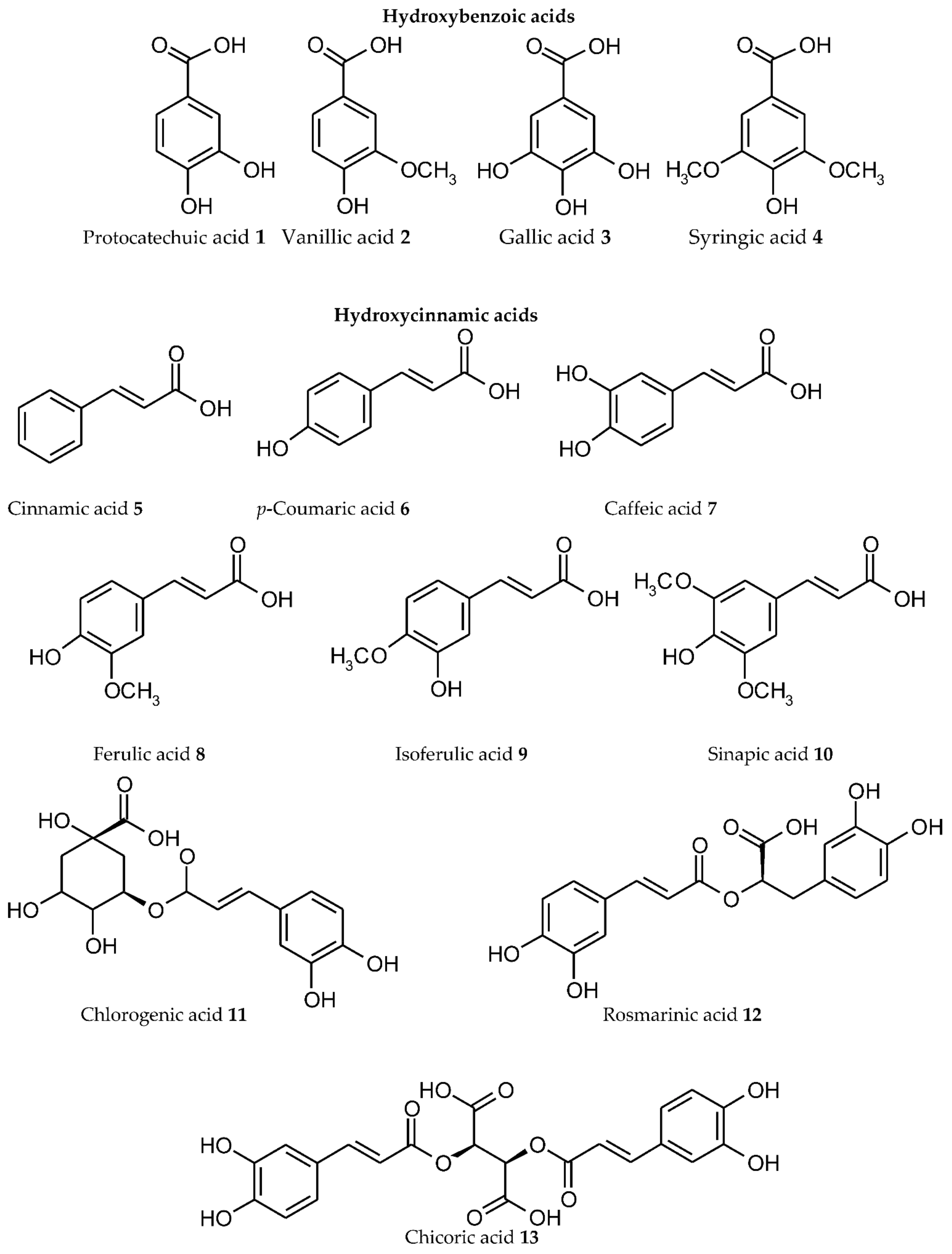







Figure 1. Structure of individual representative ligands capable of binding metal/metalloid ions from various plant phenolic subgroups.

Figure 2. The elements confirmed to form phenolic ligand–Men+ complexes (highlighted in red).
Table 1. Complexes of plant phenolic ligands with metal(loid) ions.
| Phenolic Ligand | Metal(loid) Ion | Number of Metal Ions | References |
|---|---|---|---|
| Phenolic acid | |||
| Hydroxybenzoic acids | |||
| Protocatechuic acid 1 | Al(III), U(VI) | 2 | [34][35] |
| Vanillic acid 2 | Zn(II), Y(III), La(III), Ce(III), Pr(III), Nd(III), Sm(III), Eu(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Tm(III), Yb(III), Lu(III), Np(V) |
17 | [36][37][38] |
| Gallic acid 3 | Fe(II), Zn(II), Fe(III), Eu(III) | 4 | [39][40][41][42] |
| Syringic acid 4 | Li(I), Na(I), K(I), Rb(I), Cs(I), Fe(II), Fe(III), Y(III), La(III), Ce(III), Pr(III), Nd(III), Sm(III), Eu(III), Gd(III), Tb(III), Dy(III), Ho(III), Er(III), Tm(III), Yb(III), Lu(III) |
22 | [43][44][45] |
| Hydroxycinnamic acids | |||
| Cinnamic acid 5 | Li(I), Na(I), K(I), Rb(I), Cs(I), Hg(I), Ca(II), Co(II), Ni(II), Cu(II), Zn(II), Ru(II), Cd(II), La(III), Eu(III), Tb(III), VO(IV), Th(IV) |
19 | [46][47][48][49][50][51][52][53] |
| p-Coumaric acid 6 | Li(I), Na(I), K(I), Rb(I), Cs(I), Co(II), Ni(II), Cu(II), Zn(II),Al(III) | 10 | [33][54][55] |
| Caffeic acid 7 | Li(I), Na(I), K(I), Rb(I), Cs(I), Cu(II), Pb(II), Pt(II), Al(III), Fe(III), Cr(III), Eu(III) |
12 | [33][56][57][58][59] |
| Ferulic acid 8 | Ca(II), Mn(II), Cu(II), Zn(II), Cd(II), Al(III), VO(IV), V(V) | 8 | [33][48][60][61] |
| Isoferulic acid 9 | Na(I), Mg(II), Mn(II) | 3 | [62] |
| Sinapic acid 10 | Cu(II), Pt(II), V(V) | 3 | [57][63] |
| Chlorogenic acid 11 | Li(I), Na(I), K(I), Rb(I), Cs(I), Ca(II), Zn (II), Fe(III), VO(IV) |
9 | [64][65][66][67][68] |
| Rosmarinic acid 12 | Li(I), Na(I), K(I), Rb(I), Cs(I), Ca(II), Cu(II) | 7 | [67][69][70] |
| Chicoric acid 13 | Co(II), Ni(II), Cu(II), Zn(II) | 4 | [71] |
| Coumarins | |||
| Coumarin 14 | La(III), Ce(III), Nd(III), Sm(III), Dy(III) | 5 | [59] |
| Umbellipherone 15 | Ce(III) | 1 | [72] |
| Daphnetin 16 | Cu(II), Zn(II), Ge(IV) | 3 | [73] |
| Chalcones | |||
| Butein 17 | Cu(II), Zn(II) | 2 | [74] |
| Dihydrochalcones | |||
| Phloretin 18 | Ru(III) | 1 | [75] |
| Flavanones | |||
| Naringenin 19, naringin 20 |
Fe(II), Cu(II), Ni(II), Zn(II), Pt(II), Fe(III), Cr(III), La(III), Y(III), Eu(III), Ce(IV), VO(IV), V(V) | 12 | [27][28][57][76][77][78] |
| Eriodictyol 21 | Fe(II), Fe(III) | 2 | [79] |
| Hesperitin 22, hesperidin 23 |
Ni(II), Cu(II), Zn(II), Al(III), VO(IV), | 5 | [27] |
| Flavanonols | |||
| Taxifolin 24 | Fe(II), Ni(II), Cu(II), Zn(II), Fe(III) |
5 | [28][76][80][81][82] |
| Dihydromyricetin 25 | Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II) | 6 | [83][84] |
| Flavonols | |||
| Kaempferol 26 | Fe(II), Cu(II), Zn(II), Pb(II), Fe(III), VO(IV) | 6 | [27][28][32][85] |
| Quercetin 27, rutin 28, quercitrin 29, isoquercitrin 30 |
Mg(II), Ca(II), Sc(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Mo(II), Pd(II), Cd(II), Hg(II), Sn(II), Pb(II), Al(III), Cr(III), Fe(III), Ga(III), Y(III), Rh(III), Sb(III), La(III), Pr(III), Nd(III), Eu(III), Gd(III), Tb(III), Dy(III), Tm(III), Au(III), Ge(IV), Zr(IV), Ru(IV), Sn(IV), Os(IV), Cr(VI), Mo(VI), W(VI), Tc(VII), Os(VIII), VO(IV), UO2(II), |
43 | [27][28][29][32][86][87][88][89][90][91][92] |
| Isorhamnetin 31 | Fe(II), Cu(II) | 2 | [93] |
| Tamarixetin 32 | Fe(II), Cu(II) | 2 | [93] |
| Fisetin 33 | Fe(II), Cu(II), Zn(II), Fe(III), VO(IV) | 4 | [28][94] |
| Morin 34 | Mg(II), Ca(II), Mn(II), Co(II), Ni(II), Cu(II), Zn(II), Sr(II), Pd(II), Cd(II), Ba(II), Sn(II), Pt(II), Al(III), Cr(III), Fe(III), Au(III), La(III), Eu(III), Gd(III), Lu(III), Zr(IV), VO(IV), Mo(VI), W(VI), Ti(COO)2 2+ |
26 | [27][28][29][95] |
| Myricetin 35, myricitrin 36 |
Cu(II), Zn(II), Al(III), Fe(III) | 4 | [27][29][88] |
| Galangin 37 | Fe(II), Cu(II), Zn(II), Al(III) | 4 | [85] |
| Flavan-3-ols | |||
| (+)-Catechin 38, (-)-epicatechin 39 |
Fe(II), Cu(II), Zn(II), Hg(II), Al(III), Fe(III), Cr(III), La(III), Yb(III), Gd(III) |
10 | [28][96][97][98][99][100][101][102][103] |
| (+)-Epigallocatechin 40 | Fe(II) | 1 | [100] |
| (-)-Epicatechin 3-gallate 41 |
Fe(II), Cu(II), Zn(II), Al(III), Fe(III) |
3 | [31][98] |
| (-)-Epigallocatechin 3-gallate 42 |
Fe(II), Mn(II), Cu(II), Zn(II), Pt(II), Al(III), Fe(III) |
7 | [31][98][100][104][105] |
| Theaflavin 43 | Al(III), Fe(III) | 2 | [106][107] |
| Flavones | |||
| Primuletin 44 | Zn(II), Cu(II); Pb(II), Al(III), Fe(III) |
5 | [28][85] |
| Chrysin 45 | Cu(II), Pd(II), Al(III), Fe(III), La(III), Ho(III), Er(III), Yb(III), Ce(IV), VO(IV) | 10 | [27][28][85] |
| Apigenin 46 | Cu(II), Pb(II), VO(IV) | 3 | [27][85][108] |
| Luteolin 47 | Mn(II), Fe(II), Cu(II), Al(III), Fe(III), Y(III), Ho(III), Yb(III), Lu(III), VO(IV) | 10 | [27][28][32][108][109] |
| Tricetin 48 | Fe(II), Fe(III) | 2 | [79] |
| Baicalein 49, baicalin 50 |
Fe(II), Cu(II), Fe(III), VO(IV) | 4 | [27][28][108] |
| Acacetin 51 | Fe(III) | 1 | [110] |
| Isoflavones | |||
| Daidzein 52 | Ce(IV) | 1 | [28] |
| Genistein 53 | Cu(II), Fe(III) | 2 | [27][111] |
| Biochanin A 54 | Cu(II), Fe(III) | 2 | [111] |
| Anthocyanidins | |||
| Cyanidin 55 and its glycosides | Cs(I), Mg(II), Ca(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Sr(II), Zn(II), Cd(II), Sn(II), Ba(II), Hg(II), Pb(II), B(III), Al(III), V(III), Cr(III), Fe(III), Ga(III), As(III), Bi(III), Ge(IV), VO3−, MoO42−, WO42− |
27 | [13][112][113][114][115] |
| Delphinidin 56 and its glycosides | Mg(II), Zn(II), Sn(II), Al(III), Cr(III), Fe(III), Ga(III) |
7 | [13][115][116] |
| Petunidin 57 and its glycosides | Mg(II), Sn(II), Al(III), Cr(III), Fe(III), Ga(III) | 6 | [116][117] |
| Xanthonoids | |||
| Mangiferin 58 | Fe(II), Cu(II), Zn(II), Fe(III), Se(IV), Ge(IV) |
6 | [73][118][119] |
| Stilbenes | |||
| Resveratrol 59 | Fe(II), Cu(II), Zn(II), Al(III), Fe(III) |
5 | [31][82][120][121] |
| Curcuminoids | |||
| Curcumin 60 | Mg(II), Ca(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Se(II), Pd(II), Cd(II), Sn(II), Hg(II), Pb(II), Al(III), Cr(III), Fe(III), Ga(III), Y(III), Ru(III), In(III), Re(III), Sm(III), Eu(III), Dy(III), Au(III), VO(IV), Nb(V) |
28 | [82][122][123][124] |
| Lignans | |||
| Secoisolariciresinol diglucoside 61 | Ag(I), Ca(II), Fe(II), Ni(II), Cu(II), Pb(II) | 6 | [125] |
| Flavonolignans | |||
| Silibinin(silybin) 62 | Ni(II), Cu(II), Zn(II), Fe(III), Ga(III), VO(IV) | 6 | [108][126][127][128] |
| Lignins | |||
| Ligno-cellulosic substrate | Mn(II), Cu(II), Fe(III) | 3 | [129] |
| Tannins | |||
| Condensed tannins | Fe(II), Cu(II), Zn(II), Al(III) | 4 | [130][131] |
| Oenothein B 63 | Al(III) | 1 | [132] |
| Ellagic acid 64 | Mg(II), Ca(II), Mn(II), Fe(II), Co(II), Cu(II), Fe(III) |
7 | [133] |
| Tannic acid 65 | Mg(II), Mn(II), Fe(II), Co(II), Ni(II), Cu(II), Zn(II), Mo(II), Cd(II), Al(III), V(III), Cr(III), Fe(III), Ru(III), Rh(III), Ce(III), Eu(III), Gd(III), Tb(III), Ti(IV), Zr(IV) |
21 | [105][134][135][136][137] |
Phenolic acids, as the first structural subgroup of the plant PC metabolic pathway, could be divided into hydroxybenzoic and hydroxycinnamic acids depending on the direction of their biosynthesis [138]. Among natural hydroxybenzoic acids, the complexes with Men+ have been identified for protocatechuic acid 1, vanillic acid 2, gallic acid 3, and syringic acid 4. Protocatechuic acid 1, depending on the pH, coordinates with Al(III) and U(VI) ions via the carboxyl group or the ortho-dihydroxyl group [34][35]. For vanillic acid 2, the complexation with 17 Men+ ions has been identified (Table 1). For gallic acid 3, the coordination with Men+ can involve the carboxylate and neighbouring phenolic hydroxyl groups [42]. The largest number of complexes with Men+ (22 ions) among hydroxybenzoic acids was identified for syringic acid 4.
Transformation of cinnamic acid 5 in the shikimate pathway results in the formation of different hydroxycinnamic acids (p-coumaric acid 6, caffeic acid 7, ferulic acid 8, isoferulic acid 9, sinapic acid 10, chlorogenic acid 11, rosmarinic acid 12, chicoric acid 13) [138]. Cinnamic acid 5 forms complexes with 19 Men+ ions using its carboxyl group. In the binding of p-coumaric acid 6 with Men+, its hydroxyl group could additionally be involved. In caffeic acid 7, its o-dihydroxyl group as an additional chelating site increases the ability of this molecule for complexation. The ability to form metallocomplexes has been confirmed for their methoxy derivatives (ferulic acid 8, isoferulic acid 9, sinapic acid 10). The esters of caffeic acid with quinic acid (chlorogenic acid 11), dihydroxyphenyl-lactic acid (rosmarinic acid 12), and tartaric acid (chicoric acid 13) retain the capacity for complexation with Men+.
For the following subgroups, the complexation with Men+ has been exemplified by their representative compounds: coumarins—coumarin 14, umbellipherone 15, daphnetin 16; chalcones—butein 17; dihydrochalcones—phloretin 18 (Table 1, Figure 1).
Flavanones form metallocomplexes in the form of both aglycons (naringenin 19, eriodictyol 21, hesperitin 22) and glycosides (naringin 20, hesperidin 23). For flavanonols, the metal chelating capacity has been confirmed for their derivatives with catechol (taxifolin 24) and gallic (dihydromyricetin 25) fragments.
Among the most-studied PC bioligands are flavonols (kaempferol 26, quercetin 27, rutin 28, quercitrin 29, isoquercitrin 30, isorhamnetin 31, tamarixetin 32, fisetin 33, morin 34, myricetin 35, myricitrin 36, galangin 37) (Table 1, Figure 1). It is noteworthy that the greatest amount of coordinated metals (43 different Men+ ions) has been identified for quercetin 27 and its glycosides (rutin 28, quercitrin 29, isoquercitrin 30). This pronounced capacity of quercetin to chelate metals is associated with its structural features, which determine the possibility of different variants for the interaction with Men+. Thus, the quercetin molecule contains three potential binding sites (Figure 3): (1) between the 3-hydroxy and 4-carbonyl groups in the C ring; (2) between the 5-hydroxy (in A ring) and 4-carbonyl groups (in the C ring); (3) between the 3’- and 4’-hydroxy groups in the B ring [29].
Complexation of flavan-3-ols with Men+ is carried out by catechol and gallic binding sites ((+)-catechin 38, its stereoisomer (-)-epicatechin 39, (+)-epigallocatechin 40, esters with gallic acid–(-)-epicatechin 3-gallate 41, (-)-epigallocatechin 3-gallate 42). In theaflavin 43, Men+ binding may also involve its tropolone moiety [107].
For flavones (primuletin 44, chrysin 45, apigenin 46, luteolin 47, tricetin 48, baicalein 49, baicalin 50, acacetin 51) without 3-hydroxy groups in the C ring, the complexation with Men+ may involve the binding sites between 5-hydroxy (in A ring) and 4-carbonyl (in the C ring) or the catechol and gallic moieties. In this subgroup, the greatest number of metallocomplexes was identified for chrysin 45 and luteolin 47 (each binds 10 various Men+ ions).
Isoflavone ligands are represented by daidzein 52, genistein 53, and its O-methylated derivative biochanin A 54.
Metal chelating capacity has been demonstrated for anthocyanins and their glycosides with two or three hydroxyl groups in the B ring: cyanidin 55, delphinidin 56, petunidin 57. In contrast to other flavonoids, a specific peculiarity of ACNs is a pH-dependent dynamic equilibrium of aqueous solutions between several structural forms, which are capable of Men+ binding [13]. Among these ligands, the greatest number of metal complexes was identified for cyanidin 55 and its glycosides (27 Men+, in cationic and anionic forms).
For xanthonoids, metallocomplex formation was exemplified by mangiferin 58 (glucosylxanthone) and for stilbenes by resveratrol 59.
Among curcuminoids, the most comprehensively studied ligand is curcumin 60, which may bind 28 various Men+ due to its capacity of keto-enol tautomerism.
The ability of lignans for complexation has been confirmed for secoisolariciresinol diglucoside 61 and of flavonolignans for silibinin 62 (10 Men+ ions each).
The metal binding capacity of lignin as a polymeric phenol was studied for a ligno-cellulosic substrate with Mn(II), Cu(II), and Fe(III) ions (Merdy et al., 2003).
The presence of a great number of hydroxy groups in the structure of tannins (oligomeric and polymeric phenols) determines their high capacity for complexation with Men+. This fact has been established for their different forms: condensed tannins (proanthocyanidins), oenothein B 63 (dimeric macrocyclic ellagitannin), ellagic acid 64, tannic acid 65. The latter is one of the most-studied PC ligands (21 Men+).
Thus, the attempt at systematizing the available experimental results revealed that metallocomplexes can be formed by numerous representative ligands from 18 subgroups of plant PCs, and they are capable of binding 69 different Men+ ions (63 chemical elements) in total (Figure 2).
2.1.2. Metal Chelating Ability
The metal chelating ability is recognized as a generally accepted integrated indicator of the complexing capacity of PCs; it is used as one of the indicators in antioxidant assays [139]. The main aspects of this approach were summarized in the reviews [82][140]. The approach is based on the ability of selected metallochromic indicators to form complexes with Men+, which absorb light in the visible wavelengths range. Upon the addition of the tested PC ligand, competitive binding with Men+ occurs, with a subsequent decrease in the absorption, which is expressed as equivalents of standard chelators or the percentage metal chelating [82]. The binding ability of ligands could also be evaluated by stability constants [140]. For example, in Fe chelation, ferrozine and 2,2′-bipyridine are used as metallochromic indicators and EDTA and deferoxamine as standard metal chelators [82][141]. This approach enables the evaluation of the dependence between the structure of the PC ligand and its metal chelating activity; thus, a comparative analysis of this indicator extracts of medicinal plants is possible [141][142].
2.2. Chelating Effects In Vivo
In the studies of in vivo binding between phenolic metabolites and Men+, two aspects should be highlighted: (1) production of blue anthocyanins (ACNs) in blue flowers and (2) the defensive role of chelation in plant tolerance to toxic metal exposure. It is noteworthy that the vast majority of the in vivo studies on this topic are devoted to ACNs as metal chelators. This is due to the fact of the availability of non-destructive methods for the binding identification based on the spectral characteristics of ACN-Men+ complexes in plant tissues [13]. Blue flower coloration is associated with copigmentation of ACNs and the formation of pigment–copigment–Men+ complexes (Yoshida et al., 2009). Copigmentation can be performed with and without the participation of metal ions [143]. Such studies could be systematized according to two directions, which differ in their levels of elucidation of the content and structural organization of the pigment complex. The first direction is the evaluation of various aspects of the formation of non-stoichiometric ACNs’ metallocomplexes, which are stabilized due to copigmentation with caffeoyl or coumaroyl derivatives of quinic acids or glycosylated flavonoids [144]. For Hydrangea macrophylla, Phacelia campanularia, and Tulipa gesneriana flowers, the major pigments of those complexes are the delphinidin glucosides, while the pigments of Meconopsis grandis flowers are primarily composed of cyanidin glucosides [144]. The binding with metal ions (Fe3+, Al3+, Mg2+) is considered as a necessary condition for the formation of those pigment complexes [144].
Another direction is systemic studies, which have resulted in the establishment of the unique structure of metalloanthocyanins. According to the term’s definition, metalloanthocyanin is a self-assembled, supramolecular complex metal-containing pigment, which comprises 6 ACN molecules, 6 flavone molecules, and 2 metal ions [144]. During blue flowers’ colour formation, three major mechanisms can be implemented, i.e., self-association, copigmentation, and metal complexation [144]. To date, the following metallochelates have been isolated and identified from blue flowers: protocyanin (Centaurea cyanus), commelinin (Commelina communis), protodelphin (Salvia paterns), cyanosalvianin (Salvia uliginosa), nemophilin (Nemophila menziesii) [144]. The constitutive components of those pigments are the ACNs having a chelating centre with two (cyanydin) or three (delphinidin) hydroxyls, flavonoid apigenin derivatives, and Mg2+ and Fe2+ ions [144]. In protocyanin, an additional coordination link of Ca2+ ions with flavone molecules has been established [144]. The advantages of the supramolecular structure for plants are the stability of the pigment complex at physiological pH and the increased tolerance to UV radiation, which play an important role in the implementation of the main function of ACNs during plant blooming under sun irradiation. The simultaneous presence of non-associated and chelated ACN molecules explains the phenomenon of purple coloration due to the mixing of the two colour stimuli, red and blue [13]. Different ratios between those ACN forms, when present in vivo, create various superpositions of their colour stimuli, thus resulting in colour variability with different hues of purple plant coloration, a feature that has an important evolutionary significance, as it allows a wide diversity of plant colours and better alignment with pollinators [145][146]. One peculiarity of metallo-anthocyanins is the ability to replace coordinated biogenic Men+ with abiogenic Me ions, while the spectral characteristics of the metallocomplexes are retained. Thus, commelinin-like pigments can be formed by replacing Mg2+ with Cd2+, Zn2+, Co2+, Ni2+, and Mn2+ [144].
The ACNs’ capability of binding various Men+ ions during the formation of pigment complexes in flowers allows hypothesizing that the chelating properties could be engaged for a different purpose—to decrease the toxicity of endogenous metals, thus increasing plant metal tolerance [147]. This hypothesis was confirmed using maize as a metal-excluder plant; the in vivo chelating effect of cyanidin-3-glucoside (Cya-3-glu) in maize root tissues was found for nine exogenous Men+ (Mg2+, Fe2+, Cd2+, Ni2+, Pb2+, Al3+, VO3−, MoO42−, Cr2O72−) [11][148]. The reversible nature of Cya-3-glu–Pb2+ binding was found in maize roots, which can be controlled by manipulating the pH in the root solution [13]. An increase in the Pb2+ concentration in the root nutrient solution resulted in the increased formation of Cya-3-glu–Pb2+ complexes in maize roots in a dose-dependent manner [148].
The formation of ACN–metal complexes in the hypocotyls of Brassica plants was found upon their treatment with MoO42− and WO42− ion solutions [149][150].
The capability of binding Men+ was also demonstrated for other PCs localized in various plant tissues. Thus, the study of ACNs’ distribution over the roots of Lotus pedunculatus Cav. confirmed the hypothesis about metal binding and detoxifying by proanthocyanins in plant vacuoles [151]. Al(III) metallocomplexes with epigallocatechin gallate and proanthocyanins were identified in the leaves, stems, and roots of Camellia sinensis [131] and an oenothein B (dimeric macrocycle ellagitannin) in the roots of Eucalyptus camaldulensis [132]. Cd2+ binding by polymerized polyphenols was demonstrated in the leaves of water plants [152]. According to Rocha et al. [153], the reduction of mercury toxicity in plants can be associated with the chelating activity of gallic acid.
The confirmation of the role of PC ligands in plant–metal homeostasis is the identification of the complexes of Cu(II) with quercetin, luteolin, and syringic acid in the berries of Euterpe oleraceae and Vaccinum myrtyllus [154].
Aluminium stimulates maize plants to secrete into the rhizosphere various endogenous PCs (catechin, catechol, quercetin) capable of complexing with Al3+, thus implementing one of the mechanisms of plant tolerance to the metal excess in the root nutrition medium [155]. The role of root-secreted coumarins was shown in iron-deficient plants by the acquisition of Fe through reduction and chelation [156][157]. It is noteworthy that the binding effects/capacity of the chelators with different structural groups (including PCs) by trace elements are considered as one of the mechanisms of the soil–plant interface [158]. In this relation, it should be highlighted that metal(loid)-induced accumulation of PCs by plants is associated with their protecting role in plant metal tolerance [25].
References
- Richards, C.L.; Hanzawa, Y.; Katari, M.S.; Ehrenreich, I.M.; Engelmann, K.E.; Purugganan, M.D. Perspective on ecological and evolutionary systems biology. Annu. Rev. Plant Biol. 2009, 35, 331–351.
- Hill, M.K. Understanding Environmental Pollution, 3rd ed.; Cambridge University Press: New York, NY, USA, 2010; 534p, ISBN 978-0-521-73669-5.
- Hasanuzzaman, M.; Fujita, M. Heavy metals in the environment: Current status, toxic effects on plants and phytoremediation. In Phytotechnologies: Remediation of Environmental Contaminants; Anjum, N.A., Pereira, M.E., Ahmad, I., Duarte, A.C., Umar, S., Khan, N.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 7–73. ISBN 1439875189.
- Sharma, A.; Kapoor, D.; Gautam, S.; Landi, M.; Kandhol, N.; Araniti, F.; Ramakrishnan, M.; Satish, L.; Singh, V.P.; Sharma, P.; et al. Heavy metal induced regulation of plant biology: Recent insights. Physiol. Plant. 2022, 174, e13688.
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635.
- Corso, M.; de la Torre, V.S.G. Biomolecular approaches to understanding metal tolerance and hyperaccumulation in plants. Metallomics 2020, 12, 840–859.
- Hall, J.Á. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11.
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39.
- Rauser, W.E. Structure and function of metal chelators produced by plants. Cell Biochem. Biophys. 1999, 31, 19–48.
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.M.; Adam, V.; Fujita, M.; Kizek, R.; et al. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015, 6, 192.
- Fedenko, V.S. Cyanidin as endogenous chelator of metal ions in maize seedling roots. Ukr. Biochem. J. 2008, 80, 102–106.
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17.
- Fedenko, V.S.; Shemet, S.A.; Landi, M. UV–vis spectroscopy and colorimetric models for detecting anthocyanin-metal complexes in plants: An overview of in vitro and in vivo techniques. J. Plant Physiol. 2017, 212, 13–28.
- Jones, O.A.; Dias, D.A.; Callahan, D.L.; Kouremenos, K.A.; Beale, D.J.; Roessner, U. The use of metabolomics in the study of metals in biological systems. Metallomics 2015, 7, 29–38.
- Pirzadah, T.B.; Malik, B.; Hakeem, K.R. Integration of “omic” approaches to unravel the heavy metal tolerance in plants. In Essentials of Bioinformatics; Hakeem, K., Shaik, N., Banaganapalli, B., Elango, R., Eds.; Springer: Cham, Switzerland, 2019; Volume III, pp. 79–92. ISBN 978-3-030-19318-8.
- Hanus-Fajerska, E.; Wiszniewska, A.; Kamińska, I.; Koźmińska, A. Metallomic approach to enhance agricultural application of halophytes. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 1953–1969. ISBN 978-3-030-57635-6.
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213.
- Raza, A.; Tabassum, J.; Zahid, Z.; Charagh, S.; Bashir, S.; Barmukh, R.; Khan, R.S.A.; Barbosa, F., Jr.; Zhang, C.; Chen, H.; et al. Advances in “omics” approaches for improving toxic metals/metalloids tolerance in plants. Front. Plant Sci. 2022, 12, 794373.
- Lay, J.O., Jr.; Liyanage, R.; Borgmann, S.; Wilkins, C.L. Problems with the “omics”. TrAC Trends Anal. Chem. 2006, 25, 1046–1056.
- Williams, R.J.P. Chemical selection of elements by cells. Coord. Chem. Rev. 2001, 216, 583–595.
- Haraguchi, H. Metallomics as integrated biometal science. J. Anal. At. Spectrom. 2004, 19, 5–14.
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106.
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452.
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038.
- Fedenko, V.S.; Shemet, S.A.; Guidi, L.; Landi, M. Metal/metalloid-induced accumulation of phenolic compounds in plants. In Metal Toxicity in Higher Plants; Landi, M., Shemet, S.A., Fedenko, V.S., Eds.; Nova Science Publishers: New York, NY, USA, 2020; pp. 67–115. ISBN 978-1-53616-790-0.
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598.
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid–metal ion complexes: A novel class of therapeutic agents. Med. Res. Rev. 2014, 34, 677–702.
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877.
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 757–771.
- Uivarosi, V.; Munteanu, A. Flavonoid complexes as promising anticancer metallodrugs. In Flavonoids-from Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: Rijeka, Croatia, 2017; pp. 305–337. ISBN 978-953-51-3423-7.
- Borowska, S.; Brzoska, M.M.; Tomczyk, M. Complexation of bioelements and toxic metals by polyphenolic compounds–implications for health. Curr. Drug Targets 2018, 19, 1612–1638.
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal complexes of flavonoids: Their synthesis, characterization and enhanced antioxidant and anticancer activities. Future Med. Chem. 2019, 11, 2845–2867.
- Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A Review on Coordination Properties of Al (III) and Fe (III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights. Molecules 2021, 26, 2603.
- Rossberg, A.; Reich, T.; Bernhard, G. Complexation of uranium (VI) with protocatechuic acid—Application of iterative transformation factor analysis to EXAFS spectroscopy. Anal. Bioanal. Chem. 2003, 376, 631–638.
- Cornard, J.P.; Lapouge, C.; André, E. pH influence on the complexation site of Al (III) with protocatechuic acid. A spectroscopic and theoretical approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 108, 280–287.
- Kula, A. Thermal analysis of lanthanide (III) and Y(III) complexes with 4-hydroxy-3-methoxybenzoic acid. J. Therm. Anal. Calorim. 2005, 81, 381–385.
- Vulpius, D.; Geipel, G.; Baraniak, L.; Bernhard, G. Complex formation of neptunium (V) with 4-hydroxy-3-methoxybenzoic acid studied by time-resolved laser-induced fluorescence spectroscopy with ultra-short laser pulses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 603–608.
- Oke, I.M.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Makhafola, T.J.; Eze, K.C.; Noreljaleel, A.E.M.; Chukwuma, C.I. Vanillic acid–Zn (II) complex: A novel complex with antihyperglycaemic and anti-oxidative activity. J. Pharm. Pharmacol. 2021, 73, 1703–1714.
- Fazary, A.E.; Taha, M.; Ju, Y.H. Iron complexation studies of gallic acid. J. Chem. Eng. Data 2009, 54, 35–42.
- Taha, M.; Khan, I.; Coutinho, J.A. Complexation and molecular modeling studies of europium (III)–gallic acid–amino acid complexes. J. Inorg. Biochem. 2016, 157, 25–33.
- Motloung, D.M.; Mashele, S.S.; Matowane, G.R.; Swain, S.S.; Bonnet, S.L.; Noreljaleel, A.E.; Oyedemi, S.O.; Chukwuma, C.I. Synthesis, characterization, antidiabetic and antioxidative evaluation of a novel Zn (II)-gallic acid complex with multi-facet activity. J. Pharm. Pharmacol. 2020, 72, 1412–1426.
- Frešer, F.; Hostnik, G.; Tošović, J.; Bren, U. Dependence of the Fe (II)-Gallic Acid Coordination Compound Formation Constant on the pH. Foods 2021, 10, 2689.
- Iwan, M.; Kula, A.; Rzączyńska, Z.; Pikus, S.; Flisiuk, D.; Gomoła, M. Synthesis and properties of lanthanide (III) complexes with 4-hydroxy-3, 5-dimethoxybenzoic acid. Chem. Pap. 2007, 61, 376–382.
- Świsłocka, R. Experimental (FT-IR, FT-Raman, 1H, 13C NMR) and theoretical study of alkali metal syringates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 111, 290–298.
- Kumar, A.; Kumar, D.; Kumari, K.; Mkhize, Z.; Seru, L.K.; Bahadur, I.; Singh, P. Metal-ligand complex formation between ferrous or ferric ion with syringic acid and their anti-oxidant and anti-microbial activities: DFT and molecular docking approach. J. Mol. Liq. 2021, 322, 114872.
- Allan, J.R.; Carson, B.R.; Gerrard, D.L.; Hoey, S. Thermal, spectral and magnetic studies of some compounds of cobalt (II), nickel (II) and copper (II) with cinnamic acid. Thermochim. Acta 1989, 154, 315–322.
- Kalinovskaya, I.V.; Karasev, V.E.; Zadorozhnaya, A.N.; Lifar, L.I. Luminescence spectral properties of europium (III) and terbium (III) complexes with cinnamic acid. Russ. J. Coord. Chem. 2001, 27, 516–519.
- Ferrer, E.G.; Salinas, M.V.; Correa, M.J.; Vrdoljak, F.; Williams, P.A. ALP inhibitors: Vanadyl (IV) complexes of ferulic and cinnamic acid. Z. Naturforsch. B 2005, 60, 305–311.
- Kalinowska, M.; Świsłocka, R.; Lewandowski, W. The spectroscopic (FT-IR, FT-Raman and 1H, 13C NMR) and theoretical studies of cinnamic acid and alkali metal cinnamates. J. Mol. Struct. 2007, 834, 572–580.
- Kalinowska, M.; Lewandowski, W.; Świsłocka, R.; Regulska, E. The FT-IR, FT-Raman, 1H and 13C NMR study on molecular structure of sodium (I), calcium (II), lanthanum (III) and thorium (IV) cinnamates. Spectroscopy 2010, 24, 277–281.
- Kalinowska, M.; Świsłocka, R.; Lewandowski, W. Zn (II), Cd (II) and Hg (I) complexes of cinnamic acid: FT-IR, FT-Raman, 1H and 13C NMR studies. J. Mol. Struct. 2011, 993, 404–409.
- Graminha, A.E.; Honorato, J.; Dulcey, L.L.; Godoy, L.R.; Barbosa, M.F.; Cominetti, M.R.; Menezes, A.C.; Batista, A.A. Evaluation of the biological potential of ruthenium (II) complexes with cinnamic acid. J. Inorg. Biochem. 2020, 206, 111021.
- Chukwuma, C.I.; Mashele, S.S.; Swain, S.S. Antidiabetic and Antioxidative Properties of Novel Zn (II)-cinnamic Acid Complex. Med. Chem. 2021, 17, 913–925.
- Świsłocka, R.; Kowczyk-Sadowy, M.; Kalinowska, M.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H and 13C NMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates. Spectroscopy 2012, 27, 35–48.
- Koç, S.; Köse, D.A.; Avcı, E.; Köse, K. Synthesis and Thermal Characterization of p-Coumaric Acid Complexes of CoII, NiII, CuII and ZnII Metal Cations and Biological Applications. Hittite J. Sci. Eng. 2016, 3, 15–22.
- Khvan, A.M.; Kristallovich, E.L.; Abduazimov, K.A. Complexation of caffeic and ferulic acids by transition-metal ions. Chem. Nat. Compd. 2001, 37, 72–75.
- Fazary, A.E.; Ju, Y.H.; Al-Shihri, A.S.; Bani-Fwaz, M.Z.; Alfaifi, M.Y.; Alshehri, M.A.; Saleh, K.A.; Elbehairi, S.E.I.; Fawy, K.F.; Abd-Rabboh, H.S. Platinum and vanadate bioactive complexes of glycoside naringin and phenolates. Open Chem. 2017, 15, 189–199.
- Singh, K.; Kumar, A. Kinetics of complex formation of Fe (III) with caffeic acid: Experimental and theoretical study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 211, 148–153.
- Arciszewska, Ż.; Gama, S.; Kalinowska, M.; Świderski, G.; Świsłocka, R.; Gołębiewska, E.; Naumowicz, M.; Worobiczuk, M.; Cudowski, A.; Pietryczuk, A.; et al. Caffeic Acid/Eu (III) Complexes: Solution Equilibrium Studies, Structure Characterization and Biological Activity. Int. J. Mol. Sci. 2022, 23, 888.
- Rensing, C.; McDevitt, S.F. The Copper Metallome in Prokaryotic Cells. In Metallomics and the Cell. Metal Ions in Life Sciences; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 12, pp. 417–450. ISBN 978-94-007-5560-4.
- Kalinowska, M.; Piekut, J.; Bruss, A.; Follet, C.; Sienkiewicz-Gromiuk, J.; Świsłocka, R.; Rzączyńska, Z.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), Thermogravimetric and Antimicrobial Studies of Ca (II), Mn (II), Cu (II), Zn (II) and Cd (II) Complexes of Ferulic Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 122, 631–638.
- Kalinowska, M.; Gołębiewska, E.; Mazur, L.; Lewandowska, H.; Pruszyński, M.; Świderski, G.; Wyrwas, M.; Pawluczuk, N.; Lewandowski, W. Crystal structure, spectroscopic characterization, antioxidant and cytotoxic activity of new Mg (II) and Mn (II)/Na (I) complexes of isoferulic acid. Materials 2021, 14, 3236.
- Sengupta, P.; Pal, U.; Mondal, P.; Bose, A. Multi-spectroscopic and computational evaluation on the binding of sinapic acid and its Cu (II) complex with bovine serum albumin. Food Chem. 2019, 301, 125254.
- Naso, L.G.; Valcarcel, M.; Roura-Ferrer, M.; Kortazar, D.; Salado, C.; Lezama, L.; González-Baró, A.C.; Williams, P.A.M.; Ferrer, E.G. Promising antioxidant and anticancer (human breast cancer) oxidovanadium (IV) complex of chlorogenic acid. Synthesis, characterization and spectroscopic examination on the transport mechanism with bovine serum albumin. J. Inorg. Biochem. 2014, 135, 86–99.
- Kalinowska, M.; Bajko, E.; Matejczyk, M.; Kaczyński, P.; Łozowicka, B.; Lewandowski, W. The study of anti-/pro-oxidant, lipophilic, microbial and spectroscopic properties of new alkali metal salts of 5-o-caffeoylquinic acid. Int. J. Mol. Sci. 2018, 19, 463.
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn (II) complex of plant phenolic chlorogenic acid: Antioxidant, antimicrobial and structural studies. Materials 2020, 13, 3745.
- Palierse, E.; Przybylski, C.; Brouri, D.; Jolivalt, C.; Coradin, T. Interactions of Calcium with Chlorogenic and Rosmarinic Acids: An Experimental and Theoretical Approach. Int. J. Mol. Sci. 2020, 21, 4948.
- Yang, R.; Tian, J.; Liu, Y.; Zhu, L.; Sun, J.; Meng, D.; Wang, Z.; Wang, C.; Zhou, Z.; Chen, L. Interaction mechanism of ferritin protein with chlorogenic acid and iron ion: The structure, iron redox, and polymerization evaluation. Food Chem. 2021, 349, 129144.
- Świsłocka, R.; Regulska, E.; Karpińska, J.; Świderski, G.; Lewandowski, W. Molecular structure and antioxidant properties of alkali metal salts of rosmarinic acid. Experimental and DFT studies. Molecules 2019, 24, 2645.
- Kola, A.; Hecel, A.; Lamponi, S.; Valensin, D. Novel Perspective on Alzheimer’s Disease Treatment: Rosmarinic Acid Molecular Interplay with Copper (II) and Amyloid β. Life 2020, 10, 118.
- Świderski, G.; Jabłońska-Trypuć, A.; Kalinowska, M.; Świsłocka, R.; Karpowicz, D.; Magnuszewska, M.; Lewandowski, W. Spectroscopic, Theoretical and antioxidant study of 3D-transition metals (Co (II), Ni (II), Cu (II), Zn (II)) complexes with cichoric acid. Materials 2020, 13, 3102.
- Manolov, I.; Kostova, I.; Netzeva, T.; Konstantinov, S.; Karaivanova, M. Cytotoxic activity of cerium complexes with coumarin derivatives. Molecular modeling of the ligands. Arch. Pharm. 2000, 333, 93–98.
- Pi, J.; Zeng, J.; Luo, J.J.; Yang, P.H.; Cai, J.Y. Synthesis and biological evaluation of Germanium (IV)–polyphenol complexes as potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2902–2908.
- Sulpizio, C.; Müller, S.T.; Zhang, Q.; Brecker, L.; Rompel, A. Synthesis, characterization, and antioxidant activity of Zn2+ and Cu2+ coordinated polyhydroxychalcone complexes. Monatsh. Chem. 2016, 147, 1871–1881.
- Jin, G.; Zhao, Z.; Chakraborty, T.; Mandal, A.; Roy, A.; Roy, S.; Guo, Z. Decrypting the molecular mechanistic pathways delineating the chemotherapeutic potential of ruthenium-phloretin complex in colon carcinoma correlated with the oxidative status and increased apoptotic events. Oxid. Med.Cell. Longev. 2020, 2020, 7690845.
- Shubina, V.S.; Shatalina, Y.V. Absorption spectroscopy study of acid-base and metal-binding properties of flavanones. J. Appl. Spectrosc. 2013, 80, 761–766.
- Alexiou, A.D.; Decandio, C.C.; Almeida, S.D.N.; Ferreira, M.J.; Romoff, P.; Rocha, R.C. Metal-ligand coordination and antiradical activity of a trichromium (III) complex with the flavonoid naringenin. J. Coord. Chem. 2017, 70, 2148–2160.
- Restrepo-Guerrero, A.G.; Goitia-Semenco, H.; Naso, L.G.; Rey, M.; Gonzalez, P.J.; Ferrer, E.G.; Williams, P.A. Antioxidant and Anticancer Activities and Protein Interaction of the Oxidovanadium (IV) Naringin Complex. Inorganics 2022, 10, 13.
- Bijlsma, J.; de Bruijn, W.J.; Velikov, K.P.; Vincken, J.P. Unravelling discolouration caused by iron-flavonoid interactions: Complexation, oxidation, and formation of networks. Food Chem. 2022, 370, 131292.
- Shi, S.; Zhang, Y.; Chen, X.; Peng, M. Investigation of flavonoids bearing different substituents on ring C and their Cu2+ complex binding with bovine serum albumin: Structure–affinity relationship aspects. J. Agric. Food Chem. 2011, 59, 10761–10769.
- Lutoshkin, M.A.; Kuznetsov, B.N.; Levdansky, V.A. Spectrophotometric and quantum-chemical study of acid-base and complexing properties of (±)-taxifolin in aqueous solution. Heterocycl. Commun. 2017, 23, 395–400.
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132.
- Guo, Q.; Yuan, J.; Zeng, J.; He, X.; Li, D. Synthesis of dihydromyricetin–manganese (II) complex and interaction with DNA. J. Mol. Struct. 2012, 1027, 64–69.
- Yao, Y.; Zhang, M.; He, L.; Wang, Y.; Chen, S. Evaluation of General Synthesis Procedures for Bioflavonoid–Metal Complexes in Air-Saturated Alkaline Solutions. Front. Chem. 2020, 8, 589.
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886.
- Kuntić, V.S.; Malešev, D.L.; Radović, Z.V.; Kosanić, M.M.; Mioč, U.B.; Vukojević, V.B. Spectrophotometric Investigation of Uranil (II)− Rutin Complex in 70 Ethanol. J. Agric. Food Chem. 1998, 46, 5139–5142.
- Pyrzynska, K.; Pękal, A. Flavonoids as analytical reagents. Crit. Rev. Anal. Chem. 2011, 41, 335–345.
- Atabey-Ozdemir, B.; Demirkiran, O.; Yildiz, U.; Tekin, I.O.; Coban, B. Cytotoxicity and DNA binding of copper (II) and zinc (II) complexes of flavonoids: Quercitrin, myricitrin, rutin. Bulg. Chem. Commun. 2017, 49, 901–907.
- Catapano, M.C.; Tvrdý, V.; Karlíčková, J.; Migkos, T.; Valentová, K.; Křen, V.; Mladěnka, P. The stoichiometry of isoquercitrin complex with iron or copper is highly dependent on experimental conditions. Nutrients 2017, 9, 1193.
- Barbosa, V.T.; de Menezes, J.B.; Santos, J.C.C.; de Assis Bastos, M.L.; de Araújo-Júnior, J.X.; do Nascimento, T.G.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B. Characterization and stability of the antimony-quercetin complex. Adv. Pharm. Bull. 2019, 9, 432.
- Wongso, H. Natural product-based Radiopharmaceuticals: Focus on curcumin and its analogs, flavonoids, and marine peptides. J. Pharm. Anal. 2021, 12, 380–393.
- Sahyon, H.A.; Althobaiti, F.; Ramadan, A.E.M.M.; Fathy, A.M. Quercetin-Based Rhodium (III) Complex: Synthesis, Characterization and Diverse Biological Potentials. J. Mol. Struct. 2022, 1257, 132584.
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčková, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of iron and copper by quercetin B-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based Fenton chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937.
- Li, J.; Zhu, J.; Wu, H.; Li, W. Synthesis, in vitro, and in silico studies of fisetin and quercetin and their metal complexes as inhibitors of α-glucosidase and thrombin. J. Mol. Liq. 2022, 349, 118164.
- Cruz, M.A.; Tovani, C.B.; Favarin, B.Z.; Soares, M.P.; Fukada, S.Y.; Ciancaglini, P.; Ramos, A.P. Synthesis of Sr–morin complex and its in vitro response: Decrease in osteoclast differentiation while sustaining osteoblast mineralization ability. J. Mater. Chem. B 2019, 7, 823–829.
- Bodini, M.E.; Del Valle, M.A.; Tapia, R.; Leighton, F.; Berrios, P. Zinc catechin complexes in aprotic medium. Redox chemistry and interaction with superoxide radical anion. Polyhedron 2001, 20, 1005–1009.
- Hynes, M.J.; Coinceanainn, M.Ó. The kinetics and mechanisms of the reaction of iron (III) with gallic acid, gallic acid methyl ester and catechin. J. Inorg. Biochem. 2001, 85, 131–142.
- Inoue, M.B.; Inoue, M.; Fernando, Q.; Valcic, S.; Timmermann, B.N. Potentiometric and 1H NMR studies of complexation of Al3+ with (−)-epigallocatechin gallate, a major active constituent of green tea. J. Inorg. Biochem. 2002, 88, 7–13.
- Ansari, A.A.; Sharma, R.K. Synthesis and characterization of a biologically active lanthanum (III)–catechin complex and DNA binding spectroscopic studies. Spectrosc. Lett. 2009, 42, 178–185.
- Grzesik, M.; Namiesnik, J.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of ferrous catechin complexes. Free Radic. Biol. Med. 2018, 120, S111.
- Alasady, S.A.; Muhamad, Y.H.; Ahmed, R.S. Theoretical and Thermodynamics Studies of Complexes Formation between Natural Flavonoids and Hg (II) Ion. Syst. Rev. Pharm. 2020, 11, 2393–2404.
- Fathima, A.; Manikandamathavan, V.M.; Jonnalagadda, R.R.; Nair, B.U. Chromium-catechin complex, synthesis and toxicity check using bacterial models. Heliyon 2020, 6, e04563.
- Liu, L.; Xiao, X.; Li, K.; Li, X.; Shi, B.; Liao, X. Synthesis of Catechin-Rare Earth Complex with Efficient and Broad-Spectrum Anti-Biofilm Activity. Chem. Biodivers. 2020, 17, e1900734.
- Navarro, R.E.; Santacruz, H.; Inoue, M. Complexation of epigallocatechin gallate (a green tea extract, egcg) with Mn2+: Nuclear spin relaxation by the paramagnetic ion. J. Inorg. Biochem. 2005, 99, 584–588.
- Xie, W.; Guo, Z.; Zhao, L.; Wei, Y. Metal-phenolic networks: Facile assembled complexes for cancer theranostics. Theranostics 2021, 11, 6407.
- O’Coinceanainn, M.; Astill, C.; Baderschneider, B. Coordination of aluminium with purpurogallin and theaflavin digallate. J. Inorg. Biochem. 2003, 96, 463–468.
- O’Coinceanainn, M.; Bonnely, S.; Baderschneider, B.; Hynes, M.J. Reaction of iron (III) with theaflavin: Complexation and oxidative products. J. Inorg. Biochem. 2004, 98, 657–663.
- Naso, L.G.; Martínez, V.R.; Ferrer, E.G.; Williams, P.A. Antimetastatic effects of VOflavonoid complexes on A549 cell line. J. Trace Elem. Med. Biol. 2021, 64, 126690.
- Malacaria, L.; La Torre, C.; Furia, E.; Fazio, A.; Caroleo, M.C.; Cione, E.; Marino, T.; Plastina, P. Aluminum (III), iron (III) and copper (II) complexes of luteolin: Stability, antioxidant, and anti-inflammatory properties. J. Mol. Liq. 2022, 345, 117895.
- Frański, R. Influence of iron redox abilities on the electrospray ionization collision induced dissociation of iron complexes with methoxylated flavonoids. Int. J. Mass Spectrom. 2019, 446, 116216.
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098.
- Fedenko, V.S. Cyanidin complexation with metal ions. Ukr. Biochem. J. 2006, 78, 149–153.
- Khaodee, W.; Aeungmaitrepirom, W.; Tuntulani, T. Effectively simultaneous naked-eye detection of Cu (II), Pb (II), Al (III) and Fe (III) using cyanidin extracted from red cabbage as chelating agent. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 98–104.
- Ike, J.N.; Tyopine, A.A.; Okoye, C.O.B. Application of Cyanidin in Quantitative Estimation of Metals in Fish Samples. Am. J. Anal. Chem. 2019, 10, 621–628.
- Torrini, F.; Renai, L.; Scarano, S.; Del Bubba, M.; Palladino, P.; Minunni, M. Colorimetric selective quantification of anthocyanins with catechol/pyrogallol moiety in edible plants upon zinc complexation. Talanta 2022, 240, 123156.
- Mollaamin, F.; Mohammadian, N.T.; Najaflou, N.; Monajjemi, M. Iranian Qara Qat fruit (redcurrant) in Arasbaran forests as the resource of anthocyanin pigments in formation of chelation clusters. SN Appl. Sci. 2021, 3, 1–18.
- Tang, P.; Giusti, M.M. Metal chelates of petunidin derivatives exhibit enhanced color and stability. Foods 2020, 9, 1426.
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005, 513, 47–55.
- Nuñez-Selles, A.J.; Nuevas-Paz, L.; Martínez-Sánchez, G. Inhibition of Peroxidation Potential and Protein Oxidative Damage by Metal Mangiferin Complexes. Appl. Sci. 2022, 12, 2240.
- Dias, K.; Nikolaou, S. Does the combination of resveratrol with Al (III) and Zn (II) improve its antioxidant activity? Nat. Prod. Commun. 2011, 6, 1673–1676.
- Chiavarino, B.; Crestoni, M.E.; Fornarini, S.; Taioli, S.; Mancini, I.; Tosi, P. Infrared spectroscopy of copper-resveratrol complexes: A joint experimental and theoretical study. J. Chem. Phys. 2012, 137, 024307.
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112.
- Shakeri, A.; Panahi, Y.; Johnston, T.P.; Sahebkar, A. Biological properties of metal complexes of curcumin. BioFactors 2019, 45, 304–317.
- Vergara, V.B.; Kalinich, J.F. Nutraceuticals as Potential Radionuclide Decorporation Agents. Nutrients 2021, 13, 2545.
- Fucassi, F.; Heikal, A.; Mikhalovska, L.I.; Standen, G.; Allan, I.U.; Mikhalovsky, S.V.; Cragg, P.J. Metal chelation by a plant lignan, secoisolariciresinol diglucoside. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 345–351.
- Borsari, M.; Gabbi, C.; Ghelfi, F.; Grandi, R.; Saladini, M.; Severi, S.; Borella, F. Silybin, a new iron-chelating agent. J. Inorg. Biochem. 2001, 85, 123–129.
- Tvrdý, V.; Catapano, M.C.; Rawlik, T.; Karlíčková, J.; Biedermann, D.; Křen, V.; Mladěnka, P.; Valentová, K. Interaction of isolated silymarin flavonolignans with iron and copper. J. Inorg. Biochem. 2018, 189, 115–123.
- Vimalraj, S.; Rajalakshmi, S.; Saravanan, S.; Preeth, D.R.; Vasanthi, R.L.; Shairam, M.; Chatterjee, S. Synthesis and characterization of zinc-silibinin complexes: A potential bioactive compound with angiogenic, and antibacterial activity for bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 167, 134–143.
- Merdy, P.; Guillon, E.; Frapart, Y.M.; Aplincourt, M. Iron and manganese surface complex formation with extracted lignin. Part 2: Characterisation of magnetic interaction between transition metal and quinonic radical by EPR microwave power saturation experiments. New J. Chem. 2003, 27, 577–582.
- Zeng, X.; Du, Z.; Xu, Y.; Sheng, Z.; Jiang, W. Characterization of the interactions between apple condensed tannins and biologically important metal ions . LWT 2019, 114, 108384.
- Fu, Z.; Jiang, X.; Li, W.W.; Shi, Y.; Lai, S.; Zhuang, J.; Yao, S.; Liu, Y.; Hu, J.; Gao, L.; et al. Proanthocyanidin–aluminum complexes improve aluminum resistance and detoxification of Camellia sinensis. J. Agric. Food Chem. 2020, 68, 7861–7869.
- Tahara, K.; Hashida, K.; Otsuka, Y.; Ohara, S.; Kojima, K.; Shinohara, K. Identification of a hydrolyzable tannin, oenothein B, as an aluminum-detoxifying ligand in a highly aluminum-resistant tree, Eucalyptus camaldulensis. Plant Physiol. 2014, 164, 683–693.
- Przewloka, S.R.; Shearer, B.J. The further chemistry of ellagic acid. II. Ellagic acid and water-soluble ellagates as metal precipitants. Holzforschung 2002, 56, 13–19.
- Kraal, P.; Jansen, B.; Nierop, K.G.; Verstraten, J.M. Copper complexation by tannic acid in aqueous solution. Chemosphere 2006, 65, 2193–2198.
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering multifunctional capsules through the assembly of metal–phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551.
- Liu, T.; Zhang, M.; Liu, W.; Zeng, X.; Song, X.; Yang, X.; Zhang, X.; Feng, J. Metal ion/tannic acid assembly as a versatile photothermal platform in engineering multimodal nanotheranostics for advanced applications. ACS Nano 2018, 12, 3917–3927.
- Fu, Z.; Chen, R. Study of Complexes of Tannic Acid with Fe (III) and Fe (II). J. Anal. Methods Chem. 2019, 2019, 3894571.
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370.
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781.
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100.
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701.
- Nobahar, A.; Carlier, J.D.; Miguel, M.G.; Costa, M.C. A review of plant metabolites with metal interaction capacity: A green approach for industrial applications. BioMetals 2021, 34, 761–793.
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982.
- Yoshida, K.; Mihoko, M.; Kondo, T. Blue flower color development byanthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 857–964.
- Iwashina, T. Contribution to flower colors of flavonoids including anthocyanins: A review. Nat. Prod. Commun. 2015, 10, 529–544.
- Trunschke, J.; Lunau, K.; Pyke, G.H.; Ren, Z.X.; Wang, H. Flower color evolution and the evidence of pollinator-mediated selection. Front. Plant Sci. 2021, 12, 617851.
- Fedenko, V.S.; Shemet, S.A.; Struzhko, V.S. Complexation of cyanidin with cadmium ions in solution. Ukr. Biochem. J. 2005, 77, 104–109.
- Fedenko, V.S. Dose effect of cyanidin interaction with lead ions in roots of maize seedlings. Ukr. Biochem. J. 2007, 79, 24–29.
- Hale, K.L.; McGrath, S.P.; Lombi, E.; Stack, S.M.; Terry, N.; Pickering, I.J.; George, G.N.; Pilon-Smits, E.A.H. Molybdenum sequestration in Brassica: A role for anthocyanins? Plant Physiol. 2001, 126, 1391–1402.
- Hale, K.L.; Tufan, H.A.; Pickering, I.J.; George, G.N.; Terry, N.; Pilon, M.; Pilon-Smits, E.A.H. Anthocyanins facilitate tungsten accumulation in Brassica. Physiol. Plant. 2002, 116, 351–358.
- Stoutjesdijk, P.A.; Sale, P.W.; Larkin, P.J. Possible involvement of condensed tannins in aluminium tolerance of Lotus pedunculatus. Funct. Plant Biol. 2001, 28, 1063–1074.
- Lavid, N.; Schwartz, A.; Yarden, O.; Tel-Or, E. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 2001, 212, 323–331.
- Rocha, J.E.; Guedes, T.T.; Bezerra, C.F.; Costa, M.D.S.; Campina, F.F.; de Freitas, T.S.; Souza, A.K.; Souza, C.E.S.; de Matos, Y.M.L.S.; Pereira-Junior, F.N.; et al. Identification of the gallic acid mechanism of action on mercury chloride toxicity reduction using infrared spectroscopy and antioxidant assays. Int. Biodeterior. Biodegrad. 2019, 141, 24–29.
- Wojcieszek, J.; Ruzik, L. Enzymatic extraction of copper complexes with phenolic compounds from Açaí (Euterpe oleracea Mart.) and bilberry (Vaccinium myrtillus L.) fruits. Food Anal. Methods 2016, 9, 2105–2114.
- Kidd, P.S.; Llugany, M.; Poschenrieder, C.H.; Gunse, B.; Barcelo, J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J. Exp. Bot. 2001, 52, 1339–1352.
- Tsai, H.H.; Schmidt, W. Mobilization of iron by plant-borne coumarins. Trends Plant Sci. 2017, 22, 538–548.
- Tsai, H.H.; Rodríguez-Celma, J.; Lan, P.; Wu, Y.C.; Vélez-Bermúdez, I.C.; Schmidt, W. Scopoletin 8-hydroxylase-mediated fraxetin production is crucial for iron mobilization. Plant Physiol. 2018, 177, 194–207.
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
03 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





