Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlo Alberto Schena | -- | 3120 | 2023-02-02 09:53:00 | | | |
| 2 | Rita Xu | Meta information modification | 3120 | 2023-02-02 10:29:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Schena, C.A.; De’angelis, G.L.; Carra, M.C.; Bianchi, G.; De’angelis, N. Infections in Acute Care Surgery. Encyclopedia. Available online: https://encyclopedia.pub/entry/40760 (accessed on 07 February 2026).
Schena CA, De’angelis GL, Carra MC, Bianchi G, De’angelis N. Infections in Acute Care Surgery. Encyclopedia. Available at: https://encyclopedia.pub/entry/40760. Accessed February 07, 2026.
Schena, Carlo Alberto, Gian Luigi De’angelis, Maria Clotilde Carra, Giorgio Bianchi, Nicola De’angelis. "Infections in Acute Care Surgery" Encyclopedia, https://encyclopedia.pub/entry/40760 (accessed February 07, 2026).
Schena, C.A., De’angelis, G.L., Carra, M.C., Bianchi, G., & De’angelis, N. (2023, February 02). Infections in Acute Care Surgery. In Encyclopedia. https://encyclopedia.pub/entry/40760
Schena, Carlo Alberto, et al. "Infections in Acute Care Surgery." Encyclopedia. Web. 02 February, 2023.
Copy Citation
The burden of infections in acute care surgery (ACS) is huge. Surgical emergencies alone account for three million admissions per year in the United States (US) with estimated financial costs of USD 28 billion per year. Acute care facilities and ACS patients represent boost sanctuaries for the emergence, development and transmission of infections and multi-resistant organisms.
infections
acute care surgery
antimicrobial resistance
source control
1. Introduction
Acute care surgery (ACS) is traditionally represented by a triad composed by trauma, emergency general surgery, and surgical critical care [1]. It was conceived with the goal of combining skills from trauma surgeons, emergency surgeons, and intensivists, into a single multifaceted and comprehensive discipline. Later, both elective general surgery and surgical rescue were proposed as two additional pillars of ACS [2].
The epidemiological impact of ACS is important because of the cumulative amount of emergency and trauma surgery related morbi-mortality. The burden of surgical emergencies is high; of the three million emergency admissions per year in the United States (US) approximately 30% requires surgery [3], with 896,000 deaths reported in 2010 [4]. Moreover, trauma constitutes the first cause of death in patients under 44 years of age and the fourth cause in the elderly [5]. The estimated financial burden of emergency surgery in US is USD 28 billion every year and is expected to increase up to USD 41 billion by 2060 [6][7]. Despite clinical and financial improvements after ACS model implementation and diffusion [8], ACS patients continue to represent a high-risk population experiencing poorer outcomes. Indeed, up to one-third of ACS patients present with multiple comorbidities and frailty. Emergency surgery is associated with higher rates of mortality, postoperative complications, hospital readmissions, and costs, when compared to corresponding elective procedures [9][10][11][12]. The incidence of healthcare-associated infections (HAIs) is doubled in patients undergoing surgical procedures [13]. In this complex scenario, intra-abdominal and healthcare-associated infections have a great impact, taking into account the critical conditions leading to ACS and the scarce physiologic reserve of these patients. Thus, a close cooperation between the acute care surgeon and the healthcare system is necessary to put in place a comprehensive evidence-based management of infections in the ACS setting, prevent adverse outcomes, and optimize treatment efficacy.
2. The Management of Infections in Acute Care Surgery
The global public health challenge driven by infections in ACS refers either to patient morbidity, mortality, and quality of life, and further to financial and economic reverberations. In this perspective, the impact of HAIs and IAIs is wide.
HAIs are infections developed 48 hours or more after hospital or other healthcare facility admission, or within 30 days after having received health concerns [14]. HAIs encompass surgical site infections (SSI), catheter-associated urinary tract infections, central line-associated bloodstream infections, Clostridium difficile infections, and ventilator-associated pneumonia, among many others. Acute care facilities and ACS populations represent boost sanctuaries for emergence, development, and transmission of infections and multi-resistant organisms. The use of medical devices, such as ventilators, central venous catheters, intra-abdominal drains, and urinary catheters, but also the surgery itself, should be considered as significant risk factors for HAI acquisition. Despite the considerable predictability of HAIs, their epidemiological weight is significant. In 2011, the World Health Organization (WHO) reported that HAIs affect 7% of hospitalized patients in developed and 10% in developing countries, leading to a burden of 4 million cases in Europe and 1.7 million in US with 39,000 and 99,000 directly attributable deaths, respectively [15]. SSIs are the most common HAI in surgical units with a major impact in terms of morbidity, mortality, length of hospital stay, and costs [16]. In a multicentre and international cohort study, SSIs were identified in 12.3% of patients within 30 days after gastrointestinal surgery and disease incidence fluctuated significantly between countries with high, middle, and low-income (9.4% vs. 14.0% vs. 23.2%, respectively, p < 0.001) [17]. Additionally, Magill et al. highlighted that AMR patterns were detected in up to 60% of the microbes isolated from infected surgical sites [18].
IAIs are common surgical emergencies and represent an important cause of morbidity and mortality worldwide. In the modern era, the IAIs-related mortality has dropped sharply compared to the values higher than 50% reported in the middle of the last century [19]. Indeed, Sartelli et al. reported an overall 9.2% mortality rate in a multicenter cohort of 4553 patients with complex intra-abdominal infections over 4 months [20]. Moreover, the presence of severe sepsis considerably increased IAI mortality rate [21][22][23]. A multicentre observational study on ICU patients with a confirmed IAI diagnosis attested that 68% of IAIs were hospital-acquired, with an overall AMR prevalence of 26.3%, and overall mortality of 29.1% directly related to infection severity [24]. IAIs include appendicitis, bowel and colorectal perforations, cholecystitis, diverticulitis, gastroduodenal perforations, bowel and colorectal ischemia, necrotizing pancreatitis, and many other diseases. Despite the wide spectrum of conditions, IAIs are usually classified into uncomplicated and complicated. Uncomplicated IAIs refer to infections limited to a hollow viscus, whereas complicated IAIs presuppose the involvement of a naturally sterile area of the abdomen, such as the peritoneal cavity, other abdominal organs, abdominal wall, mesentery, and retroperitoneum [25]. Differently, the US Food and Drug Administration defined the latter as disorders requiring SC procedures, with the aim to better clarify the definition and identification of complicated IAIs. In principle, uncomplicated IAIs can be handled with either surgical SC or with antibiotics alone, while complicated IAIs require both SC and antibiotic therapy [26]. For instance, complicated IAIs may include secondary or tertiary peritonitis, intra-abdominal abscesses, or intra-abdominal phlegmons.
Worldwide, hospital and patient cares require comprehensive and standardized policies, procedures, and strategies, to minimize the impact of HAIs and AMR, and to optimize IAI treatment. AS, IPC, and SC, are the three synergistic cornerstones of the multidisciplinary management of infections in acute care facilities (Figure 1).
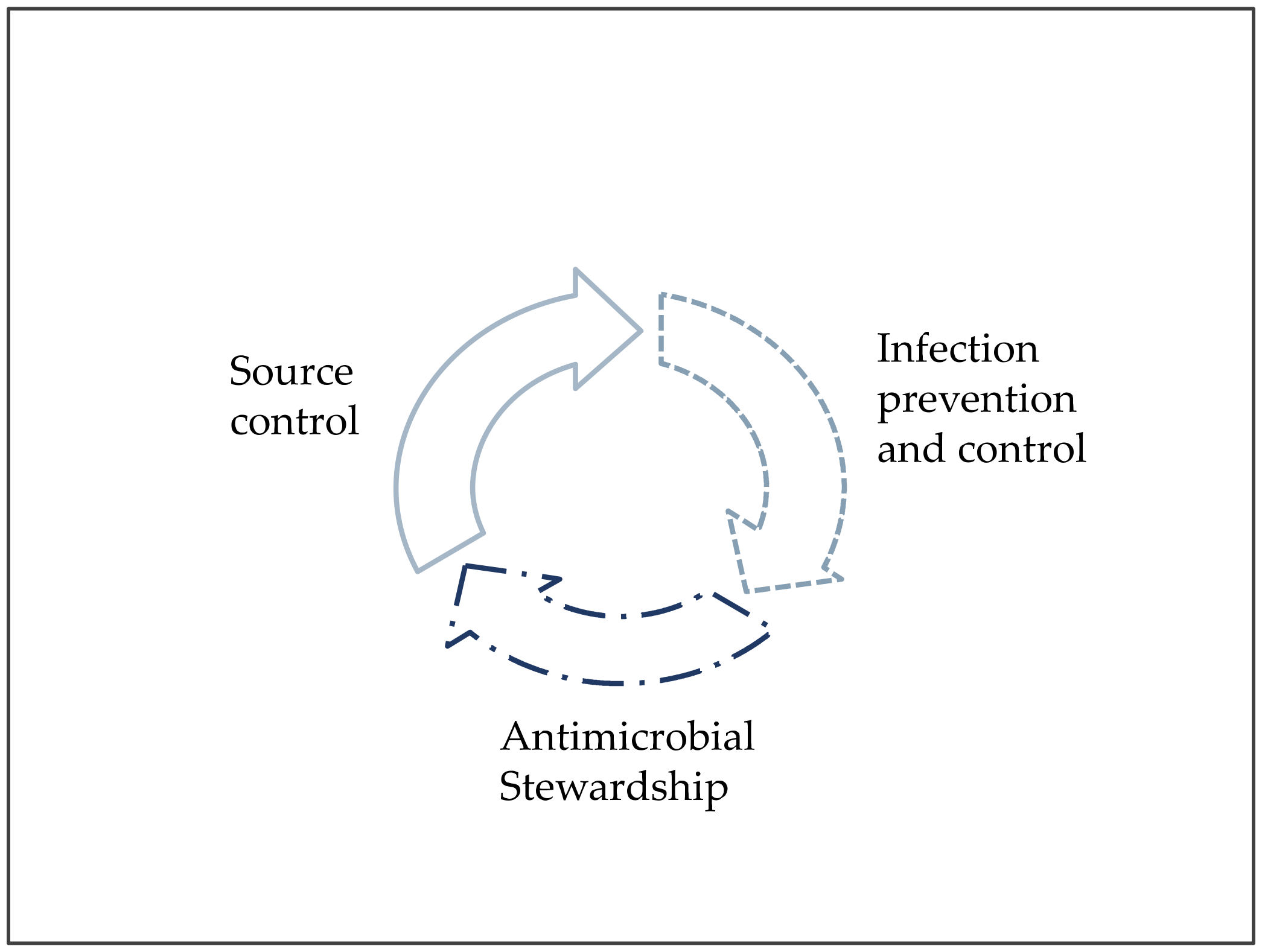
Figure 1. The three pillars of patient care against infections and antimicrobial resistance.
2.1. Source Control
SC in ACS refers to any intervention necessary to identify and eliminate the sources of infection and finally to restore normal physiological balance [27]. Combined with targeted antibiotic therapy, it represents one of the fundamentals of IAI care [26]. Delayed and partial interventions may significantly worsen SC outcomes in IAI patients; thus, prompt and goal-directed tactics are pivotal concepts to achieve the maximal efficiency of SC [19]. Sartelli et al. recently defined the four rules of an ideal SC [26]:
-
(First) Time: start as soon as possible;
-
Totalization: remove any infective source;
-
Technique: use adequate techniques;
-
(Second) Time: avoid clinical deterioration through required or planned successive procedures.
The first cornerstone is thereby the control of foci of infection within the shortest delay possible, especially in critically ill patients [28]. Elsewise, the optimal timing of IAI SC has long been debated [29]. Azuhata et al. showed the negative impact of each hour delay on survival in patients with gastrointestinal perforation and septic shock [30]. The 2017 Surgical Infection Society Revised Guidelines indicated that SC interventions must be undertaken within 24 hours from the diagnosis of IAI, except in the case of clinical evidence supporting non-interventional or delayed approach, as appropriate (strength of the recommendations: Grade 2-B), and in the case of sepsis or septic shock always seeking immediate treatment (strength of the recommendations: Grade 2-C) [25]. The same suggestion emerged from a comprehensive European review [31] and an international multi-society document on IAI [26]. According to the Surviving Sepsis Campaign guidelines, any specific anatomic diagnosis of infection requiring emergent SC should be rapidly identified or excluded and any essential SC intervention should be implemented [32].
The second cornerstone is the completeness of SC. Several studies highlighted the association between incomplete interventions and severe adverse outcomes [29][33][34][35]. Indeed, the persistence of infected fluid or contaminated tissue may nullify the benefits of resuscitation and antimicrobial therapy and prevent the physiological recovery. Van de Groep et al., investigating on a large cohort of critically ill patients with IAI, reported approximately 50% of infection persistence or recurrence after the first SC with a median of three procedures per patient and 67% SC adequateness on day 14 [36]. SC failure may occur in more than one-in-five patients [25] and in this scenario a prompt abdominal re-exploration should strongly be considered [19][26].
The selection of the optimal SC intervention should consider the risk-benefit ratio of the procedure, the site and severity of the infection, the patient’s fitness and health state, the medical expertise, and the surgical, interventional, or diagnostic staff availability. SC encompasses many procedures, namely drainage of abscess or infected collection, debridement of infected or necrotic tissue, removal of an infected device, and definitive control of the microbial source (e.g., resection of infected organs–appendicitis, cholecystitis, bowel ischemia – and suture or resection of perforated viscus–gastric and duodenal perforation, perforated diverticulitis). The international WISS study pointed out the source of infection in 4553 patients from 132 hospitals over a 4 month period, as follows: 34.2% appendicitis, 18.5% cholecystitis, 11% gastroduodenal perforations, 8.5% postoperative, 5.9% non-diverticular colonic perforation, 5.4% small bowel perforation, 5.2% diverticulitis, 2.5% post-traumatic perforation, 1.1% pelvic inflammatory disease, and 7.7% other [20].
Techniques to achieve SC encompass primary and alternative interventions which should be selected according to severity and stage of the disease, patients’ characteristics, and hospital resource availability (Table 1).
Table 1. Source control strategies for most common intra-abdominal infection scenarios.
| Primary Intervention | Alternative Intervention |
|---|---|
| Acute appendicitis [28][37][38][39] | |
| Appendectomy via laparoscopy is the preferred approach for both uncomplicated and complicated (perforation and peri-appendiceal abscess) acute appendicitis |
|
| Acute cholecystitis [40][41][42] | |
|
|
| Gastroduodenal perforation [43] | |
|
Non-operative management could be considered in extremely selected cases with sealed perforation confirmed on water-soluble contrast imaging. It consists of the association of absolute fasting + antibiotics + decompression via nasogastric tube + proton pump inhibitor therapy and requires a follow-up endoscopy at 4–6 weeks. |
| Postoperative peritonitis [26] | |
| Prompt surgical management is mandatory in case of diffuse peritonitis | Antibiotics + percutaneous drainage should be considered for localized intra-abdominal abscesses in stable patients with no signs of generalized peritonitis |
| Small bowel perforation [26] | |
|
In case of small bowel ischemia, resection and delayed anastomoses should be considered as alternative |
| Diverticulitis [44][45][46] | |
|
|
| Esophageal perforation [47] | |
| Antibiotics + absolute fasting + proton pump inhibitor therapy can be indicated in stable patients with early onset, minimal esophageal damage, and contained contamination, if highly specialized surveillance is guaranteed | Surgery should be advised in absence of non-operative management criteria (e.g., hemodynamic instability) |
| Acute cholangitis [26] | |
| Antibiotics + biliary drainage via endoscopic retrograde cholangiopancreatography (treatment of choice) or percutaneous biliary drainage (in case of ERCP * failure) are the first-line approach | Open drainage should be considered only in case of failure or contraindication to endoscopic or percutaneous interventions |
The primary goal of SC interventions is to identify the origin of IAI and to control the cause of abdominal sepsis. Peritoneal fluid cultures should be reached during SC procedures with the purpose of guiding antimicrobial therapy on the basis of antibiogram. Recent evidence reported the growing identification of multidrug-resistant microbes in IAI, thereby AMR may represent a determining factor for SC failure [48][49][50]. In the setting of complicated IAIs, a short-course antimicrobial therapy following an adequate SC intervention is a proper strategy for complicated IAIs, while it is not required for uncomplicated IAIs, such as uncomplicated cholecystitis or appendicitis [19][51][52][53][54]. The empiric antimicrobial therapy for complicated IAIs should be started as soon as possible and drug choice should be based on local ecology and AMR data, preferring antimicrobials with a spectrum of action against Enterobacteriaceae, enteric streptococci and obligate enteric anaerobes [19][25][26][32][33][55]:
-
Third-generation cephalosporines (e.g., cefotaxime, ceftriaxone, ceftazidime) in combination with metronidazole are active against Enterobacteriaceae and may be considered for uncomplicated IAIs [60][63]. Recently, two fifth-generation cephalosporines, namely ceftolozane/tazobactam and ceftazidime/avibactam, have been approved as treatments for complicated IAIs in combination with metronidazole, given to their action against several multidrug resistant bacteria [64][65][66];
Empirical antimycotic therapy may be appropriate in selected conditions (hospital-acquired IAIs, immunosuppressive state, critical illness) [19][70].
According to the Surgical Infection Society, microbiologic data and antibiogram can be used to reassess and optimize antimicrobial therapy [25]. Furthermore, the adequateness and appropriateness of antimicrobial treatment should be re-thought daily [71]. Concerning surgical SC, the usefulness of a prophylactic drain placement after digestive surgery has long been debated [72][73][74]. Intra-abdominal drains paradoxically may promote surgical site infections [75] and increase hospital length of stay and costs [76][77] in several emergency scenarios, namely acute appendectomies, or cholecystectomies. De Waele et al. recommended a limited duration of abdominal drains in the treatment of abdominal sepsis, a prompt drain removal once the source has been controlled, and to avoid culturing drains upon removal [78].
In principle, less invasive and more effective procedure should be chosen to reach SC [25]. During the last decades, minimally invasive surgery has gained wider acceptance in the treatment of IAI and abdominal sepsis ensuring concomitant advantages of diagnostic and operative tools with better post-operative outcomes (e.g., decreased postoperative infections and pain, shorter hospital stay and earlier recovery of physiological functions). Indeed, several authors recognized a laparoscopic and robotic approach as feasible, effective, and safe techniques for many emergency conditions [79][80][81][82]. Despite the clear advantages, minimally-invasive surgery in acute care setting requires adequate technical skills and specialized training.
2.2. Infection Prevention and Control
Infection prevention and control (IPC) is a pragmatic, evidence-based approach, which primary goal is preventing patients and health workers acquisition of HAIs, reducing the spread of HAIs within health care facilities, and weakening AMR [83]. For this purpose, the WHO issued several recommendations, defined as core components [83], and their respective minimum requirements [84] to ensure the effectiveness of IPC programs at national and facility level. The implementation of IPC core components should be faced using a stepwise approach [85], according to the following five steps:
-
Preparing for action;
-
Baseline assessment;
-
Developing and executing an action plan;
-
Assessing impact;
-
Sustaining the program over the long term.
IPC is a multidisciplinary strategy that involves all levels of the health system and different hospital professionals. According to the systematic review by Zingg et al., an adequate IPC programme in an acute care hospital must include as a minimum standard at least one dedicated and specialized infection-control nurse, a dedicated physician trained in IPC, and microbiological and data management support [86]. A recent worldwide cross-sectional survey on this topic revealed that a multidisciplinary IPC team composed by a median of six professionals (including most frequently microbiologists 72.4%, infectious diseases specialists 70.2%, nurses 68.4%, pharmacologists 67.6%, and surgeons 56.7%) was present in about 90% of participating hospital [87].
The effective implementation of infection control programs requires the engagement of well-designed organizational and structural models: specifically, ward occupancy have not to exceed its planned capacity; health-care workload must be adapted accordingly; and dedicated full-time nurses and physicians should be preferred over pool or agency professionals [86]. Indeed, several studies showed the association between HAIs and bed occupancy, high workload, and pool or agency professionals [88][89][90][91][92]. Furthermore, the usability of IPC programs is closely related to the development of multimodal strategies for team education and training, allowing the transposition of IPC pivotal fundamentals into documents and protocols based on local context and facilitating the good daily practice through surveillance, auditing, and personal feedback [86].
SSIs account for most HAIs among surgical patients. Additionally, SSIs are associated with longer hospitalization, higher costs, morbidity, and mortality [16], thus SSI prevention has a crucial role in all surgical departments worldwide. During the last years, the WHO [93][94][95], the US CDC [96] and the National Institute for Health and Care Excellence (NICE) [97], published evidence-based guidelines for the prevention of SSIs, dispensing recommendations for both preoperative, intraoperative, and postoperative surgical phase. The common goal of all these health institutions is to standardize patient care through the identification of the best practice, so promoting a favorable fallout on morbidity, mortality, and costs. The most recent WHO strong recommendations are reported in Table 2.
Table 2. WHO strong recommendations for the prevention of surgical site infection *.
| Core Topics |
|---|
| Perioperative intranasal applications of mupirocin 2% ointment with or without a combination of chlorhexidine gluconate body wash is indicated in patients undergoing cardiothoracic and orthopedic surgery with known nasal carriage of Staphylococcus aureus |
| Surgical antibiotic prophylaxis should be administered within 120 minutes before surgical incision, even though considering antibiotic half-life |
| Mechanical bowel preparation should not be used for the prevention of SSI in elective colorectal surgery if not associated with preoperative oral antibiotics |
| Hair removal and shaving is strongly discouraged in any surgical procedure. If inevitable, hair should be removed only with a clipper |
| Alcohol-based antiseptic solutions based on chlorhexidine gluconate are recommended for surgical site skin preparation before surgery |
| Surgical hand preparation should be performed by scrubbing with either a suitable antimicrobial soap and water or using appropriate alcohol-based solutions before wearing sterile gloves |
| Prolongation of surgical antibiotic prophylaxis after the surgical procedure is not indicated for the purpose of preventing SSI |
References
- Committee to Develop the Reorganized Specialty of Trauma and Emergency Surgery. Acute care surgery: Trauma, critical care, and emergency surgery. J. Trauma 2005, 58, 614–616.
- Kutcher, M.E.; Sperry, J.L.; Rosengart, M.R.; Mohan, D.; Hoffman, M.K.; Neal, M.D.; Alarcon, L.H.; Watson, G.A.; Puyana, J.C.; Bauzá, G.M.; et al. Surgical rescue: The next pillar of acute care surgery. J. Trauma Acute Care Surg. 2017, 82, 280–286.
- Kutcher, M.E.; Sperry, J.L.; Rosengart, M.R.; Mohan, D.; Hoffman, M.K.; Neal, M.D.; Alarcon, L.H.; Watson, G.A.; Puyana, J.C.; Bauzá, G.M.; et al. Technical Evidence Review for Emergency Major Abdominal Operation Conducted for the AHRQ Safety Program for Improving Surgical Care and Recovery. J Am. Coll. Surg. 2020, 231, 743–764.
- Stewart, B.W.K.P.; Khanduri, P.; McCord, C.; Ohene-Yeboah, M.; Uranues, S.; Vega Rivera, F.; Mock, C. Global disease burden of conditions requiring emergency surgery. Br. J. Surg. 2014, 101, e9–e22.
- Frydrych, L.M.; Keeney-Bonthrone, T.P.; Gwinn, E.; Wakam, G.K.; Anderson, M.S.; Delano, M.J. Short-term versus long-term trauma mortality: A systematic review. J. Trauma Acute Care Surg. 2019, 87, 990–997.
- Narayan, M.; Tesoriero, R.; Bruns, B.R.; Klyushnenkova, E.N.; Chen, H.; Diaz, J.J. Acute Care Surgery: Defining the Economic Burden of Emergency General Surgery. J. Am. Coll. Surg. 2016, 222, 691–699.
- Ogola, G.O.; Gale, S.C.; Haider, A.; Shafi, S. The financial burden of emergency general surgery: National estimates 2010 to 2060. J. Trauma Acute Care Surg. 2015, 79, 444–448.
- Chana, P.; Burns, E.M.; Arora, S.; Darzi, A.W.; Faiz, O.D. A Systematic Review of the Impact of Dedicated Emergency Surgical Services on Patient Outcomes. Ann. Surg. 2016, 263, 20–27.
- Havens, J.M.; Peetz, A.B.; Do, W.S.; Cooper, Z.; Kelly, E.; Askari, R.; Reznor, G.; Salim, A. The excess morbidity and mortality of emergency general surgery. J. Trauma Acute Care Surg. 2015, 78, 306–311.
- Scott, J.W.; Olufajo, O.A.; Brat, G.A.; Rose, J.A.; Zogg, C.K.; Haider, A.H.; Salim, A.; Havens, J.M. Use of National Burden to Define Operative Emergency General Surgery. JAMA Surg. 2016, 151, e160480.
- Vergis, A.; Metcalfe, J.; Stogryn, S.E.; Clouston, K.; Hardy, K. Impact of acute care surgery on timeliness of care and patient outcomes: A systematic review of the literature. Can. J. Surg. 2019, 62, 281–288.
- Ball, C.G.; Murphy, P.; Verhoeff, K.; Albusadi, O.; Patterson, M.; Widder, S.; Hameed, S.M.; Parry, N.; Vogt, K.; Kortbeek, J.B.; et al. A 30-day prospective audit of all inpatient complications following acute care surgery: How well do we really perform? Can. J. Surg. 2020, 63, E150–E154.
- Sax, H.; Uçkay, I.; Balmelli, C.; Bernasconi, E.; Boubaker, K.; Mühlemann, K.; Ruef, C.; Troillet, N.; Widmer, A.; Zanetti, G.; et al. Overall burden of healthcare-associated infections among surgical patients. Results of a national study. Ann. Surg. 2011, 253, 365–370.
- Boev, C.; Kiss, E. Hospital-Acquired Infections: Current Trends and Prevention. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65.
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide; World Health Organization: Geneva, Switzerland, 2011.
- Leaper, D.J.; Van Goor, H.; Reilly, J.; Petrosillo, N.; Geiss, H.K.; Torres, A.J.; Berger, A. Surgical site infection—A European perspective of incidence and economic burden. Int. Wound J. 2004, 1, 247–273.
- GlobalSurg, C. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: A prospective, international, multicentre cohort study. Lancet Infect. Dis. 2018, 18, 516–525.
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208.
- Sartelli, M.; Chichom-Mefire, A.; Labricciosa, F.M.; Hardcastle, T.; Abu-Zidan, F.M.; Adesunkanmi, A.K.; Ansaloni, L.; Bala, M.; Balogh, Z.J.; Beltran, M.A.; et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J. Emerg. Surg. 2017, 12, 29.
- Sartelli, M.; Abu-Zidan, F.M.; Catena, F.; Griffiths, E.A.; Di Saverio, S.; Coimbra, R.; Ordoñez, C.A.; Leppaniemi, A.; Fraga, G.P.; Coccolini, F.; et al. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: A prospective multicentre study (WISS Study). World J. Emerg. Surg. 2015, 10, 61.
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310.
- Wacha, H.; Hau, T.; Dittmer, R.; Ohmann, C. Risk factors associated with intraabdominal infections: A prospective multicenter study. Peritonitis Study Group. Langenbecks Arch. Surg. 1999, 384, 24–32.
- Mulier, S.; Penninckx, F.; Verwaest, C.; Filez, L.; Aerts, R.; Fieuws, S.; Lauwers, P. Factors affecting mortality in generalized postoperative peritonitis: Multivariate analysis in 96 patients. World J. Surg. 2003, 27, 379–384.
- Blot, S.; Antonelli, M.; Arvaniti, K.; Blot, K.; Creagh-Brown, B.; De Lange, D.; De Waele, J.; Deschepper, M.; Dikmen, Y.; Dimopoulos, G.; et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: AbSeS, a multinational observational cohort study and ESICM Trials Group Project. Intensive Care Med. 2019, 45, 1703–1717.
- Mazuski, J.E.; Tessier, J.M.; May, A.K.; Sawyer, R.G.; Nadler, E.P.; Rosengart, M.R.; Chang, P.K.; O’Neill, P.J.; Mollen, K.P.; Huston, J.M.; et al. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg. Infect. 2017, 18, 1–76.
- Sartelli, M.; Coccolini, F.; Kluger, Y.; Agastra, E.; Abu-Zidan, F.M.; Abbas, A.E.S.; Ansaloni, L.; Adesunkanmi, A.K.; Atanasov, B.; Augustin, G.; et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J. Emerg. Surg. 2021, 16, 49.
- Marshall, J.C.; al Naqbi, A. Principles of source control in the management of sepsis. Crit. Care Clin. 2009, 25, 753–768.
- De Almeida Leite, R.M.; Seo, D.J.; Gomez-Eslava, B.; Hossain, S.; Lesegretain, A.; de Souza, A.V.; Bay, C.P.; Zilberstein, B.; Marchi, E.; Machado, R.B.; et al. Nonoperative vs Operative Management of Uncomplicated Acute Appendicitis: A Systematic Review and Meta-analysis. JAMA Surg. 2022, 157, 828–834.
- Tellor, B.; Skrupky, L.P.; Symons, W.; High, E.; Micek, S.T.; Mazuski, J.E. Inadequate Source Control and Inappropriate Antibiotics are Key Determinants of Mortality in Patients with Intra-Abdominal Sepsis and Associated Bacteremia. Surg. Infect. 2015, 16, 785–793.
- Azuhata, T.; Kinoshita, K.; Kawano, D.; Komatsu, T.; Sakurai, A.; Chiba, Y.; Tanjho, K. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit. Care 2014, 18, R87.
- Sartelli, M.; Catena, F.; Ansaloni, L.; Leppaniemi, A.; Taviloglu, K.; van Goor, H.; Viale, P.; Lazzareschi, D.V.; Coccolini, F.; Corbella, D.; et al. Complicated intra-abdominal infections in Europe: A comprehensive review of the CIAO study. World J. Emerg. Surg. 2012, 7, 36.
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143.
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg. Infect. 2010, 11, 79–109.
- Bloos, F.; Thomas-Rüddel, D.; Rüddel, H.; Engel, C.; Schwarzkopf, D.; Marshall, J.C.; Harbarth, S.; Simon, P.; Riessen, R.; Keh, D.; et al. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: A prospective observational multi-center study. Crit. Care 2014, 18, R42.
- Launey, Y.; Duteurtre, B.; Larmet, R.; Nesseler, N.; Tawa, A.; Mallédant, Y.; Seguin, P. Risk factors for mortality in postoperative peritonitis in critically ill patients. World J. Crit. Care Med. 2017, 6, 48–55.
- Van de Groep, K.; Verhoeff, T.L.; Verboom, D.M.; Bos, L.D.; Schultz, M.J.; Bonten, M.J.; Cremer, O.L. Epidemiology and outcomes of source control procedures in critically ill patients with intra-abdominal infection. J. Crit. Care 2019, 52, 258–264.
- Di Saverio, S.; Birindelli, A.; Kelly, M.D.; Catena, F.; Weber, D.G.; Sartelli, M.; Sugrue, M.; De Moya, M.; Gomes, C.A.; Bhangu, A.; et al. WSES Jerusalem guidelines for diagnosis and treatment of acute appendicitis. World J. Emerg. Surg. 2016, 11, 34.
- Di Saverio, S.; Podda, M.; De Simone, B.; Ceresoli, M.; Augustin, G.; Gori, A.; Boermeester, M.; Sartelli, M.; Coccolini, F.; Tarasconi, A.; et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J. Emerg. Surg. 2020, 15, 27.
- Rushing, A.; Bugaev, N.; Jones, C.; Como, J.J.; Fox, N.; Cripps, M.; Robinson, B.; Velopulos, C.; Haut, E.R.; Narayan, M. Management of acute appendicitis in adults: A practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2019, 87, 214–224.
- Mayumi, T.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Solomkin, J.S.; Schlossberg, D.; Pitt, H.A.; Yoshida, M.; Gomi, H.; Miura, F.; et al. Tokyo Guidelines 2018: Management bundles for acute cholangitis and cholecystitis. J. Hepatobiliary Pancreat. Sci. 2018, 25, 96–100.
- Pisano, M.; Allievi, N.; Gurusamy, K.; Borzellino, G.; Cimbanassi, S.; Boerna, D.; Coccolini, F.; Tufo, A.; Di Martino, M.; Leung, J.; et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J. Emerg. Surg. 2020, 15, 61.
- Ansaloni, L.; Pisano, M.; Coccolini, F.; Peitzmann, A.B.; Fingerhut, A.; Catena, F.; Agresta, F.; Allegri, A.; Bailey, I.; Balogh, Z.J.; et al. 2016 WSES guidelines on acute calculous cholecystitis. World J. Emerg. Surg. 2016, 11, 25.
- Tarasconi, A.; Coccolini, F.; Biffl, W.L.; Tomasoni, M.; Ansaloni, L.; Picetti, E.; Molfino, S.; Shelat, V.; Cimbanassi, S.; Weber, D.G.; et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J. Emerg. Surg. 2020, 15, 3.
- Sartelli, M.; Weber, D.G.; Kluger, Y.; Ansaloni, L.; Coccolini, F.; Abu-Zidan, F.; Augustin, G.; Ben-Ishay, O.; Biffl, W.L.; Bouliaris, K.; et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J. Emerg. Surg. 2020, 15, 32.
- Cirocchi, R.; Sapienza, P.; Anania, G.; Binda, G.A.; Avenia, S.; di Saverio, S.; Tebala, G.D.; Zago, M.; Donini, A.; Mingoli, A.; et al. State-of-the-art surgery for sigmoid diverticulitis. Langenbecks Arch. Surg. 2022, 407, 1–14.
- Nascimbeni, R.; Amato, A.; Cirocchi, R.; Serventi, A.; Laghi, A.; Bellini, M.; Tellan, G.; Zago, M.; Scarpignato, C.; Binda, G.A. Management of perforated diverticulitis with generalized peritonitis. A multidisciplinary review and position paper. Tech. Coloproctology 2021, 25, 153–165.
- Chirica, M.; Kelly, M.D.; Siboni, S.; Aiolfi, A.; Riva, C.G.; Asti, E.; Ferrari, D.; Leppäniemi, A.; Ten Broek, R.P.; Brichon, P.Y.; et al. Esophageal emergencies: WSES guidelines. World J. Emerg. Surg. 2019, 14, 26.
- Christou, N.V.; Turgeon, P.; Wassef, R.; Rotstein, O.; Bohnen, J.; Potvin, M. Management of intra-abdominal infections. The case for intraoperative cultures and comprehensive broad-spectrum antibiotic coverage. The Canadian Intra-abdominal Infection Study Group. Arch. Surg. 1996, 131, 1193–1201.
- Labricciosa, F.M.; Sartelli, M.; Abbo, L.M.; Barbadoro, P.; Ansaloni, L.; Coccolini, F.; Catena, F. Epidemiology and Risk Factors for Isolation of Multi-Drug-Resistant Organisms in Patients with Complicated Intra-Abdominal Infections. Surg. Infect. 2018, 19, 264–272.
- Seguin, P.; Fedun, Y.; Laviolle, B.; Nesseler, N.; Donnio, P.Y.; Malledant, Y. Risk factors for multidrug-resistant bacteria in patients with post-operative peritonitis requiring intensive care. J. Antimicrob. Chemother. 2010, 65, 342–346.
- Sartelli, M.; Catena, F.; Ansaloni, L.; Coccolini, F.; di Saverio, S.; Griffiths, E.A. Duration of Antimicrobial Therapy in Treating Complicated Intra-Abdominal Infections: A Comprehensive Review. Surg. Infect. 2016, 17, 9–12.
- Sawyer, R.G.; Claridge, J.A.; Nathens, A.B.; Rotstein, O.D.; Duane, T.M.; Evans, H.L.; Cook, C.H.; O’Neill, P.J.; Mazuski, J.E.; Askari, R.; et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N. Engl. J. Med. 2015, 372, 1996–2005.
- Montravers, P.; Tubach, F.; Lescot, T.; Veber, B.; Esposito-Farèse, M.; Seguin, P.; Paugam, C.; Lepape, A.; Meistelman, C.; Cousson, J.; et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: The DURAPOP randomised clinical trial. Intensive Care Med. 2018, 44, 300–310.
- Lee, R.A.; Stripling, J.T.; Spellberg, B.; Centor, R.M. Short course antibiotics for common infections: What do we Know and where do we Go from Here? Clin. Microbiol. Infect. 2022.
- Sartelli, M.; Catena, F.; Coccolini, F.; Pinna, A.D. Antimicrobial Management of Intra-Abdominal Infections: Literature’s Guidelines. World J. Gastroenterol. 2012, 18, 865–871.
- Hawser, S.P.; Bouchillon, S.K.; Hoban, D.J.; Badal, R.E.; Canton, R.; Baquero, F. Incidence and antimicrobial susceptibility of Escherichia coli and Klebsiella pneumoniae with extended-spectrum beta-lactamases in community- and hospital-associated intra-abdominal infections in Europe: Results of the 2008 Study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob. Agents Chemother. 2010, 54, 3043–3046.
- Morrissey, I.; Hackel, M.; Badal, R.; Bouchillon, S.; Hawser, S.; Biedenbach, D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals 2013, 6, 1335–1346.
- Veeraraghavan, B.; Bakthavatchalam, Y.D.; Sahni, R.D. Orally Administered Amoxicillin/Clavulanate: Current Role in Outpatient Therapy. Infect. Dis. Ther. 2021, 10, 15–25.
- Harris, P.N.; Tambyah, P.A.; Paterson, D.L. beta-lactam and beta-lactamase inhibitor combinations in the treatment of extended-spectrum beta-lactamase producing Enterobacteriaceae: Time for a reappraisal in the era of few antibiotic options? Lancet Infect. Dis. 2015, 15, 475–485.
- Sartelli, M.; Weber, D.G.; Ruppé, E.; Bassetti, M.; Wright, B.J.; Ansaloni, L.; Catena, F.; Coccolini, F.; Abu-Zidan, F.M.; Coimbra, R.; et al. Antimicrobials: A global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J. Emerg. Surg. 2016, 11, 33.
- Surat, G.; Meyer-Sautter, P.; Rusch, J.; Braun-Feldweg, J.; Germer, C.T.; Lock, J.F. Comparison of Duration and Empiric Antibiotic Choice of Post-Operative Treatment in Abdominal Sepsis. Surg. Infect 2022, 23, 444–450.
- Perry, C.M.; Markham, A. Piperacillin/tazobactam: An updated review of its use in the treatment of bacterial infections. Drugs 1999, 57, 805–843.
- Belley, A.; Morrissey, I.; Hawser, S.; Kothari, N.; Knechtle, P. Third-generation cephalosporin resistance in clinical isolates of Enterobacterales collected between 2016-2018 from USA and Europe: Genotypic analysis of beta-lactamases and comparative in vitro activity of cefepime/enmetazobactam. J. Glob. Antimicrob. Resist. 2021, 25, 93–101.
- Van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation beta-Lactam/beta-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241.
- Zhanel, G.G.; Chung, P.; Adam, H.; Zelenitsky, S.; Denisuik, A.; Schweizer, F.; Lagacé-Wiens, P.R.; Rubinstein, E.; Gin, A.S.; Walkty, A.; et al. Ceftolozane/tazobactam: A novel cephalosporin/beta-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 2014, 74, 31–51.
- Solomkin, J.; Hershberger, E.; Miller, B.; Popejoy, M.; Friedland, I.; Steenbergen, J.; Yoon, M.; Collins, S.; Yuan, G.; Barie, P.S.; et al. Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI). Clin. Infect. Dis. 2015, 60, 1462–1471.
- Paterson, D.L.; Isler, B.; Harris, P.N.A. PRO: Carbapenems should be used for ALL infections caused by ceftriaxone-resistant Enterobacterales. JAC Antimicrob. Resist. 2021, 3, dlab013.
- Rodriguez-Bano, J.; Gutierrez-Gutierrez, B.; Pascual, A. CON: Carbapenems are NOT necessary for all infections caused by ceftriaxone-resistant Enterobacterales. JAC Antimicrob. Resist. 2021, 3, dlaa112.
- Vidal, L.; Gafter-Gvili, A.; Borok, S.; Fraser, A.; Leibovici, L.; Paul, M. Efficacy and safety of aminoglycoside monotherapy: Systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2007, 60, 247–257.
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50.
- Global Alliance for Infections in Surgery Working Group. A Global Declaration on Appropriate Use of Antimicrobial Agents across the Surgical Pathway. Surg. Infect. 2017, 18, 846–853.
- Messager, M.; Sabbagh, C.; Denost, Q.; Regimbeau, J.M.; Laurent, C.; Rullier, E.; Cunha, A.S.; Mariette, C. Is there still a need for prophylactic intra-abdominal drainage in elective major gastro-intestinal surgery? J. Visc. Surg. 2015, 152, 305–313.
- He, S.; Xia, J.; Zhang, W.; Lai, M.; Cheng, N.; Liu, Z.; Cheng, Y. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database Syst. Rev. 2021, 12, CD010583.
- EuroSurg Collaborative. Intraperitoneal drain placement and outcomes after elective colorectal surgery: International matched, prospective, cohort study. Br. J. Surg. 2022, 109, 520–529.
- Mujagic, E.; Zeindler, J.; Coslovsky, M.; Hoffmann, H.; Soysal, S.D.; Mechera, R.; von Strauss, M.; Delko, T.; Saxer, F.; Glaab, R.; et al. The association of surgical drains with surgical site infections—A prospective observational study. Am. J. Surg. 2019, 217, 17–23.
- Li, Z.; Zhao, L.; Cheng, Y.; Cheng, N.; Deng, Y. Abdominal drainage to prevent intra-peritoneal abscess after open appendectomy for complicated appendicitis. Cochrane Database Syst. Rev. 2018, 5, CD010168.
- Gurusamy, K.S.; Koti, R.; Davidson, B.R. Routine abdominal drainage versus no abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database Syst. Rev. 2013, 9, CD006004.
- De Waele, J.J.; Boelens, J.; van de Putte, D.; Veld, D.H.I.; Coenye, T. The Role of Abdominal Drain Cultures in Managing Abdominal Infections. Antibiotics 2022, 11, 697.
- De’Angelis, N.; Khan, J.; Marchegiani, F.; Bianchi, G.; Aisoni, F.; Alberti, D.; Ansaloni, L.; Biffl, W.; Chiara, O.; Ceccarelli, G.; et al. Robotic surgery in emergency setting: 2021 WSES position paper. World J. Emerg. Surg. 2022, 17, 4.
- Sauerland, S.; Agresta, F.; Bergamaschi, R.; Borzellino, G.; Budzynski, A.; Champault, G.; Fingerhut, A.; Isla, A.; Johansson, M.; Lundorff, P.; et al. Laparoscopy for abdominal emergencies: Evidence-based guidelines of the European Association for Endoscopic Surgery. Surg. Endosc. 2006, 20, 14–29.
- Mandrioli, M.; Inaba, K.; Piccinini, A.; Biscardi, A.; Sartelli, M.; Agresta, F.; Catena, F.; Cirocchi, R.; Jovine, E.; Tugnoli, G.; et al. Advances in laparoscopy for acute care surgery and trauma. World J. Gastroenterol. 2016, 22, 668–680.
- Rodriguez, R.M.J.; Segura-Sampedro, J.J.; Flores-Cortés, M.; López-Bernal, F.; Martín, C.; Diaz, V.P.; Ciuro, F.P.; Ruiz, J.P. Laparoscopic approach in gastrointestinal emergencies. World J. Gastroenterol. 2016, 22, 2701–2710.
- World Health Organization. Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level; World Health Organization: Geneva, Switzerland, 2016.
- World Health Organization. Minimum Requirements for Infection Prevention and Control Programmes; World Health Organization: Geneva, Switzerland, 2019.
- World Health Organization. Strengthening Infection Prevention and Control in Primary Care: A Collection of Existing Standards, Measurement and IMPLEMENTATION REsources; World Health Organization: Geneva, Switzerland, 2021.
- Zingg, W.; Holmes, A.; Dettenkofer, M.; Goetting, T.; Secci, F.; Clack, L.; Allegranzi, B.; Magiorakos, A.P.; Pittet, D. Hospital organisation, management, and structure for prevention of health-care-associated infection: A systematic review and expert consensus. Lancet Infect. Dis. 2015, 15, 212–224.
- Sartelli, M.; Labricciosa, F.M.; Coccolini, F.; Coimbra, R.; Abu-Zidan, F.M.; Ansaloni, L.; Al-Hasan, M.N.; Ansari, S.; Barie, P.S.; Caínzos, M.A.; et al. It is time to define an organizational model for the prevention and management of infections along the surgical pathway: A worldwide cross-sectional survey. World J. Emerg. Surg. 2022, 17, 17.
- Alonso-Echanove, J.; Edwards, J.R.; Richards, M.J.; Brennan, P.; Venezia, R.A.; Keen, J.; Ashline, V.; Kirkland, K.; Chou, E.; Hupert, M. Effect of nurse staffing and antimicrobial-impregnated central venous catheters on the risk for bloodstream infections in intensive care units. Infect. Control Hosp. Epidemiol. 2003, 24, 916–925.
- Borg, M.A.; Suda, D.; Scicluna, E. Time-series analysis of the impact of bed occupancy rates on the incidence of methicillin-resistant Staphylococcus aureus infection in overcrowded general wards. Infect. Control Hosp. Epidemiol. 2008, 29, 496–502.
- Cunningham, J.B.; Kernohan, W.G.; Rush, T. Bed occupancy, turnover intervals and MRSA rates in English hospitals. Br. J. Nurs. 2006, 15, 656–660.
- Howie, A.J.; Ridley, S.A. Bed occupancy and incidence of Methicillin-resistant Staphylococcus aureus infection in an intensive care unit. Anaesthesia 2008, 63, 1070–1073.
- Hugonnet, S.; Chevrolet, J.C.; Pittet, D. The effect of workload on infection risk in critically ill patients. Crit. Care Med. 2007, 35, 76–81.
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303.
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M.; et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287.
- Sway, A.; Solomkin, J.S.; Pittet, D.; Kilpatrick, C. Methodology and Background for the World Health Organization Global Guidelines on the Prevention of Surgical Site Infection. Surg. Infect. 2018, 19, 33–39.
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791.
- National Institute for Health and Care Excellence. Surgical Site Infections: Prevention and Treatment; National Institute for Health and Care Excellence: Great Britain, UK, 2019.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
637
Revisions:
2 times
(View History)
Update Date:
02 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




