Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dmitriy Aleksandrovich Martyushev | -- | 5761 | 2023-02-02 08:00:21 | | | |
| 2 | Catherine Yang | -3 word(s) | 5758 | 2023-02-02 08:47:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
El-Masry, J.F.; Bou-Hamdan, K.F.; Abbas, A.H.; Martyushev, D.A. Application of Nanomaterials in Enhanced Oil Recovery. Encyclopedia. Available online: https://encyclopedia.pub/entry/40756 (accessed on 07 February 2026).
El-Masry JF, Bou-Hamdan KF, Abbas AH, Martyushev DA. Application of Nanomaterials in Enhanced Oil Recovery. Encyclopedia. Available at: https://encyclopedia.pub/entry/40756. Accessed February 07, 2026.
El-Masry, Jamil Fadi, Kamel Fahmi Bou-Hamdan, Azza Hashim Abbas, Dmitriy A. Martyushev. "Application of Nanomaterials in Enhanced Oil Recovery" Encyclopedia, https://encyclopedia.pub/entry/40756 (accessed February 07, 2026).
El-Masry, J.F., Bou-Hamdan, K.F., Abbas, A.H., & Martyushev, D.A. (2023, February 02). Application of Nanomaterials in Enhanced Oil Recovery. In Encyclopedia. https://encyclopedia.pub/entry/40756
El-Masry, Jamil Fadi, et al. "Application of Nanomaterials in Enhanced Oil Recovery." Encyclopedia. Web. 02 February, 2023.
Copy Citation
The implementation of nanoparticles in Enhanced Oil Recovery (EOR) techniques is a novel method that has proven to increase the recovery of oil in place more than conventional EOR processes in most cases. The main aim of integrating nanoparticles in EOR methods is to boost the performance of each EOR technique by enhancing one or more parameters or mechanisms related to the recovery method. Sometimes, adding nanoparticles to the EOR method might reduce oil recovery due to porosity reduction, injection blockage, aggregation, and settling problems. The utilization of nanomaterials in several EOR applications comes with many benefits, such as IFT reduction, wettability alteration, and mobility improvement.
nanoparticles
nanomaterials
enhanced oil recovery
interfacial tension
1. Application of Nanomaterials in Chemical EOR
The integration of nanotechnology in chemically enhanced oil recovery can help overcome the major limitations of chemical EOR and improve its efficiency. Such limitations include chemical adsorption, retention, degradation, and precipitation due to reservoir water brines. These processes are mainly caused by the high salinity and high temperature of the reservoir, the pH and composition of the reservoir fluids, and the presence of clay minerals in the reservoir rock [1].
Surfactant and polymeric nanofluids are a mixture of nanoparticles with surfactant and polymer solutions, respectively. These two nanomaterial types are the most applied and studied in the chemical EOR.
1.1. Nanosurfactant EOR
In nanosurfactant EOR, nanoparticles can help increase the displacement efficiency through rock wettability alteration and further reduction of interfacial tension [1]. Emulsions that provide conformance efficiency and stable foams that help achieve fluid diversion to low permeability locations are created in the reservoir. Nanoparticles reduce the surfactant adsorption onto the reservoir rock surface, where the surfactants are adsorbed onto the surface of the nanoparticles resulting in surfactant-coated NPs [2]. A low surfactant-to-NP ratio results in a low fraction of surfactant-coated NPs. Moreover, a high surfactant-to-NP ratio causes a bilayer of surfactant to form on the surface of NPs. A single-chain surfactant achieves maximum hydrophobic nature and flocculation on NPs, leading to optimal performance [2].
According to several studies, IFT reduction by surfactant nanofluids is optimum in high-salinity and high-temperature environments, especially when using silica nanoparticles (SiO2) [2]. Mohajeri et al. [3] compared the performance of two surfactant nanofluids in reducing the oil/water interfacial tension [3]. The used surfactant nanofluids are zirconium dioxide (ZrO2) NP mixed with sodium dodecyl sulfate (SDS), which is an anionic surfactant and cetyltrimethylammonium bromide (CTAB) known as a cationic surfactant [4]. The results showed that SDS/ZrO2 nanofluid reduced the oil-water IFT by 81% more than the CTAB/ZrO2 combination, which reduced it by 70%. The combination of silica NPs with anionic surfactants such as SDS has shown improvements in the performance of the surfactant, whether in IFT reduction, recovery of oil, and the reduction of surfactant adsorption [2].
Studies have shown that the NP’s surface becomes hydrophobic after the adsorption of surfactants on NPs. NPs carry the surfactants from the surfactant solution to the oil-water interface, where IFT is reduced by reducing the interfacial energy. Typically, surfactant molecules desorb from the oil-water interface in surfactant flooding, but the utilization of NPs helps prevent desorption, thus maintaining better IFT reduction [2].
When the interfacial energy decreases, wettability alterations occur along with a change in the relative permeability of oil and water. Nanoparticles can strongly alter the wettability of the rock from oil-wet to water-wet, which helps recover the residual oil more efficiently. Surfactant nanofluids have proven to be effective in recovering oil from carbonate reservoirs that are hydrophobic [2]. Nwidee et al. [5] studied the wettability alteration of surfactant nanofluids, where they compared the performance of ZrO2 and NiO NPs with triton X-100 and CTAB surfactants at temperatures ranging from 0 °C up to 70 °C [5]. They found that the combination of CTAB with ZrO2 and NiO yielded better overall performance in the efficiency of wettability alteration at all temperatures.
Surfactant flooding becomes economically unfeasible due to the adsorption of the surfactant molecules on the reservoir rock surface. Oil recovery is improved with less surfactant adsorption; here lies the main function of NPs in surfactant EOR [1][2]. An experiment using SiO2 and Al2O3 NPs in SDS flooding of a kaolinite sample saturated with reservoir brine showed a significant reduction in surfactant adsorption. The addition of Al2O3 NP reduced the SDS adsorption on the surface of kaolinite by 38%, compared to 75% when using SiO2 with SDS. Surfactant nanofluids have also improved oil recovery from sandstone reservoirs by increasing the effective permeability of oil, favoring oil displacement [2].
Another benefit of using NPs is to stabilize foams that are unstable in the presence of oil and reservoir brine. NPs help elongate the foam half-life and help withstand harsh reservoir conditions [1][2]. At the lamellae interface between the gas and liquid phases, NPs adsorb with strong adhesion energy by irreversible attachment.
In order to achieve highly stable surfactant nanofluids, they must withstand high temperatures and high salinity environments [6]. This can be done by modifying the adsorption of the surfactant on the surface of NPs. The most commonly used NPs for such applications are metallic, metal oxide, and nonmetal NPs. Usually, metal NPs show great stability when mixed with surfactants, unlike their metal oxide and nonmetal counterparts. Nonmetal and metal oxide NPs require surface modifications to achieve the desired stability [6][7]. For instance, active groups, such as hydroxyl, carbonyl, and carboxyl groups, are attached to the surface of NPs to modify the chemical binding of the NPs with the surfactant. This increases the nanofluid stability and hence the capacity for oil displacement [6].
Based on the experiments made so far, the performance of surfactant nanofluid flooding not only depends on the type of NPs used but also on the surfactant type [8][9][10][11]. Each NP has a specific surfactant that yields an optimal performance when applied together. Among the studied NPs, SiO2 displayed robustness and optimal performance under harsh conditions [8][9][10][11]. Furthermore, other factors such as the NPs and surfactant concentration, reservoir conditions, reservoir rock properties, and others can have a detrimental influence on the results of the nanosurfactant EOR.
There are no similar scenarios when applying nanosurfactant EOR. A nanosurfactant fluid might yield optimal performance in one reservoir while yielding the worst performance in the other. Each scenario requires extensive research to produce the best surfactant and NPs mix that yields the highest incremental recovery factor.
1.2. Nanopolymers EOR
Nanopolymers are classified into polymer-coated NPs and polymer NPs, where polymer-coated NPs are more effective when it comes to NPs agglomeration in the reservoir [12]. Polymer NPs are synthesized to avoid the dissociation of polymers in polymer solution at reservoir conditions [13]. Mixing NPs with a polymer solution enhanced the stability, performance characteristics, tolerance to harsh conditions, and thermal characteristics of the injectant compared to the sole application of NPs and polymer solutions [14]. NPs interact with polymers in 5 ways:
-
Electrosteric repulsion.
-
Electrostatic and van der Waals forces.
-
Steric repulsion.
-
Hydrophobic bonding.
-
Hydrogen-hydrogen bonds.
Polymers bind to NPs through polymer grafting on the NP’s surface by chemical bonding. This is known as polymer-grafted NPs (PGN). Another method is the suspension of NPs in the polymer solution, known as polymer nanofluid suspension (PNS) hybrid [2][14]. Nanopolymers formed by PGN are more effective than those formed by PNS. This is due to the polyelectrolytes found in PGN that are not vulnerable to bonding with the cations present in brines.
The oil extraction efficiency is better in water-wet reservoirs than in oil-wet ones, where the NPs integrated into the polymer solution can alter the reservoir’s wettability. Nanoparticles reduce the capillary forces, switch the reservoir to water-wet, and consequently improve oil mobility and relative permeability, as shown in Figure 1 [14].
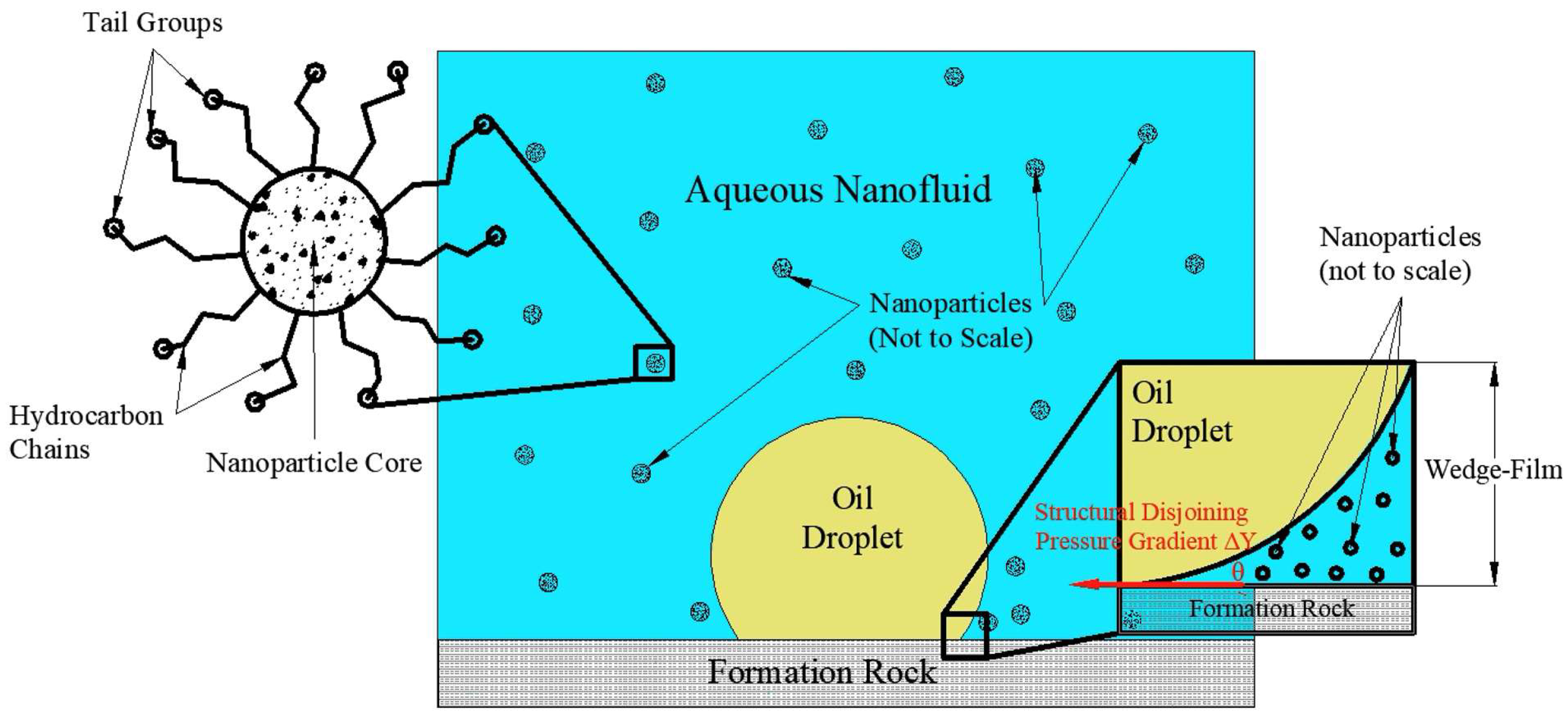
Figure 1. Development of the structural disjoining pressure gradient resulting in the separation of the oil droplets from the reservoir.
As shown in Figure 1, NPs develop a wedge coating when they come into contact with an oil-rich surface and consequently form a wedge film at the interface between the oil phase, rock, and nanofluid [2][12]. The rise in the wedge film results in a disjoining pressure gradient that alters the rock surface wettability [12][15]. Altering the rock wettability by NPs in nanofluids contribute to the improvement in the microscopic displacement efficiency.
Experiments have shown that the adsorption of polymers on the surface of the reservoir rock is reduced when adding NPs to the polymer solution. This is mainly because of the solution’s interaction and bonding between the NPs and the polymers [2][12]. Furthermore, the settlement of NPs in the reservoir pores (i.e., filtration) is reduced when using polymeric nanofluids rather than NPs alone. The adsorption of NPs in the pores of the rock can cause serious problems, where it lowers the rock’s permeability and blocks the pores and pore throats, leading to low mobility and EOR efficiency [14]. The polymer and NPs adsorption level in the reservoir is controlled by the polymer concentration (whether hydrophilic or hydrophobic) on the NP’s surface. Moreover, the rate of adsorption drops due to the forces of repulsion on the NP’s surface triggered by the polymer coating and due to decreased hydrophobic forces between the polymer-grafted NPs and the rock surface [14]. However, polymer nanofluid suspension (PNS) reduces adsorption by having NPs of the same charge as the reservoir rock surface. This will trigger repulsive forces between the nanofluid and the rock surface, improving the nanofluid stability and recovery efficiency [14].
NPs in polymeric nanofluids adsorb to the oil-water interface, consequently lowering the IFT at the interface. Stable emulsions are formed at the interface, which decreases the mobility ratio between the nanofluid and the residual oil. This improves the areal and vertical sweep efficiencies [14]. For many reasons, emulsions formed by polymer nanofluids are more effective than those formed by surfactants. For instance, the morphology of the emulsions formed by polymer nanofluids is kinetically affected by the volume of the residual oil. Furthermore, the NPs in polymer nanofluids are less susceptible to retention and can travel throughout the reservoir, maintaining stabilized emulsions. Such emulsions remain stable under extreme reservoir conditions, which is advantageous over emulsions created by surfactants [1][14].
Generally, polymers degrade under reservoir conditions, and the viscosity of the displacing fluid drops with temperature, which reduces the sweep efficiency. Another benefit of the utilization of NPs in polymer flooding is the improvement of the polymer viscosity and its stability under reservoir conditions (i.e., high pressure, temperature, and salinity). The viscosity of the polymer nanofluid is higher than that of the normal polymer solution due to the networking formed by NPs in the solution as a result of the hydrogen bonds between them [1].
NPs enhance the rheological behavior of the displacing fluid (polymer nanofluid), and this prevents the viscous fingering mechanism and ensures an appropriate mobility ratio. The viscosity of the displacing fluid drops in the reservoir due to the reaction of the cations found in the formation of water with amide and carboxylate groups in polymers [2]. In the case of polymer nanofluids, the electrostatic forces between NPs in the solution rise in the presence of brine cations. Consequently, the surface functionality is lost, which is desirable in EOR [12].
The performance of NPs application in chemical EOR has been mostly assessed with surfactants or polymers separately. The literature lacks studies on the hybrid application of NPs in surfactant-polymer solution mixtures. This hybrid application can bring a synergetic effect on the performance of EOR applications knowing that SP (surfactant-polymer) flooding has been tested and proved to yield better results than separate polymer or surfactant flooding [16][17].
1.3. Synergy between Low Salinity Waterflooding (LSWF), Surfactant, and Nanoparticles
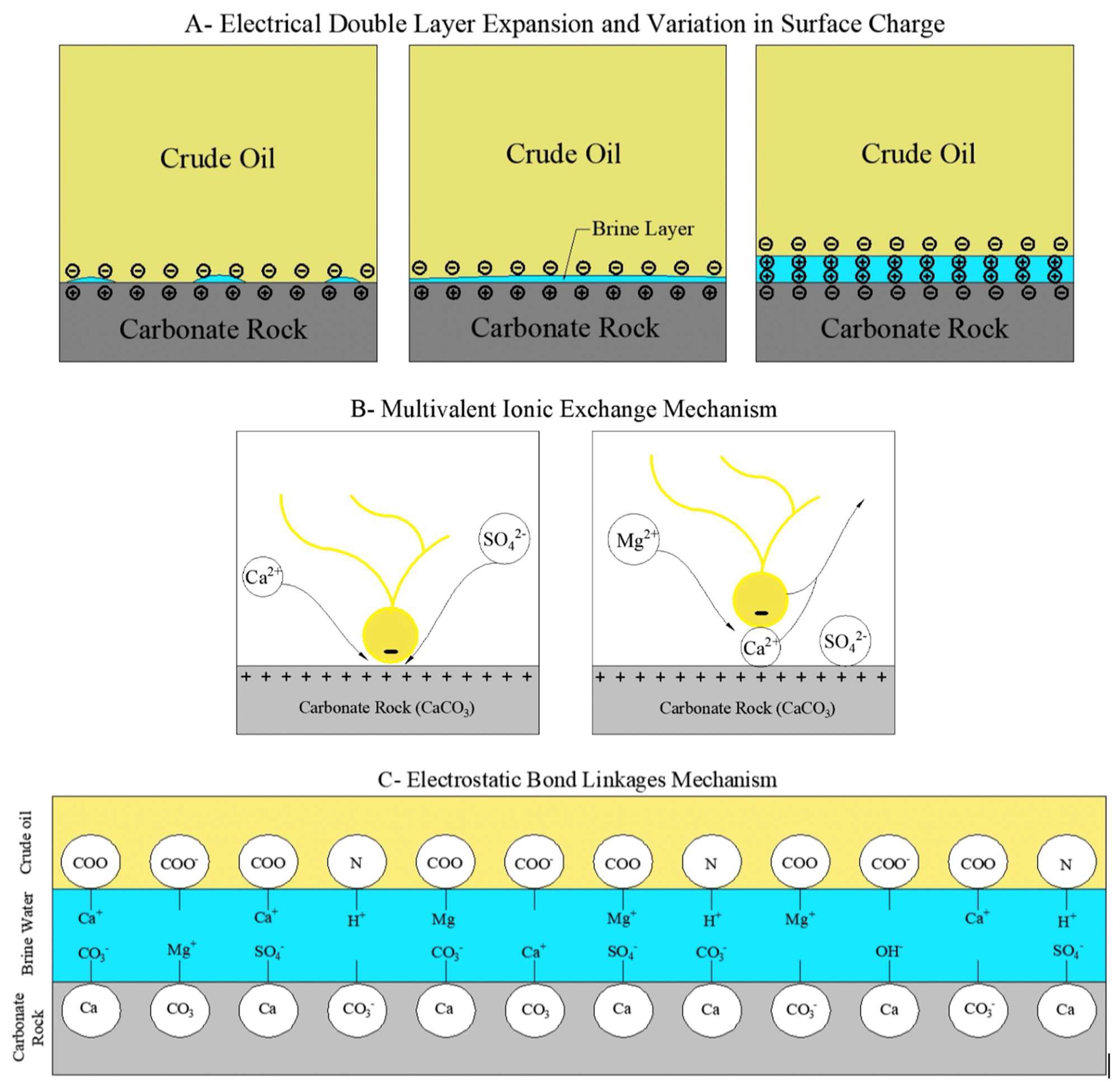
Low salinity waterflooding (LSWF) is a process that aims to modify the chemical properties of the waterflood to yield additional oil recovery during secondary recovery. LSWF mechanism offers great improvements in secondary recovery, especially in carbonate reservoirs. The effectiveness of the LSWF mechanism is critical to the mineralogy of the reservoir; the wettability alterations caused by this mechanism are controlled by the silica, clay, and anhydrite content of the reservoir rock [18]. The mechanisms of LSWF in carbonate reservoirs are classified into rock-fluid and fluid-fluid interactions. Rock-fluid mechanisms are mineral dissolution and wettability alteration through electrostatic interactions by electrical double-layer expansion and variation of surface charge, multivalent ionic exchange, and electrostatic bond linkages. These mechanisms are further demonstrated in Figure 2. As for the fluid–fluid interactions, these include the formation of water in oil microemulsions, IFT reduction, and fluid coalescence [18].
LSWF was proposed to be integrated into EOR mechanisms and nanoparticle-assisted flooding. Studies on low-salinity polymer flooding showed improvements in mobility control, mobility ratio, displacement sweep efficiency, and volumetric sweep efficiency, in addition to rock wettability. On the other hand, low salinity surfactant flooding promotes additional IFT reduction and wettability alteration, leading to a drop in capillary forces. Moreover, implementing LSFW prior to CO2 flooding have shown to enhance the sweep efficiency due to rock wettability alteration by LSFW [18]. NPs have displayed great potential in reducing and preventing fine migration in the reservoir during LSFW [19]. NPs are being implemented in LSFW with EOR chemicals, such as surfactants, to modify the fluid system’s rheological properties, reduce surfactant adsorption on the rock surface, and minimize fine migrations. The LSFW success is critical to the NPs selection, where NPs must prevent pore throat plugging and formation damage by fines. The addition of surfactants to LSFW reduces the fluid system’s IFT, alters rock wettability, and eventually enhances oil recovery [18][19]. The stability of NPs during LSFW depends on the salinity of the injectant. NPs are stable in deionized water at room temperature, where the main forces present in the solution are Van der Waals attractive forces and hydration repulsive forces. Solution stability also depends on the type of present ions. However, the stability of NPs in low-high saline water is more complex, where NPs can either coagulate or achieve stabilization [19]. NPs stability in saline water depends on the NPs concentration, NPs size, and solution pH. Salt ions lead to NPs coagulation by preventing particle repulsion; moreover, proton exchange is dominant in the low-salinity solution, eventually increasing the surface potential. Higher NPs concentration reduces the pH of the system, whereas higher solution acidity and smaller NPs sizes improve the stability of NPs. The addition of salt ions to the solution reduces the zeta potential towards zero leading to NPs coagulation. For an effective surfactant EOR mechanism, injectant salinity is an important factor to consider. The dominant mechanisms when integrating surfactants with LSFW include capillary pressure, osmosis, wettability alteration, diffusion, IFT reduction, and electrical double-layer effect [19]. NPs stability increases when implemented in the LSFW mechanism, which also increases oil recovery.
The synergetic effects of including a surfactant in the LSFW mechanism include further wettability alterations and IFT reduction, especially in carbonate reservoirs. As for the synergetic effects of NPs in LSFW, studies have shown additional rock wettability switch to water-wet in addition to further IFT reduction. Moreover, an increase in solution viscosity, higher displacement efficiency, less chalk dissolution, and less formation damage were observed. Further increase in water salinity reduces NPs stability and the ability of wettability alteration of the mechanism [19]. The synergetic effects of combining NPs, surfactants, and LSFW on IFT reduction and wettability alteration are controlled by the NPs’ charge, concentration, and size. The optimal performance of the LSFW mechanism with NPs and surfactants in the carbonate reservoirs is achieved by alternating injection [19].
1.4. Factors That Influence the Nanofluid’s Performance
Many factors affect the performance of nanofluids, such as:
-
Type: Each NP type alters unique properties. For instance, nonmetal NPs alter the wettability by reducing the IFT at the oil-water interface, whereas other NPs, such as metal oxides, affect other properties of the reservoir, such as oil viscosity or permeability [6].
-
Concentration: This factor affects the interfacial tension and the disjoining pressure of the nanofluid. A higher concentration of NPs in the nanofluid generally results in a higher repulsion between the NPs and, consequently, higher disjoining pressure. Furthermore, as the NPs concentration increases, the IFT drops further. However, a high NP concentration may lead to agglomerations, which is undesired. Therefore, there is an optimal NPs concentration that compromises between the NPs agglomerations and nanofluid performance [6][7].
-
Wettability: Hydrophobic NPs are more effective than their hydrophilic counterparts when it comes to the detachment of oil droplets from the reservoir rock. Hydrophilic NPs can lead to oil expansion and rapid detachment, which also makes the hydrophilic NPs a good candidate for EOR applications [6][7].
-
Charge: The charge of the NPs directly affects the disjoining pressure, where the ability to separate the oil droplets from the rock surface is higher for nanofluids with charged NPs [6].
-
Formation water salinity: The stability of the NPs in a nanofluid is related to the salinity of the environment (i.e., formation water and the carrying fluid). Higher salinity causes faster agglomeration. Generally, surface modifications are made to the NPs to tolerate the salinity of the environment. The lower the salinity of the environment, the better the performance of the nanofluids and the displacement efficiency [7].
-
Formation temperature: The stability of the NPs in a nanofluid decreases with the increase in formation temperature, which also leads to faster NPs agglomeration. Nonetheless, the temperature does not affect the NP’s retention [7].
-
Carrying fluid pH: Fluids with a pH close to the isoelectric point (i.e., pH where the net electric charge of molecules in the solution is zero) will have unstable nanofluids. Nanofluids are more stable when the solution pH is closer to 7 (neutral) [7].
-
Crude oil composition: The composition of reservoir fluids influences the structure of NPs suspension in the nanofluid. Moreover, the incremental recovery factor from nanofluids is affected by the percentage of heavy components in the reservoir fluids [21].
-
Rock mineral type and properties: The performance of a nanofluid depends on the reservoir lithology and properties; for instance, the performance of a particular nanofluid injected in a sandstone reservoir will yield a different performance than in a carbonate one. Moreover, rock wettability influences the adsorption of nanoparticles where oil-wet reservoirs cause lower NPs adsorption than the water and neutral-wet ones [7][21].
2. Application of Nanomaterials in Thermal EOR
Thermal EOR is performed exclusively on heavy oil reservoirs containing asphaltenes and resins. Hydrocarbons in such reservoirs are characterized by long carbon chains, consequently having high molecular weight, high viscosity, and high boiling point [12][22]. These properties make the recovery of such oil difficult using conventional fluids such as gas or water. Nanoparticles such as nickel, cobalt, and iron are integrated into thermal EOR in the form of nanocatalysts. These nanocatalysts help decrease the heavy oil viscosity. Additionally, they are applied in the aquathermolysis oil treatment process. This process involves the improvement of the oil quality by reducing its viscosity under the effect of nanocatalysts, where the quality of the oil normally increases when the size of the nanocatalysts decreases [12][22]. The reaction that reduces the viscosity of the heavy oil is usually slow, and the volume of heavy oil exposed to nanocatalysts is usually large due to the high surface-to-volume ratio of nanocatalysts [12]. The reactions that occur during the aquathermolysis process include hydrodenitrogenation, hydrogenation, hydrocracking, and hydrodesulfurization. The dominant reaction that takes place during aquathermolysis is aliphatic sulfur bond hydrolysis [12].
Lighter hydrocarbon fractions aggregate to form heavier ones, where nanoparticles combine with the lighter hydrocarbon compounds avoiding their aggregation and growth. This is mainly because the interaction between NPs and the lighter hydrocarbons is stronger than that between the hydrocarbons themselves [23]. The adsorption of heavy HC compounds on NPs involves heat absorption or release and is a spontaneous process. Furthermore, this interaction alters the NPs’ surface acidity, creating a modified surface chemical structure. The medium of the NPs’ surface turns from acidic to basic then to neutral when interacting with asphaltenes, as a result of the strong interaction between the acidic NPs and asphaltenes yielding weak chemical bonds. The adsorption of different iron oxide NPs, such as hematite, magnetite, and iron oxide NPs was investigated. Results have shown that the hematite NPs have the highest adsorption capacity and can achieve equilibrium faster. Moreover, the asphaltene adsorption on hematite was an exothermic process, while the asphaltene adsorption on magnetite was endothermic. This implies that the structural properties of NPs control the asphaltene adsorption on NPs [23]. Another study performed by Marei et al. [24] suggests that the size of the NPs can influence the adsorption performance of NPs, where the adsorption capacity of larger NPs is higher than that of smaller ones [24]. Moreover, the surface properties of NPs and their texture vary widely with NPs size, hence influencing the adsorption behavior. When comparing alumina and silica NPs, silicon dioxide was found to have the highest asphaltene adsorption potential. It was also found that the presence of resins can affect the adsorption performance of asphaltenes on NPs [23].
The asphaltene’s large molecule dissociates into the smaller molecule when it approaches the adsorption potential zone of the nanoparticle, as illustrated in Figure 3. The main reason for asphaltene adsorption on the NP surface is the interaction between the functional group of the deployed NPs and the main functional group of the asphaltene, which is composed of aliphatic and aromatic compounds [23].
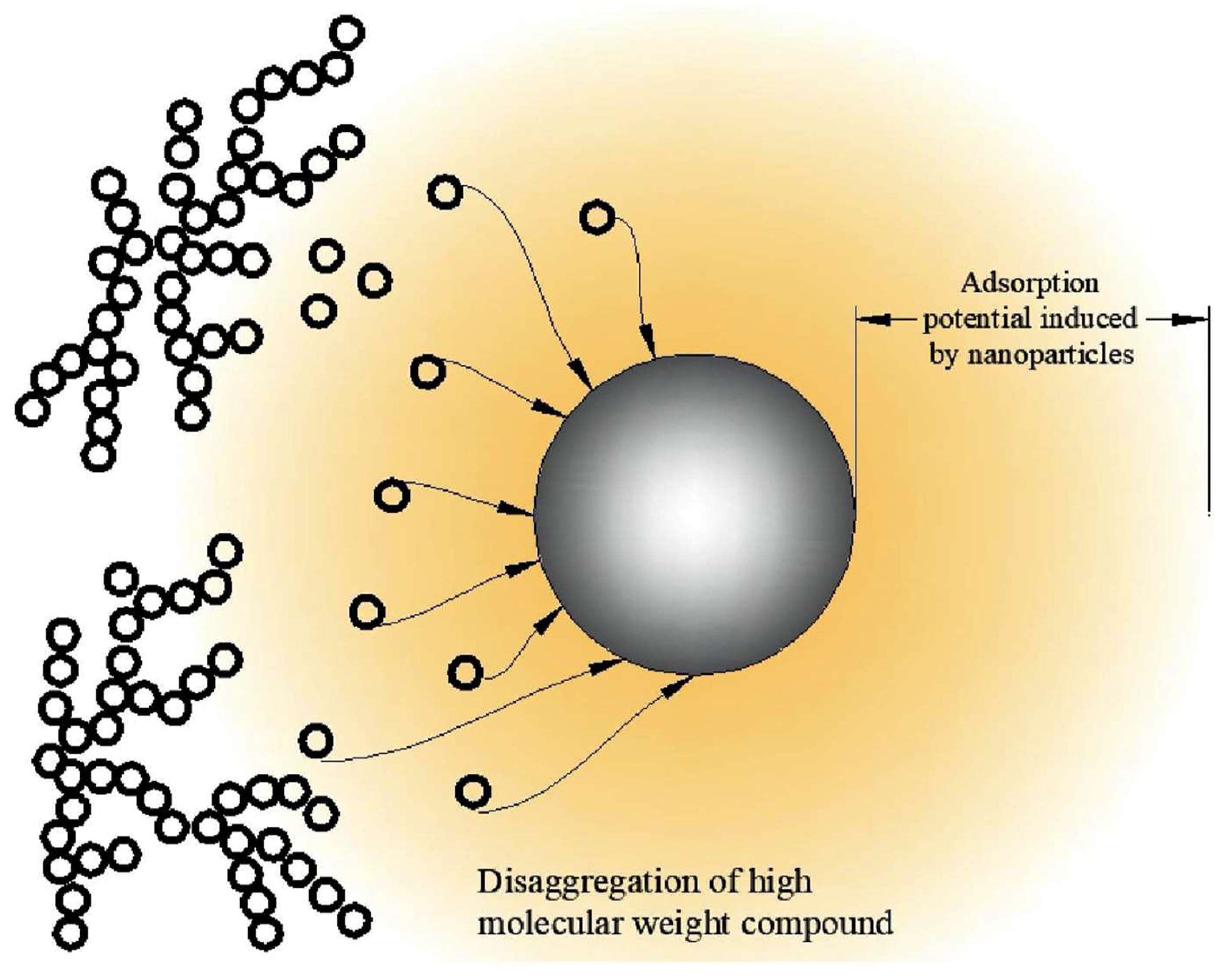
Figure 3. The disaggregation of asphaltene molecule through NP adsorption mechanism.
2.1. Application of NPs in In-Situ Combustion Thermal EOR
In-situ combustion involves the injection of oxygen-enriched air into the reservoir. Oxygen will trigger the combustion of heavy crude oil in the reservoir at the reservoir temperature. The presence of catalyst nanoparticles in this process accelerates the reactions involved in in-situ combustion. These reactions include oxidation, steam thrust, polymerization, distillation, vaporization, and catalytic disintegration [23].
Metal and metal oxide NPs are the most studied NPs in in-situ combustion. It was observed that nickel oxide and cobalt oxide (Co3O4) NPs reduced the temperature of oxidation for n-C7 asphaltenes from 450 °C to 325 °C compared to 365 °C for Fe3O4 NPs. When an asphaltene molecule gets adsorbed on the surface of the NP, the temperature of its combustion and oxidation will drop significantly, which will assist the in-situ combustion process [25]. The utilization of copper NPs in the in-situ combustion inhibits the asphaltene oxidation reaction at low temperatures. Moreover, hydrophobic copper oxide NPs raise oxygen consumption during in-situ combustion [23]. In general, NPs utilization in in-situ combustion lowers the activation energy of residual oil, improves the oxidation efficiency (which lowers the asphaltenes’ temperature of ignition), and enhances coke ignition [23].
For silica NPs, it was found that the oxidation performance of asphaltene depends on the acidity of the nanocatalysts’ surface. The application of acidic and basic NPs triggers the production of methane and carbon monoxide. Bimetallic NPs have a larger catalytic activity than monometallic ones. Hence, their utilization helps reduce the rate of asphaltene oxidation at lower temperatures [23]. The oxidation temperature and the activation energy of asphaltenes rise with lower resin content in crude oil. Furthermore, when resin accumulates on the NPs’ surface, the oxidation efficiency increases. The catalytic performance of NPs drops with higher asphaltene aggregation, increasing the activation energy of asphaltenes [23].
2.2. Application of NPs in Steam Injection Thermal EOR
Steam injection in a heavy oil reservoir leads to hydrogenation, methanation, gasification, aquathermolysis, and steam reforming of heavy hydrocarbon molecules. Aquathermolysis is initiated with the break of C-S bonds found in n-C7 asphaltenes since this bond is the weakest in the asphaltene molecule. As a result, hydrogen sulfide gas is generated [23]. Steam injection thermal oil recovery includes steam-assisted gravity drainage, continuous steam injection, and cyclic steam stimulation, all of which do not provide more than a 50% recovery factor. Therefore, the role of nanoparticles is of great importance since it helps reduce the temperature of asphaltene dissociation and enhances oil recovery [23].
Metal oxide NPs improve the properties of heavy crude oil when contacting asphaltenes during steam injection. Such NPs reduce the reaction temperature of asphaltene, even though their catalytic activity differs from one another. The catalytic activity of metal oxide NPs depends on the surface-to-volume ratio. The upgrading efficiency of NPs is controlled by the metal concentration and the degree of particle homogeneity in porous media [23].
The gasification and the hydrothermolysis decomposition reactions in steam injection EOR are boosted when using a composite of different NP types. Moreover, composite NPs prevent heavy hydrocarbon addition reactions, which are achieved by decreasing the decomposition intensity at high temperatures [23]. Furthermore, the utilization of composite NPs can significantly reduce the decomposition temperature of asphaltene to a greater extent than that done by a single NPs species. Another benefit of utilizing NPs in steam injection is the reduction of interfacial tension, which improves oil mobility [23]. The integration of composite NPs can further improve tar gasification and elimination. Moreover, it can reduce the viscosity of heavy oil, leading to Newtonian rheological behavior when contacting steam and NPs.
2.3. Application of NPs in Electromagnetic Heating Thermal EOR
In this EOR process, electric currents of different frequencies are applied to heat the heavy oil reservoir, hence reducing oil viscosity. The use of dielectric nanofluids in association with this method can alter the oil-water interface and increase oil production. Magnetic NPs have shown better performance when integrated with this EOR method. Moreover, the viscosity of the utilized dielectric nanofluids effectively enhances the sweep efficiency of oil and hence increases oil recovery [23]. Metal oxide NPs can absorb induced microwaves, consequently increasing the temperature of the reservoir. As a result, microwave heating of metal oxide NPs can reduce the viscosity of heavy crude oil. However, there is an optimal concentration of NPs in the nanofluid that reduces the viscosity of crude oil; beyond this concentration, the oil viscosity will rise again [23].
3. Application of Nanomaterials in Miscible/Immiscible EOR
Unfavorable phenomena might occur during this EOR mechanism, for instance, viscous fingering, gravity underride and override, and undesired mobility ratio. Water alternating gas flooding has been applied as an alternative to reduce the influence of these phenomena [12][26]. Furthermore, NPs have been tested and used in WAG EOR, which enhanced the macroscopic and microscopic sweep efficiencies of this mechanism [26].
Moradi et al. [26] investigated the performance of the WAG EOR mechanism when integrating SiO2 NPs. They compared the performance of WAG EOR with and without NPs. The outcome of the study was a 20% improvement in the recovery factor when utilizing SiO2 NPs compared to the conventional WAG process. This is due to the adsorption of the NPs on the surface of the reservoir rock, hence altering the wettability from oil-wet to water-wet. Moreover, the IFT at the oil-water interface has dropped as a result of NPs alignment at the interface [26]. Zhang et al. [27] studied the fluid miscibility of the mineral water-oil interface in the presence and absence of surfactant-decorated NPs. Contact angle and IFT experiments were conducted on different concentrations of SiO2 NPs coated with CTAB surfactant. The results of the experiments revealed that optimal miscibility is achieved by high pressure, low temperature, small NPs sizes (<40 nm), high surfactant concentration, more wetting state, and 0.5–0.6wt.% NPs concentration. Moreover, additional surfactants would require higher NP concentration, and larger NP sizes would require less NP concentration to achieve optimal miscibility. This is further demonstrated in Figure 4, where surfactants at the oil-water align around the NP’s surface. Deploying such NPs in the WAG mechanism under the mentioned conditions enhanced the recovery factor by more than 20% compared to conventional WAG EOR [27].
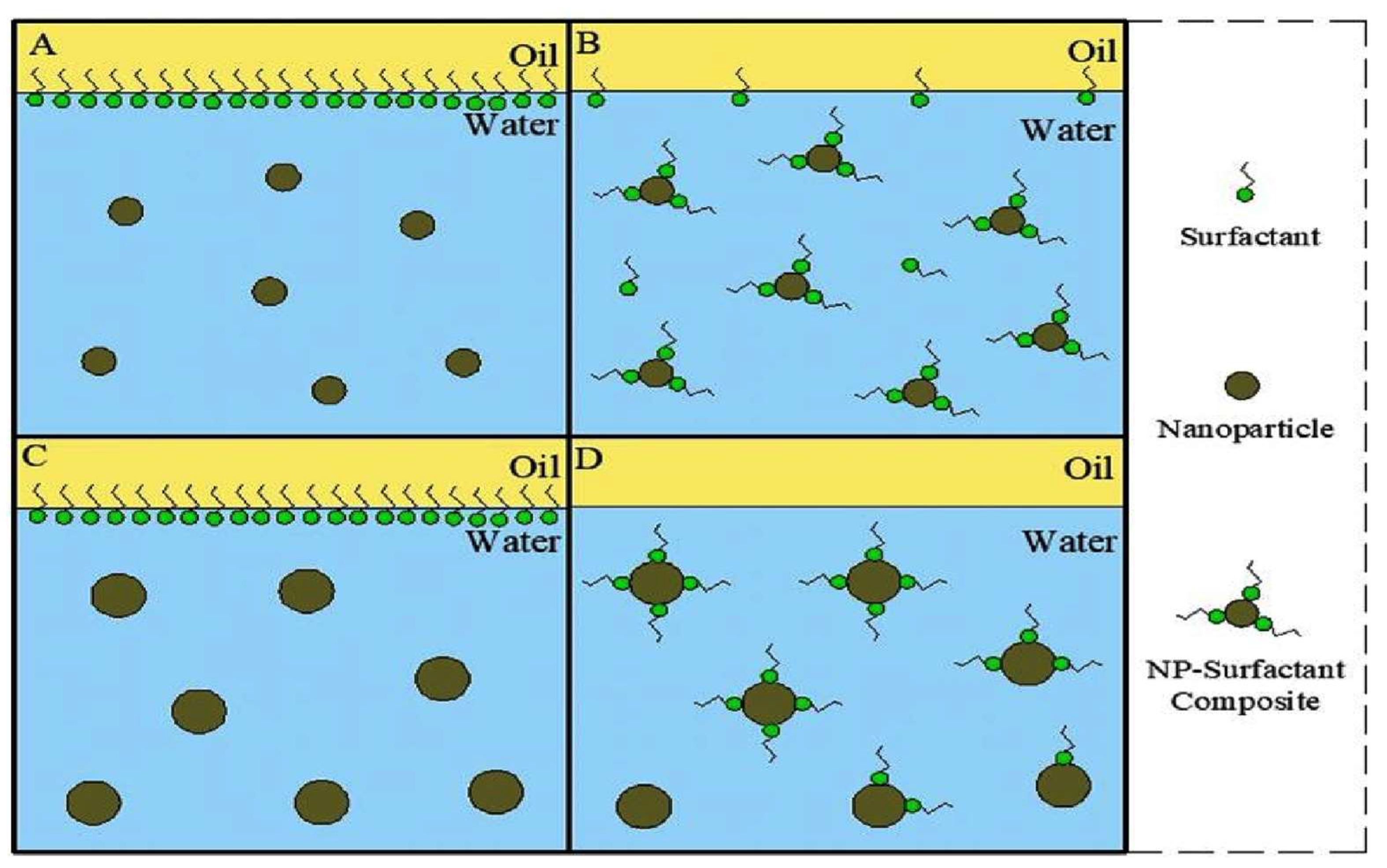
Figure 4. Adsorption mechanism of surfactants at the oil-water interface on NPs surface: (A,B) Small size NPs are introduced, where the surfactants’ concentration was enough to achieve optimal miscibility; (C,D) Larger NPs are introduced, where the surfactants’ concentration was not enough to reach optimal miscibility.
The use of NPs can prevent viscous fingering and improve gas viscosity, density, mobility ratio, and recovery factor. Dezfuli et al. [28] demonstrated the benefits of implementing NPs in supercritical CO2 flooding and the optimum NPs volume fraction to be deployed [28]. The results of this study showed that the optimal volume fraction of silica NPs to be used in light oil reservoirs is 3.5%. Beyond this volume fraction, the increase in oil recovery starts to drop until it reaches a maximum value at a 4 wt.% NPs volume fraction [28]. The corresponding maximum recovery factor achieved by supercritical CO2 injection with 4 wt.% silica NPs is 30.47%. Similarly, for heavy oil reservoirs, the optimal silica NPs volume fraction range is between 0 and 5 wt.%, and the maximum achieved ultimate recovery factor corresponding to supercritical CO2 flooding with 5 wt.% silica NPs is 27.82% [28]. It is obvious that this value is technically feasible but commercially expensive.
Al-Shargabi et al. [29] illustrated the utilization of NPs from coal ash to stabilize the CO2 foam in the reservoir and hence control its mobility [29]. CO2 flooding effectiveness is improved after the incorporation of NPs in the injection fluid. Several factors affect the performance of NPs-assisted CO2 foam. These factors include NPs size, NPs wettability, reservoir temperature, formation water salinity, NPs hydrophilic concentration, flow features, hydrocarbons, NPs type, and NPs retention [29]. Small NP sizes are better for the stability of the CO2 foam due to more adsorption of surfactants on the NP’s surface. The NP’s wettability is controlled by the ratio of adhesion and cohesion forces, which in turn will affect the stability of the CO2 foam and the fluid mobility in the reservoir. Reservoir conditions such as salinity and temperature also affect the performance of NPs enhanced CO2 foam flooding, where more salinity reduces the surfactant adsorption at the interface, foam half-life, and hence the foam stability.
Similarly, the stability of CO2 foam drops with the rise in the medium temperature [29]. CO2 foam is more stable in more hydrophilic NPs and NPs with modified surfaces. Moreover, it was found that NPs assisted CO2 foam for all NP types is more stable than surfactant foams without NPs. The NP-CO2 foam stability is further controlled by the shape, density, size, wettability, and surface charge of NPs [29].
The main reason for the destabilization of the CO2 foam is the surfactant adsorption on the rock surface and the absence of a stable front. Kalyanaraman et al. [30] investigated the improvement of CO2 foam stability by the incorporation of polyelectrolytes and polyelectrolyte complex NPs (polyethyleneimine and dextran sulfate). It was found that the durability of surfactant-NP-CO2 foam is higher when crude oil is present, and its viscosity is better than that of the conventional CO2 foam created by surfactant. Moreover, the addition of polyelectrolyte complex NPs recovered 58.33% of the residual oil in place compared to 47.6% for surfactant-generated CO2 foam. Surfactant-NP-CO2 foam system was injected after flooding with surfactant-CO2 foam, and an additional 9.1% of residual oil was recovered [30]. A highly stable lamella was developed at the interface by the integration of polyelectrolyte complex NPs during supercritical CO2 foam flooding. This stable lamella ensures better foam stability and better compatibility of supercritical CO2 foam with water. The results of Nazari et al. [31] proved that the optimal surfactant-polyelectrolyte complex NPs ratio yields a highly stable supercritical CO2 foam in a high salinity environment and improves the oil recovery factor. Moreover, the study showed that the lowest saturation of residual oil and the highest increase in oil recovery are obtained by the optimization of electrolyte concentrations in the surfactant-polyelectrolyte complex NPs-supercritical CO2 foam flooding [31].
Lai et al. [32] studied the performance of CO2/N2 responsive NPs during the CO2 EOR mechanism. These NPs are created by silica NPs modification with 3-aminopropyltrimethoxysilane through the Eschweiler–Clark reaction. Results of the study showed that responsive NPs are a viable candidate for enhancing CO2 flooding performance, where more than 26% of the OOIP was recovered. Furthermore, the wettability of the rock changed from oil-wet to water-wet, which was beneficial for the recovery of residual oil [32].
Another problem encountered during CO2 miscible flooding is the asphaltene deposition on the reservoir rock surface. This would alter the reservoir rock properties and affect oil recovery. NPs such as ferric oxide and aluminum oxide were proposed by Azizkhani & Gandomkar, 2020, as direct asphaltene inhibitors during miscible CO2 flooding. Results of the study showed that such direct asphaltene inhibitors could reduce the number of precipitated asphaltenes during the miscible NPs-assisted CO2 flooding mechanism under reservoir conditions. Moreover, CO2 miscible flooding with ferric oxide showed a better performance than with aluminum oxide. This is due to the higher solubility involved in Fe3O4-CO2 flooding. Higher concentrations of NPs in the injected fluid result in lower precipitation of asphaltene during the flooding [33]. A similar study was performed by Hassanpour et al. [34] by comparing the performance of TiO2 and Fe2O3. NPs in reducing asphaltene precipitation. The conclusion of this study also revealed that ferric oxide NPs showed a better performance than titanium dioxide in reducing asphaltene precipitation at the CO2-water interface. Moreover, the optimal NPs concentration for this purpose was found to be 1%. Utilizing titanium oxide and ferric oxide NPs reduced the asphaltene precipitation by 17% and 18%, respectively [34]. Consequently, NPs are great candidates for reducing the asphaltene precipitation during miscible flooding, which in turn favors oil recovery.
NPs can also assist miscible EOR mechanisms in reducing the viscosity of residual oil and increasing the recovery factor. A new viscosity-reducing mechanism for heavy oil reservoirs was proposed by Shah [35]. This mechanism involves the integration of thermally conductive metallic NPs in supercritical CO2 (viscosity-reducing injectant) flooding EOR coupled with soluble surfactants for further viscosity reduction. The thermal properties of the NPs enabled further increase in the injectant temperature and eventually lower oil viscosity. Oil viscosity reduction is further assisted by the chemical properties of the associated surfactant and the miscible properties of the injected supercritical CO2 [35].
4. Cost Analysis of NPs for EOR Applications
The process of manufacturing nanomaterials is complex and expensive, especially if it is non-standardized [36][37]. Usually, the quantity of NPs used in laboratories is small and its cost implications are less consequential [38]. However, the number of nanomaterials to be used in a field-scale application depends on several factors: for instance, the required (optimal) NPs concentration in the injection fluid, the field size, rock and reservoir fluid properties, and the properties of the used nanomaterial (i.e., size, shape, and charge). Presently, economic analyses comparing oil prices with NPs costs are still scarce, which prevents the implementation of NPs in field applications. Field-scale application of NPs requires large NPs quantities, and eventually, the realistic economic implications of NPs at different oil regimes must be further investigated [38]. Typical prices of some common NPs in US$/gram are summarized in Table 1.
Table 1. Indicative prices of most common nanoparticles in $/g [39].
| Nanoparticle Type | Price in $/g |
|---|---|
| Aluminum Oxide (Al2O3) | 0.7 |
| Aluminum (Al) | 3.8 |
| Copper Oxide (CuO) | 0.75 |
| Copper (Cu) | 5 |
| Silica Dioxide (SiO2) | 0.7 |
| Silver (Ag) | 4 |
| Gold (Au) | 55 |
| Titanium Dioxide (TiO2) | 0.8 |
| Carbon Nanotubes | 9.3–12.5 |
References
- Lashari, N.; Ganat, T. Emerging Applications of Nanomaterials in Chemical Enhanced Oil Recovery: Progress and Perspective. Chin. J. Chem. Eng. 2020, 28, 1995–2009.
- Gbadamosi, A.; Junin, R.; Manan, M.; Agi, A.; Oseh, J. Nanotechnology Application in Chemical Enhanced Oil Recovery: Current Opinion and Recent Advances. In Enhanced Oil Recovery Processes—New Technologies; Books on Demand: Norderstedt, Germany, 2019.
- Mohajeri, M.; Hemmati, M.; Shekarabi, A.S. An Experimental Study on Using a Nanosurfactant in an EOR Process of Heavy Oil in a Fractured Micromodel. J. Pet. Sci. Eng. 2015, 126, 162–173.
- Mohajeri, S.; Dolati, A.; Yazdanbakhsh, K. Synthesis and Characterization of a Novel Non-Enzymatic Glucose Biosensor Based on Polyaniline/Zinc Oxide/Multi-Walled Carbon Nanotube Ternary Nanocomposite. J. Electrochem. Sci. Eng. 2019, 9, 207–222.
- Nwidee, L.N.; Lebedev, M.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S. Wettability Alteration of Oil-Wet Limestone Using Surfactant-Nanoparticle Formulation. J. Colloid Interface Sci. 2017, 504, 334–345.
- Liu, D.; Zhang, X.; Tian, F.; Liu, X.; Yuan, J.; Huang, B. Review on Nanoparticle-Surfactant Nanofluids: Formula Fabrication and Applications in Enhanced Oil Recovery. J. Dispers. Sci. Technol. 2022, 43, 745–759.
- Udoh, T.H. Improved Insight on the Application of Nanoparticles in Enhanced Oil Recovery Process. Sci. Afr. 2021, 13, e00873.
- Ortega, A.; Hernández, A.; Puello, J.; Marin-Batista, J. Effect of Liquefied Petroleum Gas (LPG) on Heavy Oil Recovery Process. Chem. Eng. Trans. 2017, 57, 1297–1302.
- Azin, R.; Izadpanahi, A. Fundamentals and Practical Aspects of Gas Injection; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 3030772004.
- Perera, M.S.A.; Gamage, R.P.; Rathnaweera, T.D.; Ranathunga, A.S.; Koay, A.; Choi, X. A Review of CO2 -Enhanced Oil Recovery with a Simulated Sensitivity Analysis. Energies 2016, 9, 481.
- Verma, M.K. Fundamentals of Carbon Dioxide-Enhanced Oil Recovery (CO2-EOR): A Supporting Document of the Assessment Methodology for Hydrocarbon Recovery Using CO2-EOR Associated with Carbon Sequestration; US Department of the Interior, US Geological Survey: Washington, DC, USA, 2015.
- Sircar, A.; Rayavarapu, K.; Bist, N.; Yadav, K.; Singh, S. Applications of Nanoparticles in Enhanced Oil Recovery. Pet. Res. 2021, 7, 77–90.
- Davarpanah, A. Parametric Study of Polymer-Nanoparticles-Assisted Injectivity Performance for Axisymmetric Two-Phase Flow in EOR Processes. Nanomaterials 2020, 10, 1818.
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Yekeen, N.; Agi, A.; Oseh, J.O. Recent Advances and Prospects in Polymeric Nanofluids Application for Enhanced Oil Recovery. J. Ind. Eng. Chem. 2018, 66, 1–19.
- Agi, A.; Junin, R.; Arsad, A.; Abbas, A.; Gbadamosi, A.; Azli, N.B.; Oseh, J. Synergy of the Flow Behaviour and Disperse Phase of Cellulose Nanoparticles in Enhancing Oil Recovery at Reservoir Condition. PLoS ONE 2019, 14, e0220778.
- Samanta, A.; Ojha, K.; Sarkar, A.; Mandal, A. Surfactant and Surfactant-Polymer Flooding for Enhanced Oil Recovery. Adv. Pet. Explor. Dev. 2011, 2, 13–18.
- Druetta, P.; Picchioni, F. Surfactant-Polymer Flooding: Influence of the Injection Scheme. Energy Fuels 2018, 32, 12231–12246.
- Tetteh, J.T.; Brady, P.V.; Barati Ghahfarokhi, R. Review of Low Salinity Waterflooding in Carbonate Rocks: Mechanisms, Investigation Techniques, and Future Directions. Adv. Colloid Interface Sci. 2020, 284, 102253.
- Olayiwola, S.O.; Dejam, M. A Comprehensive Review on Interaction of Nanoparticles with Low Salinity Water and Surfactant for Enhanced Oil Recovery in Sandstone and Carbonate Reservoirs. Fuel 2019, 241, 1045–1057.
- Tetteh, J.T.; Alimoradi, S.; Brady, P.V.; Barati Ghahfarokhi, R. Electrokinetics at Calcite-Rich Limestone Surface: Understanding the Role of Ions in Modified Salinity Waterflooding. J. Mol. Liq. 2020, 297, 111868.
- Li, S.; Ng, Y.H.; Lau, H.C.; Torsæter, O.; Stubbs, L.P. Experimental Investigation of Stability of Silica Nanoparticles at Reservoir Conditions for Enhanced Oil-Recovery Applications. Nanomaterials 2020, 10, 1522.
- Bou-Hamdan, K.F. Applications of Nanomaterials in the Oil and Gas Industry. In Handbook of Research on Green Synthesis and Applications of Nanomaterials; Garg, R., Garg, R., Eddy, N., Eds.; IGI Global: Hershey, PA, USA, 2022; pp. 173–198.
- Medina, O.E.; Olmos, C.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review. Energies 2019, 12, 4671.
- Marei, N.N.; Nassar, N.N.; Vitale, G. The Effect of the Nanosize on Surface Properties of NiO Nanoparticles for the Adsorption of Quinolin-65. Phys. Chem. Chem. Phys. 2016, 18, 6839–6849.
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative Oxidation of Adsorbed Asphaltenes onto Transition Metal Oxide Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 145–149.
- Moradi, B.; Pourafshary, P.; Jalali Farahani, F.; Mohammadi, M.; Emadi, M.A. Application of SiO2 Nano Particles to Improve the Performance of Water Alternating Gas EOR Process. In Proceedings of the Society of Petroleum Engineers—SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 24–26 November 2015.
- Zhang, K.; Jia, N.; Li, S.; Liu, L. How Surfactant-Decorated Nanoparticles Contribute to Thermodynamic Miscibility. Nanotechnology 2018, 29, 475701.
- Dezfuli, M.G.; Jafari, A.; Gharibshahi, R. Optimum Volume Fraction of Nanoparticles for Enhancing Oil Recovery by Nanosilica/Supercritical CO2 Flooding in Porous Medium. J. Pet. Sci. Eng. 2020, 185, 106599.
- Al-Shargabi, M.; Davoodi, S.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Carbon Dioxide Applications for Enhanced Oil Recovery Assisted by Nanoparticles: Recent Developments. ACS Omega 2022, 7, 9984–9994.
- Kalyanaraman, N.; Arnold, C.; Gupta, A.; Tsau, J.S.; Ghahfarokhi, R.B. Stability Improvement of CO2 Foam for Enhanced Oil-Recovery Applications Using Polyelectrolytes and Polyelectrolyte Complex Nanoparticles. J. Appl. Polym. Sci. 2017, 134, 44491.
- Nazari, N.; Hosseini, H.; Tsau, J.S.; Shafer-Peltier, K.; Marshall, C.; Ye, Q.; Barati Ghahfarokhi, R. Development of Highly Stable Lamella Using Polyelectrolyte Complex Nanoparticles: An Environmentally Friendly ScCO2 Foam Injection Method for CO2 Utilization Using EOR. Fuel 2020, 261, 116360.
- Lai, N.; Zhu, Q.; Qiao, D.; Chen, K.; Wang, D.; Tang, L.; Chen, G. CO2/N2-Responsive Nanoparticles for Enhanced Oil Recovery During CO2 Flooding. Front. Chem. 2020, 8.
- Azizkhani, A.; Gandomkar, A. A Novel Method for Application of Nanoparticles as Direct Asphaltene Inhibitors during Miscible CO2 Injection. J. Pet. Sci. Eng. 2020, 185, 106661.
- Hassanpour, S.; Malayeri, M.R.; Riazi, M. Asphaltene Precipitation during Injection of CO2 Gas into a Synthetic Oil in the Presence of Fe3O4 and TiO2 Nanoparticles. J. Chem. Eng. Data 2018, 63, 1266–1274.
- Shah, R.D. Application of Nanoparticle Saturated Injectant Gases for EOR of Heavy Oils. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009; Volume 7.
- Cheraghian, G.; Rostami, S.; Afrand, M. Nanotechnology in Enhanced Oil Recovery. Processes 2020, 8, 1073.
- Foroozesh, J.; Kumar, S. Nanoparticles Behaviors in Porous Media: Application to Enhanced Oil Recovery. J. Mol. Liq. 2020, 316, 113876.
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An Overview of Chemical Enhanced Oil Recovery: Recent Advances and Prospects. Int. Nano Lett. 2019, 9, 171–202.
- Sergis, A.; Hardalupas, Y. Anomalous Heat Transfer Modes of Nanofluids: A Review Based on Statistical Analysis. Nanoscale Res. Lett. 2011, 6, 391.
More
Information
Subjects:
Geology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
02 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




